Paracetamol (Acetaminophen) and the Developing Brain
Abstract
:1. Introduction
2. Pharmacology of Paracetamol
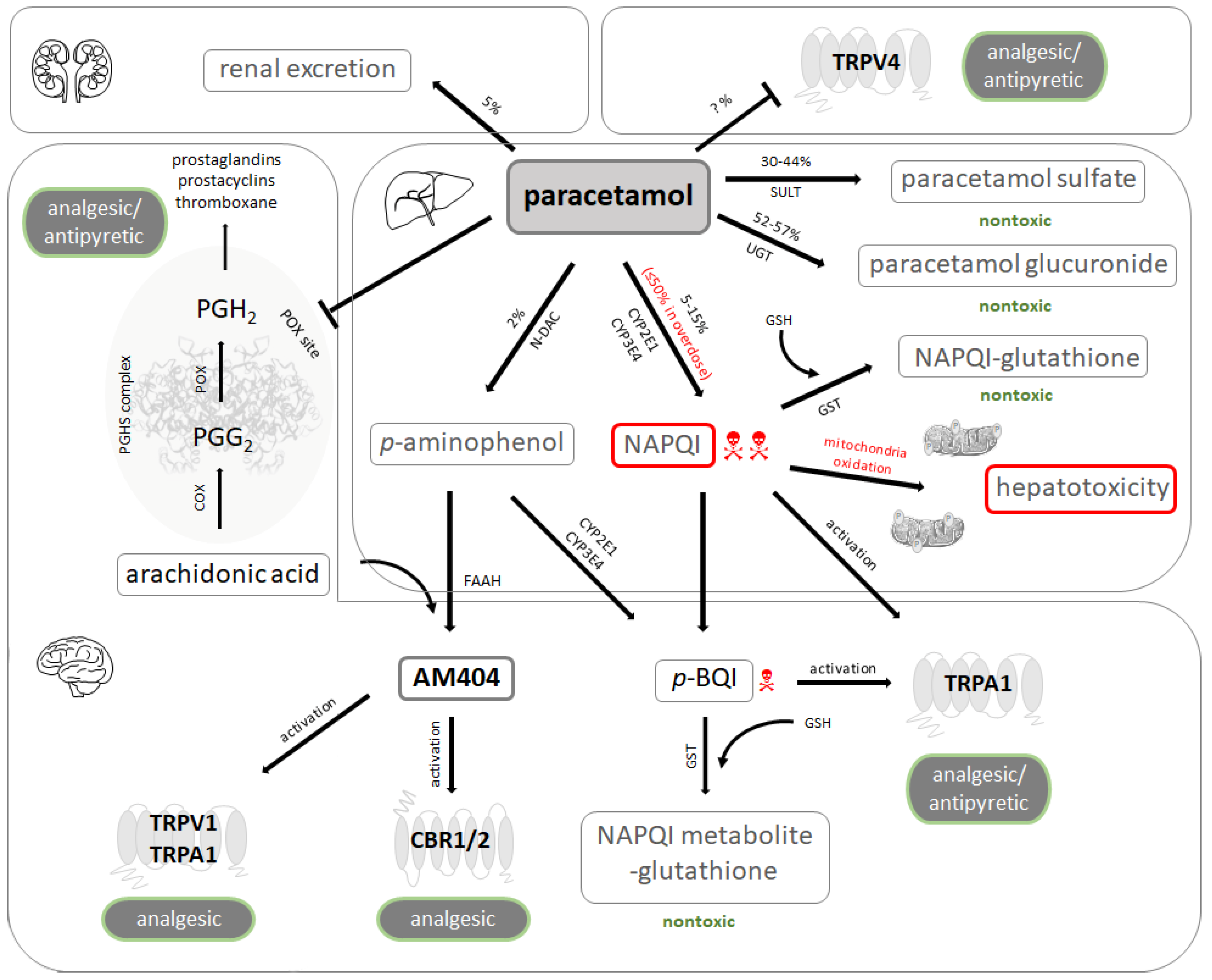
2.1. Inhibition of Prostaglandin Synthesis
2.2. Interaction with Central Receptors Involved in Nociception
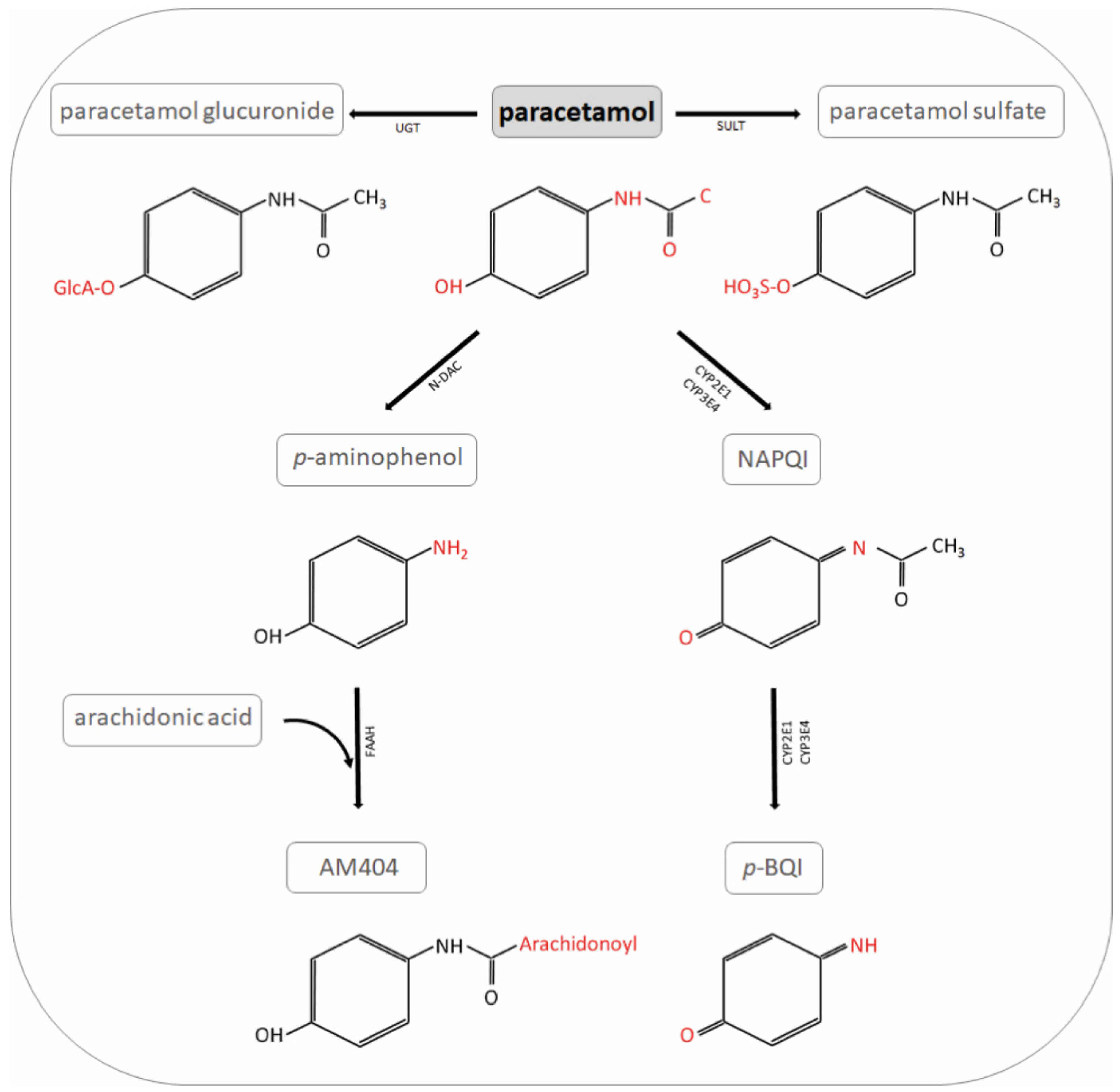
2.3. Pharmacokinetics and Toxicology
3. Epidemiological Studies Investigating the Impact of Paracetamol on the Developing Brain
3.1. Paracetamol Use during Pregnancy
3.2. Postnatal Use of Paracetamol in Term and Preterm Newborn Infants
4. Animal Models
5. Chronic Exposure to Ultra-Low Concentrations of Paracetamol
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| AM404 | N-arachidonoyl-phenolamine |
| ASD | Autism spectrum disorder |
| CBR1/2 | Cannabinoid receptors type 1 and 2 |
| COX | Cyclooxygenase |
| FAAH | Fatty acid amide hydrolase |
| FDA | Food and Drug Administration |
| GABA | γ-amino-butyric acid |
| GSH | Glutathione |
| GST | Glutathione S-transferase. |
| IQ | Intelligence quotient |
| HR | Hazard ratio |
| MRI | Magnetic resonance imaging |
| NAPQI | N-acetyl-p-benzo-quinone-imine |
| N-DAC | N-deacetylase |
| OR | Odds ratio |
| p-BQI | p-benzoquinone |
| PG G2 | Prostaglandin G2 |
| PG H2 | Prostaglandin H2 |
| PGHS | Prostaglandin endoperoxide-H synthase |
| POX | Peroxidase |
| SULT | Sulfotransferase |
| TRPA | Transient receptor potential ankyrin |
| TRPV | Transient vanilloid-subtype receptor |
| UGT | UDP-glucuronyl transferase |
References and Notes
- Bandoli, G.; Palmsten, K.; Chambers, C.D. Acetaminophen use in pregnancy: Examining prevalence, timing, and indication of use in a prospective birth cohort. Paediatr. Perinat. Epidemiol. 2019, 34, 237–246. [Google Scholar] [CrossRef]
- Mitra, S.; Florez, I.D.; Tamayo, M.E.; Mbuagbaw, L.; Vanniyasingam, T.; Veroniki, A.A.; Zea, A.M.; Zhang, Y.; Sadeghirad, B.; Thabane, L. Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen with Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants. JAMA 2018, 319, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Shah, P.S. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 1, CD010061. [Google Scholar] [CrossRef] [PubMed]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2020, 48, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Weisz, D.; McNamara, P. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Semin. Perinatol. 2018, 42, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Seibold, S.A.; Rieke, C.J.; Song, I.; Cukier, R.I.; Smith, W.L. Prostaglandin Endoperoxide H Synthases. J. Biol. Chem. 2007, 282, 18233–18244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.L.; Urade, Y.; Jakobsson, P.-J. Enzymes of the Cyclooxygenase Pathways of Prostanoid Biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, A.; Ajmone-Cat, M.A.; Nicolini, A.; Sciulli, M.G.; Minghetti, L. Paracetamol effectively reduces prostaglandin E2 synthesis in brain macrophages by inhibiting enzymatic activity of cyclooxygenase but not phospholipase and prostaglandin E synthase. J. Neurosci. Res. 2003, 71, 844–852. [Google Scholar] [CrossRef]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2007, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Davies, M.; Day, R.; Mohamudally, A.; Scott, K. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013, 21, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Dominguez, R.; Warner, T.; Vojnovic, I.; Mitchell, J.A. Cellular mechanisms of acetaminophen: Role of cyclo-oxygenase. FASEB J. 2005, 19, 1–15. [Google Scholar] [CrossRef]
- Wong, C.T.; Bestard-Lorigados, I.; Crawford, D.A. Autism-related behaviors in the cyclooxygenase-2-deficient mouse model. Genes Brain Behav. 2018, 18, e12506. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, F.; Higashi, S.; Ando, E.; Ohsumi, T.; Watanabe, S.; Takeuchi, H. Modification of TRPV4 activity by acetaminophen. Heliyon 2020, 6, e03301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallet, C.; Barriere, D.; Ermund, A.; Jönsson, B.; Eschalier, A.; Zygmunt, P.M.; Högestätt, E.D. TRPV1 in Brain Is Involved in Acetaminophen-Induced Antinociception. PLoS ONE 2010, 5, e12748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, C.; Andersson, D.A.; Bevan, S.J. TRPA1 mediates the hypothermic action of acetaminophen. Sci. Rep. 2015, 5, 12771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Högestätt, E.D.; Jönsson, B.; Ermund, A.; Andersson, D.; Björk, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of Acetaminophen to the Bioactive N-Acylphenolamine AM404 via Fatty Acid Amide Hydrolase-dependent Arachidonic Acid Conjugation in the Nervous System. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrière, D.A.; Mallet, C.; Blomgren, A.; Simonsen, C.; Daulhac, L.; Libert, F.; Chapuy, E.; Étienne, M.; Högestätt, E.D.; Zygmunt, P.M.; et al. Fatty Acid Amide Hydrolase-Dependent Generation of Antinociceptive Drug Metabolites Acting on TRPV1 in the Brain. PLoS ONE 2013, 8, e70690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.V.; Long, J.H.; Shah, S.; Rahman, J.; Perrett, D.; Ayoub, S.S.; Mehta, V. First evidence of the conversion of paracetamol to AM404 in human cerebrospinal fluid. J. Pain Res. 2017, 10, 2703–2709. [Google Scholar] [CrossRef] [Green Version]
- Barrière, D.A.; Boumezbeur, F.; Dalmann, R.; Cadeddu, R.; Richard, D.; Pinguet, J.; Daulhac, L.; Sarret, P.; Whittingstall, K.; Keller, M.; et al. Paracetamol is a centrally acting analgesic using mechanisms located in the periaqueductal grey. Br. J. Pharmacol. 2019, 177, 1773–1792. [Google Scholar] [CrossRef]
- Upadhya, S.C.; Tirumalai, P.S.; Boyd, M.R.; Mori, T.; Ravindranath, V. Cytochrome P4502E (CYP2E) in Brain: Constitutive Expression, Induction by Ethanol and Localization by Fluorescence in Situ Hybridization. Arch. Biochem. Biophys. 2000, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.; Andersson, A.; Bucher, B.; Galzi, J.-L.; Sterner, O.; Bevan, S.J.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeschke, H.; Adelusi, O.B.; Ramachandran, A. Ferroptosis and Acetaminophen Hepatotoxicity: Are We Going Down Another Rabbit Hole? Gene Expr. 2021, 20, 169–178. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Hinson, J.A. The development and hepatotoxicity of acetaminophen: Reviewing over a century of progress. Drug Metab. Rev. 2020, 52, 472–500. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Braithwaite, I.; McKinlay, C.J.D.; Dalziel, S.R. Comparison of Acetaminophen (Paracetamol) With Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years. JAMA Netw. Open 2020, 3, e2022398. [Google Scholar] [CrossRef]
- Posadas, I.; Santos, P.; Blanco, A.; Muñoz-Fernández, M.; Ceña, V. Acetaminophen Induces Apoptosis in Rat Cortical Neurons. PLoS ONE 2010, 5, e15360. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Garrettson, L.K.; Soda, D.M. Letter: Evidence of placental transfer of acetaminophen. Pediatrics 1975, 55, 895. [Google Scholar] [PubMed]
- Kumpulainen, E.; Kokki, H.; Halonen, T.; Heikkinen, M.; Savolainen, J.; Laisalmi, M. Paracetamol (Acetaminophen) Penetrates Readily into the Cerebrospinal Fluid of Children After Intravenous Administration. Pediatrics 2007, 119, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Rebordosa, C.; Lee, P.-C.; Olsen, J. Acetaminophen Use During Pregnancy, Behavioral Problems, and Hyperkinetic Disorders. JAMA Pediatr. 2014, 168, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Liew, Z.; Ritz, B.; Virk, J.; Olsen, J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism Res. 2015, 9, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Virk, J.; Arah, O.A.; Olsen, J. Prenatal Use of Acetaminophen and Child IQ. Epidemiology 2016, 27, 912–918. [Google Scholar] [CrossRef]
- Liew, Z.; Bach, C.C.; Asarnow, R.F.; Ritz, B.; Olsen, J. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int. J. Epidemiol 2016, 45, 2009–2017. [Google Scholar] [CrossRef]
- Inoue, K.; Ritz, B.; Ernst, A.; Tseng, W.L.; Yuan, Y.; Meng, Q.; Ramlau-Hansen, C.H.; Strandberg-Larsen, K.; Arah, O.A.; Obel, C.; et al. Behavioral Problems at Age 11 Years After Prenatal and Postnatal Exposure to Acetaminophen: Parent-Reported and Self-Reported Outcomes. Am. J. Epidemiol. 2020, 190, 1009–1020. [Google Scholar] [CrossRef]
- Liew, Z.; Kioumourtzoglou, M.-A.; Roberts, A.L.; O’Reilly, J.; Ascherio, A.; Weisskopf, M.G. Use of Negative Control Exposure Analysis to Evaluate Confounding: An Example of Acetaminophen Exposure and Attention-Deficit/Hyperactivity Disorder in Nurses’ Health Study II. Am. J. Epidemiol 2019, 188, 768–775. [Google Scholar] [CrossRef]
- Parker, S.E.; Collett, B.R.; Werler, M.M. Maternal acetaminophen use during pregnancy and childhood behavioural problems: Discrepancies between mother- and teacher-reported outcomes. Paediatr. Perinat. Epidemiol 2019, 34, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Brandlistuen, R.E.; Ystrom, E.; Nulman, I.; Koren, G.; Nordeng, H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int. J. Epidemiol 2013, 42, 1702–1713. [Google Scholar] [CrossRef] [Green Version]
- Vlenterie, R.; Wood, M.E.; Brandlistuen, R.E.; Roeleveld, N.; van Gelder, M.M.; Nordeng, H. Neurodevelopmental problems at 18 months among children exposed to paracetamolin utero: A propensity score matched cohort study. Int. J. Epidemiol. 2016, 45, 1998–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ystrom, E.; Gustavson, K.; Brandlistuen, R.E.; Knudsen, G.P.; Magnus, P.; Susser, E.; Smith, G.D.; Stoltenberg, C.; Surén, P.; Håberg, S.E.; et al. Prenatal Exposure to Acetaminophen and Risk of ADHD. Pediatrics 2017, 140, e20163840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trønnes, J.N.; Wood, M.; Lupattelli, A.; Ystrom, E.; Nordeng, H. Prenatal paracetamol exposure and neurodevelopmental outcomes in preschool-aged children. Paediatr. Perinat. Epidemiol 2019, 34, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.M.D.; Waldie, K.E.; Wall, C.; Murphy, R.; Mitchell, E.A.; The ABC Study Group. Associations between Acetaminophen Use during Pregnancy and ADHD Symptoms Measured at Ages 7 and 11 Years. PLoS ONE 2014, 9, e108210. [Google Scholar] [CrossRef] [Green Version]
- Stergiakouli, E.; Thapar, A.; Smith, G.D. Association of Acetaminophen Use During Pregnancy with Behavioral Problems in Childhood. JAMA Pediatr. 2016, 170, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Golding, J.; Gregory, S.; Clark, R.; Ellis, G.; Iles-Caven, Y.; Northstone, K. Associations between paracetamol (acetaminophen) intake between 18 and 32 weeks gestation and neurocognitive outcomes in the child: A longitudinal cohort study. Paediatr. Perinat. Epidemiol 2019, 34, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Avella-Garcia, C.B.; Julvez, J.; Fortuny, J.; Rebordosa, C.; García-Esteban, R.; Riaño-Galan, I.; Tardon, A.; Rodríguez-Bernal, C.L.; Iñiguez, C.; Andiarena, A.; et al. Acetaminophen use in pregnancy and neurodevelopment: Attention function and autism spectrum symptoms. Int. J. Epidemiol. 2016, 45, 1987–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovo-Rodrigues, L.; Carpena, M.X.; Martins-Silva, T.; Santos, I.S.; Anselmi, L.; Barros, A.J.D.; Barros, F.C.; Bertoldi, A.D.; Matijasevich, A. Low neurodevelopmental performance and behavioural/emotional problems at 24 and 48 months in Brazilian children exposed to acetaminophen during foetal development. Paediatr. Perinat. Epidemiol 2020, 34, 278–286. [Google Scholar] [CrossRef]
- Bertoldi, A.D.; Rifas-Shiman, S.L.; Boing, A.C.; Pizzol, T.D.S.D.; Miranda, V.I.A.; Silveira, M.P.T.; Silveira, M.F.; Domingues, M.R.; Santos, I.S.; Bassani, D.G.; et al. Associations of acetaminophen use during pregnancy and the first year of life with neurodevelopment in early childhood. Paediatr. Perinat. Epidemiol 2020, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Alemany, S.; Avella-García, C.; Liew, Z.; García-Esteban, R.; Inoue, K.; Cadman, T.; López-Vicente, M.; González, L.; Galán, I.R.; Andiarena, A.; et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur. J. Epidemiol 2021, 1–12. [Google Scholar] [CrossRef]
- Hoover, R.M.; Hayes, V.A.G.; Erramouspe, J. Association Between Prenatal Acetaminophen Exposure and Future Risk of Attention Deficit/Hyperactivity Disorder in Children. Ann. Pharmacother. 2015, 49, 1357–1361. [Google Scholar] [CrossRef]
- Masarwa, R.; Levine, H.; Gorelik, E.; Reif, S.; Perlman, A.; Matok, I. Prenatal Exposure to Acetaminophen and Risk for Attention Deficit Hyperactivity Disorder and Autistic Spectrum Disorder: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Cohort Studies. Am. J. Epidemiol 2018, 187, 1817–1827. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Kriebel, D.; Herbert, M.R.; Bornehag, C.-G.; Swan, S.H. Prenatal paracetamol exposure and child neurodevelopment: A review. Horm. Behav. 2018, 101, 125–147. [Google Scholar] [CrossRef]
- Gou, X.; Wang, Y.; Tang, Y.; Qu, Y.; Tang, J.; Shi, J.; Xiao, D.; Mu, D. Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: A meta-analysis. Aust. N. Z. J. Psychiatry 2019, 53, 195–206. [Google Scholar] [CrossRef]
- Damkier, P. Simple twist of fate: In utero exposure to acetaminophen and risk of childhood Attention Deficit Hyperactivity Disorder. Paediatr. Perinat. Epidemiol 2020, 34, 230–232. [Google Scholar] [CrossRef]
- Talge, N.M. Prenatal acetaminophen exposure and neurodevelopment: State of the evidence. Paediatr. Perinat. Epidemiol 2020, 34, 227–229. [Google Scholar] [CrossRef]
- Leppert, B.; Havdahl, A.; Riglin, L.; Jones, H.J.; Zheng, J.; Smith, G.D.; Tilling, K.; Thapar, A.; Reichborn-Kjennerud, T.; Stergiakouli, E. Association of Maternal Neurodevelopmental Risk Alleles with Early-Life Exposures. JAMA Psychiatry 2019, 76, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Brucato, M.; Ladd-Acosta, C.; Li, M.; Caruso, D.; Hong, X.; Kaczaniuk, J.; Stuart, E.A.; Fallin, M.D.; Wang, X. Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Res. 2017, 10, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Bresnahan, M.A.; Che, X.; Schultz, A.F.; Ukaigwe, J.E.; Eddy, M.L.; Hirtz, D.; Gunnes, N.; Lie, K.K.; Magnus, P.; et al. Prenatal fever and autism risk. Mol. Psychiatry 2017, 23, 759–766. [Google Scholar] [CrossRef]
- Gustavson, K.; Ask, H.; Ystrom, E.; Stoltenberg, C.; Lipkin, W.I.; Surén, P.; Håberg, S.E.; Magnus, P.; Knudsen, G.P.; Eilertsen, E.; et al. Maternal fever during pregnancy and offspring attention deficit hyperactivity disorder. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zerbo, O.; Qian, Y.; Yoshida, C.; Grether, J.K.; Van De Water, J.; Croen, L.A. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2013, 45, 4015–4025. [Google Scholar] [CrossRef] [Green Version]
- Masarwa, R.; Platt, R.W.; Filion, K.B. Acetaminophen use during pregnancy and the risk of attention deficit hyperactivity disorder: A causal association or bias? Paediatr. Perinat. Epidemiol 2020, 34, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Pan, T.-L.; Wang, P.-W.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Tsai, S.-J.; Chen, T.-J.; et al. Prenatal Exposure to Acetaminophen and the Risk of Attention-Deficit/Hyperactivity Disorder: A Nationwide Study in Taiwan. J. Clin. Psychiatry 2019, 80. [Google Scholar] [CrossRef] [PubMed]
- Bornehag, C.-G.; Reichenberg, A.; Hallerback, M.U.; Wikstrom, S.; Koch, H.; Jonsson, B.; Swan, S. Prenatal exposure to acetaminophen and children’s language development at 30 months. Eur. Psychiatry 2018, 51, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Riley, A.W.; Lee, L.-C.; Hong, X.; Wang, G.; Tsai, H.-J.; Mueller, N.T.; Pearson, C.; Thermitus, J.; Panjwani, A.; et al. Maternal Biomarkers of Acetaminophen Use and Offspring Attention Deficit Hyperactivity Disorder. Brain Sci. 2018, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Azuine, R.E.; Zhang, Y.; Hou, W.; Hong, X.; Wang, G.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure with Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry 2020, 77, 180–189. [Google Scholar] [CrossRef]
- Baker, B.H.; Lugo-Candelas, C.; Wu, H.; Laue, H.E.; Boivin, A.; Gillet, V.; Aw, N.; Rahman, T.; Lepage, J.-F.; Whittingstall, K.; et al. Association of Prenatal Acetaminophen Exposure Measured in Meconium with Risk of Attention-Deficit/Hyperactivity Disorder Mediated by Frontoparietal Network Brain Connectivity. JAMA Pediatr. 2020, 174, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Modick, H.; Weiss, T.; Dierkes, G.; Brüning, T.; Koch, H.M. Ubiquitous presence of paracetamol in human urine: Sources and implications. Reproduction 2014, 147, R105–R117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laue, H.E.; Cassoulet, R.; Abdelouahab, N.; Serme-Gbedo, Y.K.; Desautels, A.S.; Brennan, K.J.; Bellenger, J.P.; Burris, H.H.; Coull, B.A.; Weisskopf, M.G.; et al. Association Between Meconium Acetaminophen and Childhood Neurocognitive Development in GESTE, a Canadian Cohort Study. Toxicol. Sci. 2018, 167, 138–144. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Drug Safety Communications. FDA has reviewed possible risks of pain medicine use during pregnancy 1-9-2015.
- European Medicine Agency. PRAC recommendations on signals. EMA/PRAC/157165/2019. 8-4-2019.
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.-G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T.; et al. Paracetamol use during pregnancy—a call for precautionary action. Nat. Rev. Endocrinol. 2021, 1–10. [Google Scholar] [CrossRef]
- Allegaert, K. A Critical Review on the Relevance of Paracetamol for Procedural Pain Management in Neonates. Front. Pediatr. 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlsson, A.; Shah, P.S. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database Syst. Rev. 2020, 1, CD011219. [Google Scholar] [CrossRef]
- Tinner, E.M.; Hoesli, I.; Jost, K.; Schöbi, N.; Megged, Y.U.; Burkhardt, T.; Krafft, A.; Bucher, H.U.; Surbek, D.; Nelle, M.; et al. Rectal Paracetamol in Newborn Infants after Assisted Vaginal Delivery May Increase Pain Response. J. Pediatr. 2013, 162, 62–66. [Google Scholar] [CrossRef]
- Eras, Z.; Uras, N.; Canpolat, F.E.; Erdeve, O.; Oguz, S.S.; Oncel, M.Y. Neurodevelopmental Outcomes of Preterm Infants Treated with Oral Paracetamol Versus Ibuprofen for Patent Ductus Arteriosus. Am. J. Perinatol. 2017, 34, 1185–1189. [Google Scholar] [CrossRef]
- Juujärvi, S.; Kallankari, H.; Pätsi, P.; Leskinen, M.; Saarela, T.; Hallman, M.; Aikio, O. Follow-up study of the early, randomised paracetamol trial to preterm infants, found no adverse reactions at the two-years corrected age. Acta Paediatr. 2018, 108, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Juujärvi, S.; Saarela, T.; Pokka, T.; Hallman, M.; Aikio, O. Intravenous paracetamol for neonates: Long-term diseases not escalated during 5 years of follow-up. Arch. Dis. Child.-Fetal Neonatal Ed. 2020, 106, 178–183. [Google Scholar] [CrossRef]
- Bittker, S.S.; Bell, K.R. Postnatal Acetaminophen and Potential Risk of Autism Spectrum Disorder among Males. Behav. Sci. 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Koehn, L.M.; Huang, Y.; Habgood, M.D.; Kysenius, K.; Crouch, P.J.; Dziegielewska, K.M.; Saunders, N.R. Effects of paracetamol (acetaminophen) on gene expression and permeability properties of the rat placenta and fetal brain. F1000Research 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Thiele, K.; Solano, M.E.; Huber, S.; Flavell, R.A.; Kessler, T.; Barikbin, R.; Jung, R.; Karimi, K.; Tiegs, G.; Arck, P.C. Prenatal Acetaminophen Affects Maternal Immune and Endocrine Adaptation to Pregnancy, Induces Placental Damage, and Impairs Fetal Development in Mice. Am. J. Pathol. 2015, 185, 2805–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, K.; Keßler, T.; Thiele, K.; Ramisch, K.; Erhardt, A.; Huebener, P.; Barikbin, R.; Arck, P.; Tiegs, G. Prenatal acetaminophen induces liver toxicity in dams, reduces fetal liver stem cells, and increases airway inflammation in adult offspring. J. Hepatol. 2015, 62, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Hegde, S.; Kechichian, T.; Gamble, P.; Rahman, M.; Stutz, S.J.; Anastasio, N.C.; AlShehri, W.; Lei, J.; Mori, S.; et al. Is There a Causal Relation between Maternal Acetaminophen Administration and ADHD? PLoS ONE 2016, 11, e0157380. [Google Scholar] [CrossRef]
- Hay-Schmidt, A.; Finkielman, O.T.E.; Jensen, B.A.H.; Høgsbro, C.F.; Holm, J.B.; Johansen, K.H.; Jensen, T.K.; Andrade, A.M.; Swan, S.H.; Bornehag, C.-G.; et al. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour. Reproduction 2017, 154, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.M.; Rigobello, C.; Vidigal, C.B.; Moura, K.F.; Barbosa, D.S.; Gerardin, D.C.C.; Ceravolo, G.; Moreira, E.G. Gestational exposure to paracetamol in rats induces neurofunctional alterations in the progeny. Neurotoxicol Teratol. 2019, 77, 106838. [Google Scholar] [CrossRef]
- Rigobello, C.; Klein, R.M.; Debiasi, J.D.; Ursini, L.G.; Michelin, A.P.; Matsumoto, A.K.; Barbosa, D.S.; Moreira, E.G. Perinatal exposure to paracetamol: Dose and sex-dependent effects in behaviour and brain’s oxidative stress markers in progeny. Behav. Brain Res. 2021, 408, 113294. [Google Scholar] [CrossRef] [PubMed]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Clancy, B.; Darlington, R.; Finlay, B. Translating developmental time across mammalian species. Neuroscience 2001, 105, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Workman, A.D.; Charvet, C.J.; Clancy, B.; Darlington, R.B.; Finlay, B.L. Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. J. Neurosci. 2013, 33, 7368–7383. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H.; Eriksson, P.; Gordh, T.; Fredriksson, A. Paracetamol (Acetaminophen) Administration During Neonatal Brain Development Affects Cognitive Function and Alters Its Analgesic and Anxiolytic Response in Adult Male Mice. Toxicol. Sci. 2013, 138, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippot, G.; Gordh, T.; Fredriksson, A.; Viberg, H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): Characterization of a critical period. J. Appl. Toxicol. 2017, 37, 1174–1181. [Google Scholar] [CrossRef]
- Philippot, G.; Hallgren, S.; Gordh, T.; Fredriksson, A.; Fredriksson, R.; Viberg, H. A Cannabinoid Receptor Type 1 (CB1R) Agonist Enhances the Developmental Neurotoxicity of Acetaminophen (Paracetamol). Toxicol. Sci. 2018, 166, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, N.; Hernandez, J.C.; Poulton, J.; Jones, J.P.; Konsoula, Z.; Smith, C.; Parker, W. Therapeutic doses of acetaminophen with co-administration of cysteine and mannitol during early development result in long term behavioral changes in laboratory rats. PLoS ONE 2021, 16, e0253543. [Google Scholar] [CrossRef]
- Blecharz-Klin, K.; Wawer, A.; Jawna-Zboińska, K.; Pyrzanowska, J.; Piechal, A.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Early paracetamol exposure decreases brain-derived neurotrophic factor (BDNF) in striatum and affects social behaviour and exploration in rats. Pharmacol. Biochem. Behav. 2018, 168, 25–32. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Silva, S.; Cardoso, V.V.; Benoliel, M.J.; Cardoso, E.; Coelho, M.R.; Martins, A.; Almeida, C.M.M. Screening and Seasonal Behavior of Analgesics, Non-steroidal Anti-inflammatory Drugs, and Antibiotics in Two Urban Wastewater Treatment Plants. Environ. Manag. 2021, 68, 411–425. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Pinto, G.; Correia, B.; Nunes, B. Embryonic development, locomotor behavior, biochemical, and epigenetic effects of the pharmaceutical drugs paracetamol and ciprofloxacin in larvae and embryos of Danio rerio when exposed to environmental realistic levels of both drugs. Environ. Toxicol. 2019, 34, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.P.; Nunes, B. Dangerous connections: Biochemical and behavioral traits in Daphnia magna and Daphnia longispina exposed to ecologically relevant amounts of paracetamol. Environ. Sci. Pollut. Res. 2021, 28, 38792–38808. [Google Scholar] [CrossRef]
- Koagouw, W.; Stewart, N.A.; Ciocan, C. Long-term exposure of marine mussels to paracetamol: Is time a healer or a killer? Environ. Sci. Pollut. Res. 2021, 28, 48823–48836. [Google Scholar] [CrossRef] [PubMed]
| Cohort | Country Code | Years of Birth | n | Age (Years) | Outcome | Assessed by | Association | References |
|---|---|---|---|---|---|---|---|---|
| Danish National Birth Cohort (DNBC) | DK | 1996–2002 | 64,322 | 7 | Behavior | Q D | Yes | [28] |
| ADHD | Yes | |||||||
| ADHD | ||||||||
| medication | Yes | |||||||
| Autism | D | Yes | [29] | |||||
| Lifestyle During Pregnancy Study (DNBC sub-cohort) | 1491 | 5 | IQ | T | Yes | [30] | ||
| Attention | T Q | Yes | [31] | |||||
| 40,934 | 11 | Behavior | Q | Yes | [32] | |||
| Nurses’ Health Study II | US | 1993–2005 | 8856 | ≥8 | ADHD | D | Yes | [33] |
| Craniofacial malformation, hemifacial microsomia study | US, CA | 1996–2002 | 560 | 6–12 | Behavior | Q | Yes/No | [34] |
| Norwegian Mother and Child Cohort Study (MoBa) | NO | 1999–2008 | 15,256 | 3 | Behavior | Q | Yes | [35] |
| Yes | ||||||||
| Yes | ||||||||
| 51,200 | 1½ | Behavior | Q | Yes | [36] | |||
| 112,973 | 3–13 | ADHD | D | Yes | [37] | |||
| 32,934 | 5 | Behavior | Q | Yes | [38] | |||
| Yes | ||||||||
| Yes | ||||||||
| Auckland Birthweight Collaborative Study | NZ | 1995–1997 | 871 | 7, 11 | Behavior | Q | Yes | [39] |
| Avon Longitudinal Study of Parents and Children (ALSPAC) | UK | 1991–1992 | 7796 | 7 | Behavior | Q | Yes | [40] |
| 12,418 | ½–15 | IQ Behavior | T | No | [41] | |||
| Q | Yes | |||||||
| INfancia y Medio Ambiente (INMA) | ES | 2004–2008 | 2644 | 5 | Behavior | T | Yes | [42] |
| Viva | US | 1999–2002 | 1217 | 3 | Cognition | T | No | [43] |
| Pelotas | BR | 2015 | 3818 | 2 | Cognition | T | No | [43] |
| 2004 | 3624 | 4 | Behavior | Q | No | [44] | ||
| ALSPAC | UK | 1991–1992 | 6200 | 7 | Behavior | Q | Yes | [45] |
| Generation R | NL | 2001–2005 | 3904 | 8 | ||||
| INMA | ES | 2004–2008 | 1513 | 4–5 | ||||
| GASPII | IT | 2003–2004 | 489 | 4 | ||||
| DNBC | DK | 1996–2002 | 61,430 | 7 | ||||
| RHEA | GR | 2007–2008 | 345 | 6 |
| Cohort | Country Code | Years of Birth | n | Age (Years) | Outcome | Association | References |
|---|---|---|---|---|---|---|---|
| Swedish Environmental Longitudinal, Mother and child, Asthma and allergy (SELMA) | SE | 2007–2010 | 754 | 3 | Language | Yes | [59] |
| Boston Birth cohort | US | 1998–2013 | 1180 | Physician-diagnosed ADHD | Yes | [60] | |
| 1998–2018 | 996 | 10 | Physician-diagnosed ADHD | Yes | [61] | ||
| Gestation and the Environment Cohort (GESTE) | CA | 2007–2009 | 195 | 6–8 | IQ | No | [64] |
| CA | 345 48 | 6–7 9–11 | Physician-diagnosed ADHD Functional MRI | Yes Yes | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bührer, C.; Endesfelder, S.; Scheuer, T.; Schmitz, T. Paracetamol (Acetaminophen) and the Developing Brain. Int. J. Mol. Sci. 2021, 22, 11156. https://doi.org/10.3390/ijms222011156
Bührer C, Endesfelder S, Scheuer T, Schmitz T. Paracetamol (Acetaminophen) and the Developing Brain. International Journal of Molecular Sciences. 2021; 22(20):11156. https://doi.org/10.3390/ijms222011156
Chicago/Turabian StyleBührer, Christoph, Stefanie Endesfelder, Till Scheuer, and Thomas Schmitz. 2021. "Paracetamol (Acetaminophen) and the Developing Brain" International Journal of Molecular Sciences 22, no. 20: 11156. https://doi.org/10.3390/ijms222011156
APA StyleBührer, C., Endesfelder, S., Scheuer, T., & Schmitz, T. (2021). Paracetamol (Acetaminophen) and the Developing Brain. International Journal of Molecular Sciences, 22(20), 11156. https://doi.org/10.3390/ijms222011156






