Supplementation with Lactiplantibacillus plantarum IMC 510 Modifies Microbiota Composition and Prevents Body Weight Gain Induced by Cafeteria Diet in Rats
Abstract
:1. Introduction
2. Results
2.1. Effects of L. plantarum IMC 510-Dietary Supplementation on Body Weight and Feeding Consumption
2.2. Effects of L. plantarum IMC 510-Dietary Supplementation on Blood Parameters
2.3. Liver and Adipose Tissue: Morphological Analysis
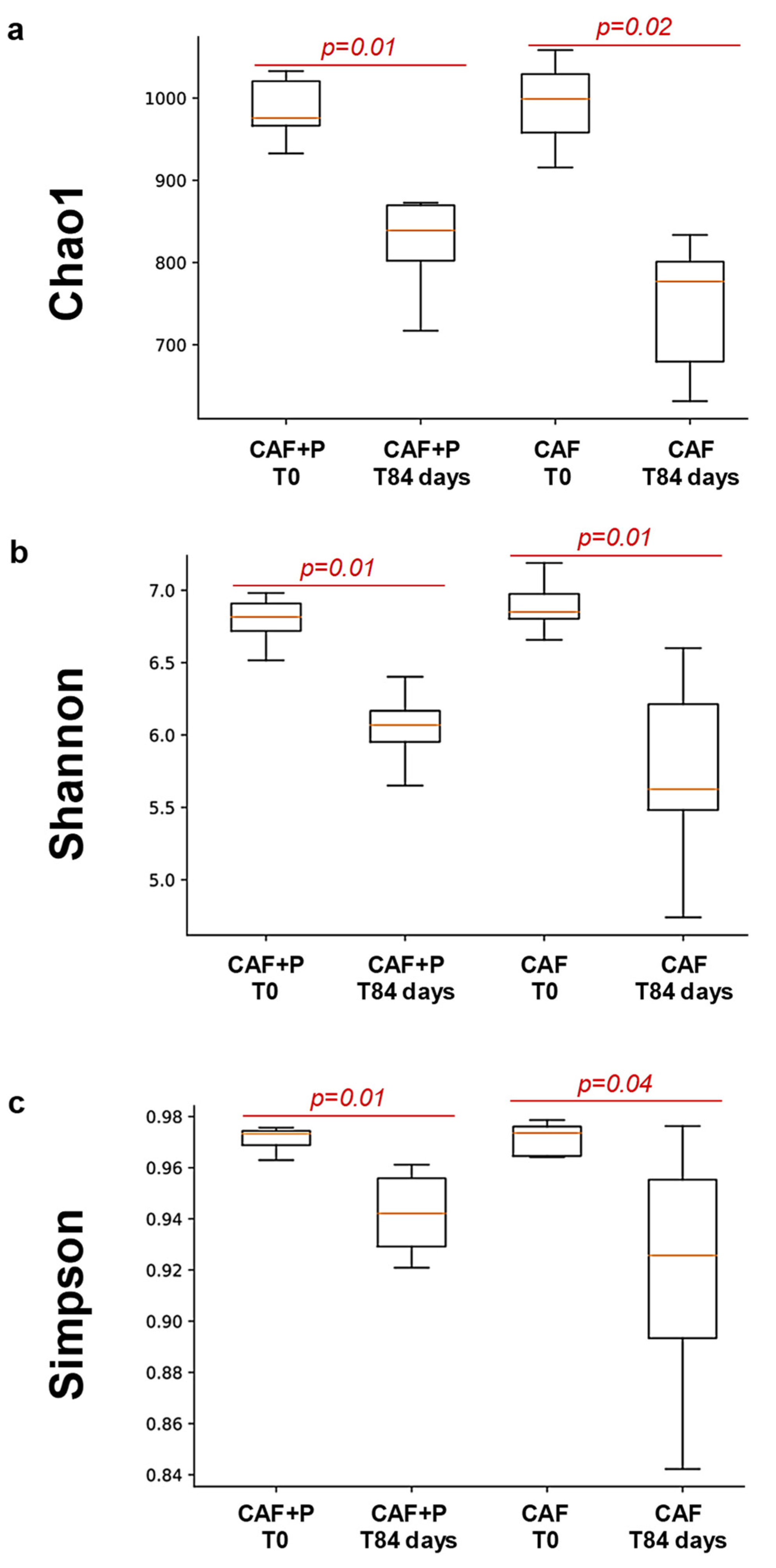
2.4. Modulation of the Gut Microbial Composition after L. plantarum IMC 510 Administration
2.4.1. Quantitative Real-Time PCR
2.4.2. Profiling of the GM by 16S Next-Generation Sequencing (NGS)
2.4.3. Intestinal Colonization of L. plantarum IMC 510
3. Discussion
4. Materials and Methods
4.1. Subjects and Diet Composition
4.2. Probiotic Supplementation
4.3. Blood Parameters
4.4. Liver and Adipose Tissue: Morphological Analysis
4.5. Microbiota Composition Analysis of Fecal Samples
4.5.1. Bacterial DNA Extraction
4.5.2. Quantitative Real-Time PCR
4.5.3. 16S NGS and Analysis
4.5.4. Recovery of L. plantarum IMC 510
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schetz, M.; De Jong, A.; Deane, A.M.; Druml, W.; Hemelaar, P.; Pelosi, P.; Pickkers, P.; Reintam-Blaser, A.; Roberts, J.; Sakr, Y.; et al. Obesity in the critically ill: A narrative review. Intensive Care Med. 2019, 45, 757–769. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [Green Version]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Office of Library and Health Literature. Services Styles for Bibliographic Citations: Guidelines for WHO-Produced Bibliographies; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [Green Version]
- Bliss, E.S.; Whiteside, E. The Gut-Brain Axis, the Human Gut Microbiota and Their Integration in the Development of Obesity. Front. Physiol. 2018, 9, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, V.E. Overview of human obesity and central mechanisms regulating energy homeostasis. Ann. Clin. Biochem. 2008, 45, 245–255. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, C.; Micioni Di Bonaventura, M.V.; Pucci, M.; Romano, A.; Gaetani, S.; Ciccocioppo, R.; Cifani, C.; Maccarrone, M. Endocannabinoid signaling and food addiction. Neurosci. Biobehav. Rev. 2014, 47, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Micioni Di Bonaventura, E.; Botticelli, L.; Tomassoni, D.; Tayebati, S.K.; Micioni Di Bonaventura, M.V.; Cifani, C. The Melanocortin System behind the Dysfunctional Eating Behaviors. Nutrients 2020, 12, 3502. [Google Scholar] [CrossRef]
- Suarez, A.N.; Liu, C.M.; Cortella, A.M.; Noble, E.E.; Kanoski, S.E. Ghrelin and Orexin Interact to Increase Meal Size Through a Descending Hippocampus to Hindbrain Signaling Pathway. Biol. Psychiatry 2020, 87, 1001–1011. [Google Scholar] [CrossRef]
- Baqai, N.; Wilding, J.P.H. Pathophysiology and aetiology of obesity. Medicine 2015, 43, 73–76. [Google Scholar] [CrossRef]
- Botticelli, L.; Micioni Di Bonaventura, E.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Romano, A.; Quaglia, W.; Cifani, C.; Micioni Di Bonaventura, M.V. Underlying Susceptibility to Eating Disorders and Drug Abuse: Genetic and Pharmacological Aspects of Dopamine D4 Receptors. Nutrients 2020, 12, 2288. [Google Scholar] [CrossRef]
- Dhurandhar, E.J.; Keith, S.W. The aetiology of obesity beyond eating more and exercising less. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 533–544. [Google Scholar] [CrossRef]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; Ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metab. Clin. Exp. 2019, 92, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Micioni Di Bonaventura, E.; Botticelli, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Cifani, C.; Micioni Di Bonaventura, M.V. Assessing the role of ghrelin and the enzyme ghrelin O-acyltransferase (GOAT) system in food reward, food motivation, and binge eating behavior. Pharmacol. Res. 2021, 172, 105847. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Brode, C.S.; Mitchell, J.E. Problematic Eating Behaviors and Eating Disorders Associated with Bariatric Surgery. Psychiatr. Clin. N. Am. 2019, 42, 287–297. [Google Scholar] [CrossRef]
- Kushner, R.F. Weight Loss Strategies for Treatment of Obesity: Lifestyle Management and Pharmacotherapy. Prog. Cardiovasc. Dis. 2018, 61, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Grandone, A.; Di Sessa, A.; Umano, G.R.; Toraldo, R.; Miraglia Del Giudice, E. New treatment modalities for obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Munoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019, 72, 26–37. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [Green Version]
- Cerdo, T.; Garcia-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [Green Version]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huang, Y.; Song, L.; Xiao, Y.; Lu, S.; Xu, J.; Li, J.; Ren, Z. Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice. J. Funct. Foods 2020, 73, 104103. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Li, X.; Meng, K.; Yang, P. Lactobacillus plantarum FRT10 alleviated high-fat diet-induced obesity in mice through regulating the PPARalpha signal pathway and gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 5959–5972. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kwon, M.H.; Kim, H.K.; Lee, H.S.; Cho, J.S.; Lee, Y.I. Anti-Obesity Effect of Lactobacillus plantarum LB818 Is Associated with Regulation of Gut Microbiota in High-Fat Diet-Fed Obese Mice. J. Med. Food 2020, 23, 750–759. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Z.; Chen, X.; Huang, Y.; Yin, B.; Guo, F.; Zhao, H.; Zhao, T.; Qu, H.; Huang, J.; et al. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. BMC Complementary Altern. Med. 2014, 14, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Jung, H.H.; Kim, Y.H.; Kim, T.H. Antiobesity Effects of Lactobacillus plantarum LMT1-48 Accompanied by Inhibition of Enterobacter cloacae in the Intestine of Diet-Induced Obese Mice. J. Med. Food 2019, 22, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Ryu, D.W.; Song, S.M.; Kim, Y.R.; Jung, H.H.; Kim, T.H.; Kim, Y.H. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci. Rep. 2020, 10, 869. [Google Scholar] [CrossRef] [Green Version]
- Drissi, F.; Raoult, D.; Merhej, V. Metabolic role of lactobacilli in weight modification in humans and animals. Microb. Pathog. 2017, 106, 182–194. [Google Scholar] [CrossRef]
- Ranji, P.; Agah, S.; Heydari, Z.; Rahmati-Yamchi, M.; Mohammad Alizadeh, A. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the serum biochemical parameters, and the vitamin D and leptin receptor genes on mice colon cancer. Iran. J. Basic Med Sci. 2019, 22, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef] [Green Version]
- de Lartigue, G.; Barbier de la Serre, C.; Espero, E.; Lee, J.; Raybould, H.E. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E187–E195. [Google Scholar] [CrossRef] [Green Version]
- Fam, B.C.; Morris, M.J.; Hansen, M.J.; Kebede, M.; Andrikopoulos, S.; Proietto, J.; Thorburn, A.W. Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diabetes Obes. Metab. 2007, 9, 840–852. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalova, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Zinocker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Shafat, A.; Murray, B.; Rumsey, D. Energy density in cafeteria diet induced hyperphagia in the rat. Appetite 2009, 52, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human Western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.J.; Kendig, M.D.; Morris, M.J. Palatable Western-style Cafeteria Diet as a Reliable Method for Modeling Diet-induced Obesity in Rodents. J. Vis. Exp. JoVE 2019, 153, e60262. [Google Scholar] [CrossRef]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; Cresci, A. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef]
- Coman, M.M.; Cresci, A. Probiotics and prebiotics for human health: Innovations and new trends. In Edizioni Accademiche Italiane trademark of OmniScriptum; GmbH & Co. KG: Saarbrucken, Deutschland/Germany, 2014. [Google Scholar]
- Verdenelli, M.C.; Ghelfi, F.; Silvi, S.; Orpianesi, C.; Cecchini, C.; Cresci, A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur. J. Nutr. 2009, 48, 355–363. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Zhao, M.; Mo, Q.; Feng, F. Weight-Reducing Effect of Lactobacillus plantarum ZJUFT17 Isolated from Sourdough Ecosystem. Nutrients 2020, 12, 977. [Google Scholar] [CrossRef] [Green Version]
- Miras, A.D.; le Roux, C.W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays News Rev. Mol. Cell. Dev. Biol. 2014, 36, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazier, T.H.; DiBaise, J.K.; McClain, C.J. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. J. Parenter. Enter. Nutr. 2011, 35, 14S–20S. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Chung, Y.W.; Gwak, H.J.; Moon, S.; Rho, M.; Ryu, J.H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e0227886. [Google Scholar] [CrossRef] [Green Version]
- McNamara, M.P.; Singleton, J.M.; Cadney, M.D.; Ruegger, P.M.; Borneman, J.; Garland, T. Early-life effects of juvenile Western diet and exercise on adult gut microbiome composition in mice. J. Exp. Biol. 2021, 224, jeb239699. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, H.B.; Lee, E.; Park, H.Y. The Effects of Gelatinized Wheat Starch and High Salt Diet on Gut Microbiota and Metabolic Disorder. Nutrients 2020, 12, 301. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Ishaq, S.L.; Zhao, F.Q.; Wright, A.G. Colonic inflammation accompanies an increase of beta-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J. Nutr. Biochem. 2016, 35, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [Green Version]
- McFarland, L.V. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: A systematic review. BMJ Open 2014, 4, e005047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Micioni Di Bonaventura, M.V.; Martinelli, I.; Moruzzi, M.; Micioni Di Bonaventura, E.; Giusepponi, M.E.; Polidori, C.; Lupidi, G.; Tayebati, S.K.; Amenta, F.; Cifani, C.; et al. Brain alterations in high fat diet induced obesity: Effects of tart cherry seeds and juice. Nutrients 2020, 12, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, R.M.; Still, W.J. Pathogenesis of atherosclerosis. Fed. Proc. 1962, 21, 12–14. [Google Scholar] [PubMed]
- Sasaki, S.; Yoneda, Y.; Fujita, H.; Uchida, A.; Takenaka, K.; Takesako, T.; Itoh, H.; Nakata, T.; Takeda, K.; Nakagawa, M. Association of blood pressure variability with induction of atherosclerosis in cholesterol-fed rats. Am. J. Hypertens. 1994, 7, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Schulz, R.; Badimon, L.; Adameova, A.; Kleinbongard, P.; Lecour, S.; Nikolaou, P.E.; Falcao-Pires, I.; Vilahur, G.; Woudberg, N.; et al. Hyperlipidaemia and cardioprotection: Animal models for translational studies. Br. J. Pharmacol. 2020, 177, 5287–5311. [Google Scholar] [CrossRef] [PubMed]
- Genangeli, M.; Heijens, A.M.M.; Rustichelli, A.; Schuit, N.D.; Micioni Di Bonaventura, M.V.; Cifani, C.; Vittori, S.; Siegel, T.P.; Heeren, R.M.A. MALDI-Mass Spectrometry Imaging to Investigate Lipid and Bile Acid Modifications Caused by Lentil Extract Used as a Potential Hypocholesterolemic Treatment. J. Am. Soc. Mass Spectrom. 2019, 30, 2041–2050. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, M.V.; Cecchini, C.; Vila-Donat, P.; Caprioli, G.; Cifani, C.; Coman, M.M.; Cresci, A.; Fiorini, D.; Ricciutelli, M.; Silvi, S.; et al. Evaluation of the hypocholesterolemic effect and prebiotic activity of a lentil (Lens culinaris Medik) extract. Mol. Nutr. Food Res. 2017, 61, 1700403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeung, W.H.; Nam, W.; Kim, H.J.; Kim, J.Y.; Nam, B.; Jang, S.S.; Lee, J.L.; Sim, J.H.; Park, S.D. Oral Administration of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 with Cinnamomi Ramulus Extract Reduces Diet-Induced Obesity and Modulates Gut Microbiota. Prev. Nutr. Food Sci. 2019, 24, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fan, C.; Fan, X.; Lu, Y.; Wang, Y.; Wang, R.; Tang, T.; Qi, K. Effects of gut microbiota on leptin expression and body weight are lessened by high-fat diet in mice. Br. J. Nutr. 2020, 124, 396–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yang, X.; Yu, S.; Zheng, R. The Leptin Resistance. Adv. Exp. Med. Biol. 2018, 1090, 145–163. [Google Scholar] [CrossRef]
- Pan, W.W.; Myers, M.G., Jr. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 2018, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- van Swieten, M.M.; Pandit, R.; Adan, R.A.; van der Plasse, G. The neuroanatomical function of leptin in the hypothalamus. J. Chem. Neuroanat. 2014, 61, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, G.A.; Paschou, S.A.; Mantzoros, C.S. Leptin and Hormones: Energy Homeostasis. Endocrinol. Metab. Clin. North Am. 2016, 45, 633–645. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin resistance in obesity: An overview of fundamental alterations. Eat. Weight Disord. EWD 2018, 23, 149–157. [Google Scholar] [CrossRef]
- Caputo, T.; Gilardi, F.; Desvergne, B. From chronic overnutrition to metaflammation and insulin resistance: Adipose tissue and liver contributions. FEBS Lett. 2017, 591, 3061–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, I.; Micioni Di Bonaventura, M.V.; Moruzzi, M.; Amantini, C.; Maggi, F.; Gabrielli, M.G.; Fruganti, A.; Marchegiani, A.; Dini, F.; Marini, C.; et al. Effects of Prunus cerasus L. Seeds and Juice on Liver Steatosis in an Animal Model of Diet-Induced Obesity. Nutrients 2020, 12, 1308. [Google Scholar] [CrossRef]
- Peng, Y.; Rideout, D.; Rakita, S.; Lee, J.; Murr, M. Diet-induced obesity associated with steatosis, oxidative stress, and inflammation in liver. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2012, 8, 73–81. [Google Scholar] [CrossRef]
- Tariq, Z.; Green, C.J.; Hodson, L. Are oxidative stress mechanisms the common denominator in the progression from hepatic steatosis towards non-alcoholic steatohepatitis (NASH)? Liver Int. Off. J. Int. Assoc. Study Liver 2014, 34, e180–e190. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients 2019, 11, 2642. [Google Scholar] [CrossRef] [Green Version]
- Al-Muzafar, H.M.; Amin, K.A. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complementary Altern. Med. 2017, 17, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, E.T.; Palacios, T.; Thomsen, M.; Vitetta, L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-alcoholic Fatty Liver Disease. Front. Microbiol. 2018, 9, 61. [Google Scholar] [CrossRef]
- Pereg, D.; Kotliroff, A.; Gadoth, N.; Hadary, R.; Lishner, M.; Kitay-Cohen, Y. Probiotics for patients with compensated liver cirrhosis: A double-blind placebo-controlled study. Nutrition 2011, 27, 177–181. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Roy, P.; Tomassoni, D.; Traini, E.; Martinelli, I.; Micioni Di Bonaventura, M.V.; Cifani, C.; Amenta, F.; Tayebati, S.K. Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence. Antioxidants 2021, 10, 858. [Google Scholar] [CrossRef]
- Cifani, C.; Avagliano, C.; Micioni Di Bonaventura, E.; Giusepponi, M.E.; De Caro, C.; Cristiano, C.; La Rana, G.; Botticelli, L.; Romano, A.; Calignano, A.; et al. Modulation of Pain Sensitivity by Chronic Consumption of Highly Palatable Food Followed by Abstinence: Emerging Role of Fatty Acid Amide Hydrolase. Front. Pharmacol. 2020, 11, 266. [Google Scholar] [CrossRef]
- Giudetti, A.M.; Micioni Di Bonaventura, M.V.; Ferramosca, A.; Longo, S.; Micioni Di Bonaventura, E.; Friuli, M.; Romano, A.; Gaetani, S.; Cifani, C. Brief daily access to cafeteria-style diet impairs hepatic metabolism even in the absence of excessive body weight gain in rats. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 9358–9371. [Google Scholar] [CrossRef]
- Silvi, S.; Verdenelli, M.; Orpianesi, C.; Cresci, A. EU project Crownalife: Functional foods, gut microflora and healthy ageing: Isolation and identification of Lactobacillus and Bifidobacterium strains from faecal samples of elderly subjects for a possible probiotic use in functional foods. J. Food Eng. 2003, 56, 195–200. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Coman, M.M.; Olek, R.A.; Fiorini, D.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Fedeli, D.; Gabbianelli, R. Changes on fecal microbiota in rats exposed to permethrin during postnatal development. Environ. Sci. Pollut. Res. Int. 2016, 23, 10930–10937. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Bacci, G.; Chiellini, C.; Fagorzi, C.; Niccolai, E.; Taddei, A.; Ricci, F.; Ringressi, M.N.; Borrelli, R.; Melli, F.; et al. Preliminary Comparison of Oral and Intestinal Human Microbiota in Patients with Colorectal Cancer: A Pilot Study. Front. Microbiol. 2017, 8, 2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Qu, F.; Zhu, L.H. Isolation of genomic DNAs from plants, fungi and bacteria using benzyl chloride. Nucleic Acids Res. 1993, 21, 5279–5280. [Google Scholar] [CrossRef] [Green Version]
- Cifani, C.; Micioni Di Bonaventura, M.V.; Pucci, M.; Giusepponi, M.E.; Romano, A.; Di Francesco, A.; Maccarrone, M.; D’Addario, C. Regulation of hypothalamic neuropeptides gene expression in diet induced obesity resistant rats: Possible targets for obesity prediction? Front. Neurosci. 2015, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocci, P.; Moruzzi, M.; Martinelli, I.; Maggi, F.; Micioni Di Bonaventura, M.V.; Cifani, C.; Mosconi, G.; Tayebati, S.K.; Damiano, S.; Lupidi, G.; et al. Tart cherry (Prunus cerasus L.) dietary supplement modulates visceral adipose tissue CB1 mRNA levels along with other adipogenesis-related genes in rat models of diet-induced obesity. Eur. J. Nutr. 2021, 60, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Giusepponi, M.E.; Kern, M.; Chakaroun, R.; Wohland, T.; Kovacs, P.; Dietrich, A.; Schon, M.R.; Krohn, K.; Pucci, M.; Polidori, C.; et al. Gene expression profiling in adipose tissue of Sprague Dawley rats identifies olfactory receptor 984 as a potential obesity treatment target. Biochem. Biophys. Res. Commun. 2018, 505, 801–806. [Google Scholar] [CrossRef]
- Pucci, M.; Micioni Di Bonaventura, M.V.; Vezzoli, V.; Zaplatic, E.; Massimini, M.; Mai, S.; Sartorio, A.; Scacchi, M.; Persani, L.; Maccarrone, M.; et al. Preclinical and Clinical Evidence for a Distinct Regulation of Mu Opioid and Type 1 Cannabinoid Receptor Genes Expression in Obesity. Front. Genet. 2019, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Graham, B.; Yakubu, F.; Lin, D.; Peters, J.C.; Hill, J.O. Metabolic differences between obesity-prone and obesity-resistant rats. Am. J. Physiol. 1990, 259, R1103–R1110. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 1997, 273, R725–R730. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Hogan, S.; Sullivan, A.C. Initiation and perpetuation of obesity and obesity resistance in rats. Am. J. Physiol. 1989, 256, R766–R771. [Google Scholar] [CrossRef]
- Giles, E.D.; Jackman, M.R.; MacLean, P.S. Modeling Diet-Induced Obesity with Obesity-Prone Rats: Implications for Studies in Females. Front. Nutr. 2016, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Pagliassotti, M.J.; Knobel, S.M.; Shahrokhi, K.A.; Manzo, A.M.; Hill, J.O. Time course of adaptation to a high-fat diet in obesity-resistant and obesity-prone rats. Am. J. Physiol. 1994, 267, R659–R664. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Anastasovska, J.; Gibson, G.; Tuohy, K.; Sharma, R.K.; Bell, J.; Frost, G. Effect of Lactobacillus acidophilus NCDC 13 supplementation on the progression of obesity in diet-induced obese mice. Br. J. Nutr. 2012, 108, 1382–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Kong, Y.; Peng, D.Q. New insights into apolipoprotein A5 in controlling lipoprotein metabolism in obesity and the metabolic syndrome patients. Lipids Health Dis. 2018, 17, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Blood Parameters (mg/dl) | ||||
|---|---|---|---|---|
| CHOW Rats | CHOW+P Rats | CAF Rats | CAF+P Rats | |
| Glycemia | 90.8 ± 1.9 | 92.9 ± 3.8 | 110.8 ± 2.2 ** | 94.1 ± 3.5 ° |
| GOT | 154.5 ± 9.0 | 150.9 ± 9.0 | 169.0 ± 8.3 | 144.9 ± 6.9 |
| GPT | 25.6 ± 2.3 | 26.7 ± 1.8 | 29.0 ± 2.7 | 23.5 ± 2.8 |
| GGT | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.3 | 0.5 ± 0.1 |
| Cholesterol | 109.0 ± 5.4 | 105.7 ± 5.7 | 128.8 ± 8.9 | 113.1 ± 7.3 |
| HDL | 29.0 ± 1.6 | 28.7 ± 2.3 | 24.3 ± 1.2 | 26.8 ± 1.8 |
| LDL | 9.2 ± 0.4 | 10.9 ± 0.7 | 19.7 ± 3.2 ** | 12.2 ± 1.8 ° |
| Triglycerides | 100.8 ± 12.3 | 108.2 ± 10.9 | 139.3 ± 21.9 | 124.9 ± 11.6 |
| CHOW Rats | CHOW+P Rats | CAF Rats | CAF+P Rats | |
|---|---|---|---|---|
| Leptin concentration | 2583.7 ± 557.4 | 2737.9 ± 276.3 | 5287.1 ± 773.4 * | 1726.7 ± 265.1 ° |
| Family | Genus | Spearman r | p (Two-Tailed) | ||
|---|---|---|---|---|---|
| Body Weight Gain (T84–T0) | Net Food Intake (T84–T0) | ||||
| CAF rats | Propionibacteriaceae | Propionibacterium | −0.6547 | - | <0.0001 |
| Porphyromonadaceae | Butyricimonas | −0.9429 | - | 0.0167 | |
| Lachnospiraceae | Lachnospiraceae UCG-005 | −0.8857 | - | 0.0333 | |
| Ruminococcaceae | Subdoligranulum | −0.3928 | - | <0.0001 | |
| Corynebacteriaceae | Corynebacterium 1 | - | −0.8857 | 0.0333 | |
| Rikenellaceae | Rikenellaceae RC9 gut group | - | −0.8857 | 0.0333 | |
| Ruminococcaceae | Faecalibacterium | - | −0.9429 | 0.0167 | |
| CAF+P rats | Coriobacteriaceae | Collinsella | 0.881 | - | 0.0072 |
| Coriobacteriaceae | Coriobacteriaceae UCG-002 | −0.7619 | - | 0.0368 | |
| Lachnospiraceae | [Eubacterium] hallii group | 0.8095 | - | 0.0218 | |
| Ruminococcaceae | Ruminococcaceae UCG-008 | 0.7619 | - | 0.0368 | |
| Ruminococcaceae | Subdoligranulum | −0.2474 | - | <0.0001 | |
| Lachnospiraceae | Lachnospiraceae NK4A136 group | - | 0.8333 | 0.0154 | |
| Lachnospiraceae | Marvinbryantia | - | 0.7857 | 0.0279 | |
| Peptostreptococcaceae | Intestinibacter | - | −0.8095 | 0.0218 | |
| Peptostreptococcaceae | Peptoclostridium | - | −0.8571 | 0.0107 | |
| Ruminococcaceae | Flavonifractor | - | 0.881 | 0.0072 | |
| Ruminococcaceae | Oscillospira | - | 0.7381 | 0.0458 | |
| Ruminococcaceae | Ruminiclostridium 6 | - | 0.8333 | 0.0154 | |
| Erysipelotrichaceae | Turicibacter | - | −0.8333 | 0.0154 | |
| CAF+P Rats | T0 | T84 |
|---|---|---|
| Lactobacillus spp. (CFU/g of feces) | (5.0 ± 1.1) × 104 | (1.3 ± 1.0) × 105 |
| L. plantarum IMC 510 (CFU/g of feces) | 0.0 ± 0.0 | (7.8 ± 1.5) × 104 |
| Percentage of positive samples for the recovery of L. plantarum IMC 510 | 0% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micioni Di Bonaventura, M.V.; Coman, M.M.; Tomassoni, D.; Micioni Di Bonaventura, E.; Botticelli, L.; Gabrielli, M.G.; Rossolini, G.M.; Di Pilato, V.; Cecchini, C.; Amedei, A.; et al. Supplementation with Lactiplantibacillus plantarum IMC 510 Modifies Microbiota Composition and Prevents Body Weight Gain Induced by Cafeteria Diet in Rats. Int. J. Mol. Sci. 2021, 22, 11171. https://doi.org/10.3390/ijms222011171
Micioni Di Bonaventura MV, Coman MM, Tomassoni D, Micioni Di Bonaventura E, Botticelli L, Gabrielli MG, Rossolini GM, Di Pilato V, Cecchini C, Amedei A, et al. Supplementation with Lactiplantibacillus plantarum IMC 510 Modifies Microbiota Composition and Prevents Body Weight Gain Induced by Cafeteria Diet in Rats. International Journal of Molecular Sciences. 2021; 22(20):11171. https://doi.org/10.3390/ijms222011171
Chicago/Turabian StyleMicioni Di Bonaventura, Maria Vittoria, Maria Magdalena Coman, Daniele Tomassoni, Emanuela Micioni Di Bonaventura, Luca Botticelli, Maria Gabriella Gabrielli, Gian Maria Rossolini, Vincenzo Di Pilato, Cinzia Cecchini, Amedeo Amedei, and et al. 2021. "Supplementation with Lactiplantibacillus plantarum IMC 510 Modifies Microbiota Composition and Prevents Body Weight Gain Induced by Cafeteria Diet in Rats" International Journal of Molecular Sciences 22, no. 20: 11171. https://doi.org/10.3390/ijms222011171
APA StyleMicioni Di Bonaventura, M. V., Coman, M. M., Tomassoni, D., Micioni Di Bonaventura, E., Botticelli, L., Gabrielli, M. G., Rossolini, G. M., Di Pilato, V., Cecchini, C., Amedei, A., Silvi, S., Verdenelli, M. C., & Cifani, C. (2021). Supplementation with Lactiplantibacillus plantarum IMC 510 Modifies Microbiota Composition and Prevents Body Weight Gain Induced by Cafeteria Diet in Rats. International Journal of Molecular Sciences, 22(20), 11171. https://doi.org/10.3390/ijms222011171









