Molecular Pathways in Prolactinomas: Translational and Therapeutic Implications
Abstract
:1. Introduction
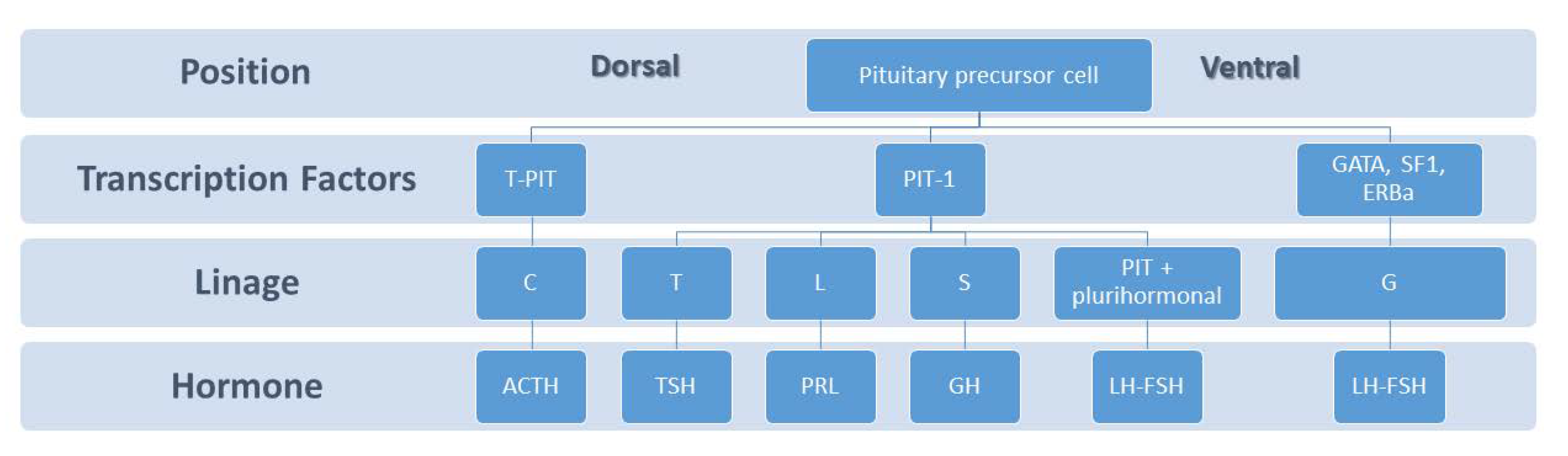
2. Pituitary Development and Lactotroph Lineage
2.1. Anatomy and Ontogeny of Pituitary
2.2. Lactotroph Lineage
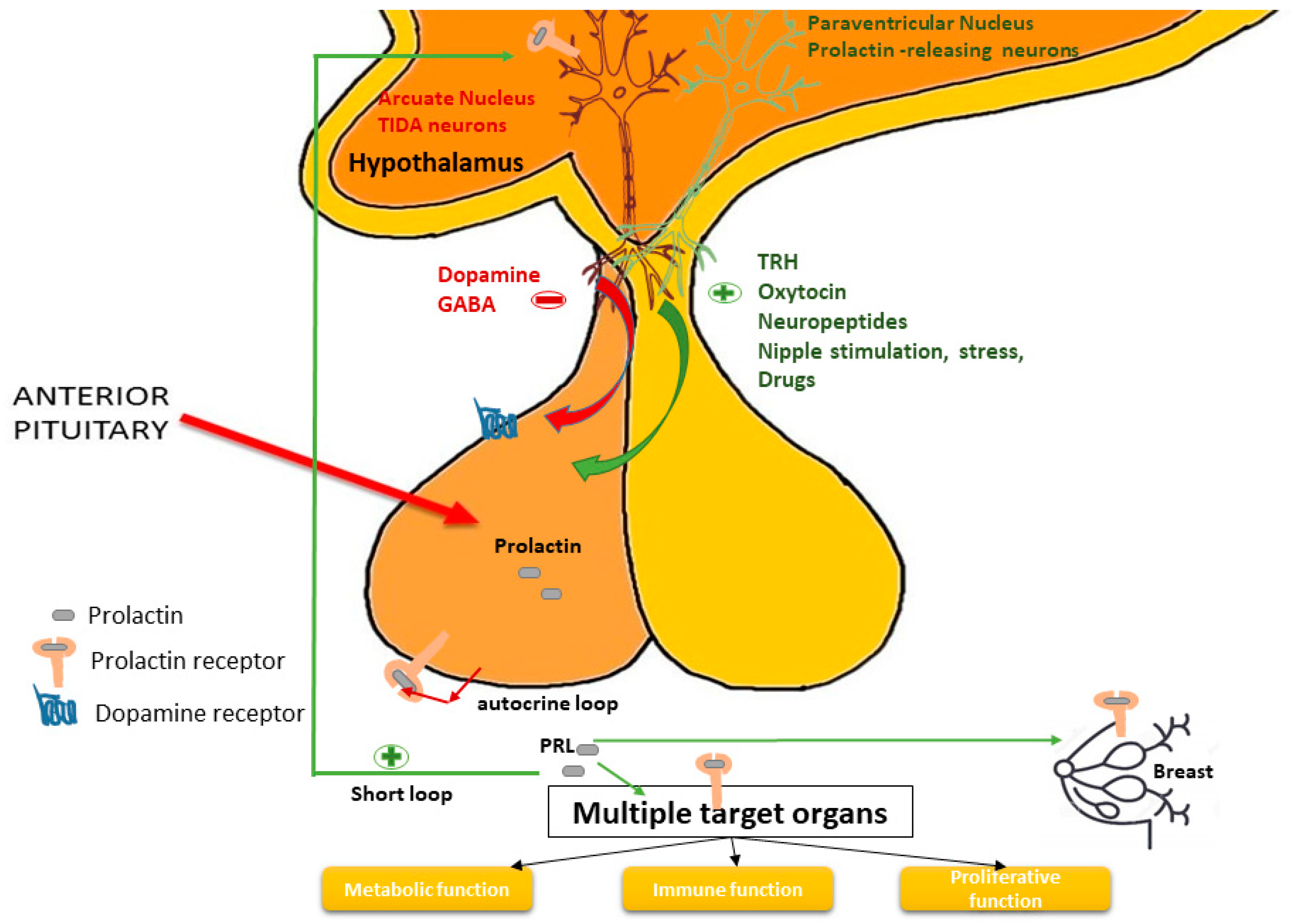
3. Prolactin Axis, Dopamine Receptor and Prolactin Receptor
3.1. Prolactin Axis
3.2. D2 Receptor, Prolactin Secretion and Antiproliferative Activity
- (1)
- D1 and D5, also called D1-like receptors. By coupling to G stimulatory sites, adenylyl cyclase cAMP is activated, which in turn activates protein kinase A (PKA), thus enhancing transcription. These receptors are abundant in the striatum, nucleus accumbens, olfactory bulb, and substantia nigra and are essential in regulating the reward system, motor activity, memory, and learning.
- (2)
- D2, D3 and D4 (D2-like receptors). By coupling to G inhibitory sites, they inhibit adenylyl cyclase and activate K+ channels. They are expressed mainly in the striatum, as well as the external globus pallidus, the core of the nucleus accumbens, hippocampus, amygdala, and cerebral cortex.
3.3. Prolactin Receptor, Intracellular Signalling and Autocrine Function
4. Tumour Development, Lessons from Mice
4.1. D2R-Deficient Mice and Dopamine Transporter-Deficient Mice
4.2. PRLR Deficient Mice
5. Gender Differences and Oestrogens in Prolactinomas
6. Somatostatin Receptors and Prolactinoma
7. Genomics in Prolactinoma
HMGA1 Gene
8. Clinical Features Predicting Prolactinoma Response to DA
9. Future Direction and Medical Options in Aggressive Prolactinomas
9.1. JAK2-STAT
9.2. PI3K-Akt-mTOR
9.3. MAPK/AMPK Pathway
9.4. Oestrogen Modulation
| Place of Action | Evidence (References) | Clinical Trials | |
|---|---|---|---|
| Capecitabine and Temozolomide in firstline | MGMT inhibits DNA synthesis and slows growth of tumour tissue | Isolated human case reports summarised in [86] | Ongoing NCT03930771 for functional and non-functional aggressive pituitary tumours |
| Pasireotide | multireceptor ligand SSTR5 > SSTR2 > SSTR3 > SSTR1 | Case reports (humans) [62,63] | No |
| Atiprimod | JAK2-STAT → STAT3 | rat cell lines GH3 [83] | No |
| 5-fluorocytosine, nortriptyline, neratinib, taxifolin, vorinostat, zileuton | PI3K-Akt-mTOR | MMQ cell lines and mRNA-miRNA data integration [84] | No |
| Everolimus | prolactinoma derived cells (human) [44] Case reports (humans) [85] | No | |
| Blockade of MAPK14 | MAPK/AMPK | mice and human prolactinoma cells [87] | No |
| Metformin | prolactinoma derived cells (human) [8] | No. A pilot study (n = 10) failed to show PRL normalisation (no data on tumour growth) | |
| Raloxifene | oestrogen receptor modulator | case reports (humans) [94] | Pilot study (n = 14), not randomised, no control group |
| Immunotherapy | PD-L1 PIT-1 | case reports (humans) [95] | No |
| Ipilimumab and nivolumab | Progressive pituitary adenoma/carcinoma NCT04042753 and NCT02834013 |
9.5. Temozolomide and Others Cytotoxic Agents
10. Concluding Remarks
11. Search Strategy and Selection Criteria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chanson, P.; Maiter, D. The Epidemiology, Diagnosis and Treatment of Prolactinomas: The Old and the New. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101290. [Google Scholar] [CrossRef]
- Vroonen, L.; Daly, A.F.; Beckers, A. Epidemiology and Management Challenges in Prolactinomas. Neuroendocrinology 2019, 109, 20–27. [Google Scholar] [CrossRef]
- McCormack, A.; Dekkers, O.M.; Petersenn, S.; Popovic, V.; Trouillas, J.; Raverot, G.; Burman, P. ESE survey collaborators Treatment of Aggressive Pituitary Tumours and Carcinomas: Results of a European Society of Endocrinology (ESE) Survey 2016. Eur. J. Endocrinol. 2018, 178, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guidelines for the Management of Aggressive Pituitary Tumours and Carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef]
- Shimon, I.; Sosa, E.; Mendoza, V.; Greenman, Y.; Tirosh, A.; Espinosa, E.; Popovic, V.; Glezer, A.; Bronstein, M.D.; Mercado, M. Giant Prolactinomas Larger than 60 Mm in Size: A Cohort of Massive and Aggressive Prolactin-Secreting Pituitary Adenomas. Pituitary 2016, 19, 429–436. [Google Scholar] [CrossRef]
- Salenave, S.; Ancelle, D.; Bahougne, T.; Raverot, G.; Kamenický, P.; Bouligand, J.; Guiochon-Mantel, A.; Linglart, A.; Souchon, P.-F.; Nicolino, M.; et al. Macroprolactinomas in Children and Adolescents: Factors Associated with the Response to Treatment in 77 Patients. J. Clin. Endocrinol. Metab. 2015, 100, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Delgrange, E.; Trouillas, J.; Maiter, D.; Donckier, J.; Tourniaire, J. Sex-Related Difference in the Growth of Prolactinomas: A Clinical and Proliferation Marker Study. J. Clin. Endocrinol. Metab. 1997, 82, 2102–2107. [Google Scholar] [CrossRef]
- Gao, H.; Wang, F.; Lan, X.; Li, C.; Feng, J.; Bai, J.; Cao, L.; Gui, S.; Hong, L.; Zhang, Y. Lower PRDM2 Expression Is Associated with Dopamine-Agonist Resistance and Tumor Recurrence in Prolactinomas. BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Dworakowska, D.; Grossman, A.B. Aggressive and Malignant Pituitary Tumours: State-of-the-Art. Endocr. Relat. Cancer 2018, 25, R559–R575. [Google Scholar] [CrossRef] [Green Version]
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2017, 28, 228–243. [Google Scholar] [CrossRef]
- Raverot, G.; Vasiljevic, A.; Jouanneau, E. Prognostic Factors of Regrowth in Nonfunctioning Pituitary Tumors. Pituitary 2018, 21, 176–182. [Google Scholar] [CrossRef]
- Delgrange, E.; Sassolas, G.; Perrin, G.; Jan, M.; Trouillas, J. Clinical and Histological Correlations in Prolactinomas, with Special Reference to Bromocriptine Resistance. Acta Neurochir. (Wien) 2005, 147, 751–757; discussion 757–758. [Google Scholar] [CrossRef]
- Trouillas, J.; Delgrange, E.; Wierinckx, A.; Vasiljevic, A.; Jouanneau, E.; Burman, P.; Raverot, G. Clinical, Pathological, and Molecular Factors of Aggressiveness in Lactotroph Tumours. Neuroendocrinology 2019, 109, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chatzellis, E.; Alexandraki, K.I.; Androulakis, I.I.; Kaltsas, G. Aggressive Pituitary Tumors. Neuroendocrinology 2015, 101, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Kang, J.; Liu, X.; Yao, Y.; Wang, H.; Wang, R. How to Classify and Define Pituitary Tumors: Recent Advances and Current Controversies. Front. Endocrinol. 2021, 12, 604644. [Google Scholar] [CrossRef]
- Ganapathy, M.K.; Tadi, P. Anatomy, Head and Neck, Pituitary Gland. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Watanabe, Y.G. Effects of Brain and Mesenchyme upon the Cytogenesis of Rat Adenohypophysis in Vitro. I. Differentiation of Adrenocorticotropes. Cell Tissue Res. 1982, 227, 257–266. [Google Scholar] [CrossRef]
- Chen, R.P.; Ingraham, H.A.; Treacy, M.N.; Albert, V.R.; Wilson, L.; Rosenfeld, M.G. Autoregulation of Pit-1 Gene Expression Mediated by Two Cis-Active Promoter Elements. Nature 1990, 346, 583–586. [Google Scholar] [CrossRef]
- Perez-Castro, C.; Renner, U.; Haedo, M.R.; Stalla, G.K.; Arzt, E. Cellular and Molecular Specificity of Pituitary Gland Physiology. Physiol. Rev. 2012, 92, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, Function, and Regulation of Secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Brown, R.S.E.; Kokay, I.C.; Phillipps, H.R.; Yip, S.H.; Gustafson, P.; Wyatt, A.; Larsen, C.M.; Knowles, P.; Ladyman, S.R.; LeTissier, P.; et al. Conditional Deletion of the Prolactin Receptor Reveals Functional Subpopulations of Dopamine Neurons in the Arcuate Nucleus of the Hypothalamus. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 9173–9185. [Google Scholar] [CrossRef]
- Jimenez, A.E.; Voogt, J.L.; Carr, L.A. Plasma Luteinizing Hormone and Prolactin Levels and Hypothalamic Catecholamine Synthesis in Steroid-Treated Ovariectomized Rats. Neuroendocrinology 1977, 23, 341–351. [Google Scholar] [CrossRef]
- Bernard, V.; Lamothe, S.; Beau, I.; Guillou, A.; Martin, A.; Tissier, P.L.; Grattan, D.; Young, J.; Binart, N. Autocrine Actions of Prolactin Contribute to the Regulation of Lactotroph Function in Vivo. FASEB J. 2018, 32, 4791–4797. [Google Scholar] [CrossRef] [Green Version]
- Glezer, A.; Bronstein, M.D. Hyperprolactinemia. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Torre, D.L.; Falorni, A. Pharmacological Causes of Hyperprolactinemia. Ther. Clin. Risk Manag. 2007, 3, 929–951. [Google Scholar]
- Kjaer, A.; Knigge, U.; Jørgensen, H.; Warberg, J. Selective Inhibition of Magnocellular Vasopressin Neurons by Hypoosmolality: Effect on Histamine- and Stress-Induced Secretion of Adrenocorticotropin and Prolactin. Neuroendocrinology 1998, 67, 330–335. [Google Scholar] [CrossRef]
- Onali, P.; Eva, C.; Olianas, M.C.; Schwartz, J.P.; Costa, E. In GH3 Pituitary Cells, Acetylcholine and Vasoactive Intestinal Peptide Antagonistically Modulate Adenylate Cyclase, Cyclic AMP Content, and Prolactin Secretion. Mol. Pharmacol. 1983, 24, 189–194. [Google Scholar]
- Veselinović, T.; Schorn, H.; Vernaleken, I.B.; Schiffl, K.; Klomp, M.; Gründer, G. Impact of Different Antidopaminergic Mechanisms on the Dopaminergic Control of Prolactin Secretion. J. Clin. Psychopharmacol. 2011, 31, 214–220. [Google Scholar] [CrossRef]
- Molitch, M.E. Drugs and Prolactin. Pituitary 2008, 11, 209–218. [Google Scholar] [CrossRef]
- Lopez-Vicchi, F.; Winne, C.D.; Brie, B.; Sorianello, E.; Ladyman, S.R.; Becu-Villalobos, D. Metabolic Functions of Prolactin: Physiological and Pathological Aspects. J. Neuroendocrinol. 2020, 32, e12888. [Google Scholar] [CrossRef]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and Autoimmunity. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Sellers, Z.P.; Bujko, K.; Schneider, G.; Kucia, M.; Ratajczak, M.Z. Novel Evidence That Pituitary Sex Hormones Regulate Migration, Adhesion, and Proliferation of Embryonic Stem Cells and Teratocarcinoma Cells. Oncol. Rep. 2018, 39, 851–859. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a Prolactin (PRL) Inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef]
- Bhatia, A.; Lenchner, J.R.; Saadabadi, A. Biochemistry, Dopamine Receptors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Karayazi Atıcı, Ö.; Govindrajan, N.; Lopetegui-González, I.; Shemanko, C.S. Prolactin: A Hormone with Diverse Functions from Mammary Gland Development to Cancer Metastasis. Semin. Cell Dev. Biol. 2021, 114, 159–170. [Google Scholar] [CrossRef]
- Gorvin, C.M. The Prolactin Receptor: Diverse and Emerging Roles in Pathophysiology. J. Clin. Transl. Endocrinol. 2015, 2, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, A.C.; Timmer, J.; Klingmüller, U. Systems Biology of JAK/STAT Signalling. Essays Biochem. 2008, 45, 109–120. [Google Scholar] [CrossRef]
- Ferraris, J.; Zárate, S.; Jaita, G.; Boutillon, F.; Bernadet, M.; Auffret, J.; Seilicovich, A.; Binart, N.; Goffin, V.; Pisera, D. Prolactin Induces Apoptosis of Lactotropes in Female Rodents. PloS One 2014, 9, e97383. [Google Scholar] [CrossRef]
- Martín-Pérez, J.; García-Martínez, J.M.; Sánchez-Bailón, M.P.; Mayoral-Varo, V.; Calcabrini, A. Role of SRC Family Kinases in Prolactin Signaling. Adv. Exp. Med. Biol. 2015, 846, 163–188. [Google Scholar] [CrossRef]
- De Dios, N.; Orrillo, S.; Irizarri, M.; Theas, M.S.; Boutillon, F.; Candolfi, M.; Seilicovich, A.; Goffin, V.; Pisera, D.; Ferraris, J. JAK2/STAT5 Pathway Mediates Prolactin-Induced Apoptosis of Lactotropes. Neuroendocrinology 2019, 108, 84–97. [Google Scholar] [CrossRef]
- Sabatini, D.M. MTOR and Cancer: Insights into a Complex Relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef]
- Dworakowska, D.; Wlodek, E.; Leontiou, C.A.; Igreja, S.; Cakir, M.; Teng, M.; Prodromou, N.; Góth, M.I.; Grozinsky-Glasberg, S.; Gueorguiev, M.; et al. Activation of RAF/MEK/ERK and PI3K/AKT/MTOR Pathways in Pituitary Adenomas and Their Effects on Downstream Effectors. Endocr. Relat. Cancer 2009, 16, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Monsalves, E.; Juraschka, K.; Tateno, T.; Agnihotri, S.; Asa, S.L.; Ezzat, S.; Zadeh, G. The PI3K/AKT/MTOR Pathway in the Pathophysiology and Treatment of Pituitary Adenomas. Endocr. Relat. Cancer 2014, 21, R331–R344. [Google Scholar] [CrossRef] [Green Version]
- Gorvin, C.M.; Newey, P.J.; Rogers, A.; Stokes, V.; Neville, M.J.; Lines, K.E.; Ntali, G.; Lees, P.; Morrison, P.J.; Singhellakis, P.N.; et al. Association of Prolactin Receptor (PRLR) Variants with Prolactinomas. Hum. Mol. Genet. 2019, 28, 1023–1037. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK Signalings: Interplay and Implication in Targeted Cancer Therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Booth, A.; Trudeau, T.; Gomez, C.; Lucia, M.S.; Gutierrez-Hartmann, A. Persistent ERK/MAPK Activation Promotes Lactotrope Differentiation and Diminishes Tumorigenic Phenotype. Mol. Endocrinol. Baltim. Md 2014, 28, 1999–2011. [Google Scholar] [CrossRef] [Green Version]
- Saiardi, A.; Bozzi, Y.; Baik, J.H.; Borrelli, E. Antiproliferative Role of Dopamine: Loss of D2 Receptors Causes Hormonal Dysfunction and Pituitary Hyperplasia. Neuron 1997, 19, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Cristina, C.; García-Tornadú, I.; Díaz-Torga, G.; Rubinstein, M.; Low, M.J.; Becú-Villalobos, D. Dopaminergic D2 Receptor Knockout Mouse: An Animal Model of Prolactinoma. Front. Horm. Res. 2006, 35, 50–63. [Google Scholar] [CrossRef]
- Bossé, R.; Fumagalli, F.; Jaber, M.; Giros, B.; Gainetdinov, R.R.; Wetsel, W.C.; Missale, C.; Caron, M.G. Anterior Pituitary Hypoplasia and Dwarfism in Mice Lacking the Dopamine Transporter. Neuron 1997, 19, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Friedman, E.; Adams, E.F.; Höög, A.; Gejman, P.V.; Carson, E.; Larsson, C.; De Marco, L.; Werner, S.; Fahlbusch, R.; Nordenskjöld, M. Normal Structural Dopamine Type 2 Receptor Gene in Prolactin-Secreting and Other Pituitary Tumors. J. Clin. Endocrinol. Metab. 1994, 78, 568–574. [Google Scholar] [CrossRef]
- Bueno, C.; Trarbach, E.B.; Bronstein, M.D.; Glezer, A. Cabergoline and Prolactinomas: Lack of Association between DRD2 Polymorphisms and Response to Treatment. Pituitary 2017, 20, 295–300. [Google Scholar] [CrossRef]
- Schuff, K.G.; Hentges, S.T.; Kelly, M.A.; Binart, N.; Kelly, P.A.; Iuvone, P.M.; Asa, S.L.; Low, M.J. Lack of Prolactin Receptor Signaling in Mice Results in Lactotroph Proliferation and Prolactinomas by Dopamine-Dependent and -Independent Mechanisms. J. Clin. Invest. 2002, 110, 973–981. [Google Scholar] [CrossRef]
- Ormandy, C.J.; Camus, A.; Barra, J.; Damotte, D.; Lucas, B.; Buteau, H.; Edery, M.; Brousse, N.; Babinet, C.; Binart, N.; et al. Null Mutation of the Prolactin Receptor Gene Produces Multiple Reproductive Defects in the Mouse. Genes Dev. 1997, 11, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Šošić-Jurjević, B.; Ajdžanović, V.; Miljić, D.; Trifunović, S.; Filipović, B.; Stanković, S.; Bolevich, S.; Jakovljević, V.; Milošević, V. Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens—An Insight From Translational Studies. Int. J. Mol. Sci. 2020, 21, 2024. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Li, C.; Gui, S.; Zhang, Y. Expression of Estrogen Receptor α and Growth Factors in Human Prolactinoma and Its Correlation with Clinical Features and Gender. J. Endocrinol. Investig. 2012, 35, 174–180. [Google Scholar] [CrossRef]
- Delgrange, E.; Vasiljevic, A.; Wierinckx, A.; François, P.; Jouanneau, E.; Raverot, G.; Trouillas, J. Expression of Estrogen Receptor Alpha Is Associated with Prolactin Pituitary Tumor Prognosis and Supports the Sex-Related Difference in Tumor Growth. Eur. J. Endocrinol. 2015, 172, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Mahboobifard, F.; Bidari-Zerehpoosh, F.; Davoudi, Z.; Panahi, M.; Dargahi, L.; Pourgholami, M.H.; Sharifi, G.; Izadi, N.; Jorjani, M. Expression Patterns of ERα66 and Its Novel Variant Isoform ERα36 in Lactotroph Pituitary Adenomas and Associations with Clinicopathological Characteristics. Pituitary 2020, 23, 232–245. [Google Scholar] [CrossRef]
- Wierinckx, A.; Delgrange, E.; Bertolino, P.; François, P.; Chanson, P.; Jouanneau, E.; Lachuer, J.; Trouillas, J.; Raverot, G. Sex-Related Differences in Lactotroph Tumor Aggressiveness Are Associated With a Specific Gene-Expression Signature and Genome Instability. Front. Endocrinol. 2018, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Yang, X.; Zhang, K.; Liu, Z.; Shao, Z.; Song, C.; Wang, X.; Li, Z. Estrogen Receptor α/Prolactin Receptor Bilateral Crosstalk Promotes Bromocriptine Resistance in Prolactinomas. Int. J. Med. Sci. 2020, 17, 3174–3189. [Google Scholar] [CrossRef]
- Shimon, I.; Yan, X.; Taylor, J.E.; Weiss, M.H.; Culler, M.D.; Melmed, S. Somatostatin Receptor (SSTR) Subtype-Selective Analogues Differentially Suppress in Vitro Growth Hormone and Prolactin in Human Pituitary Adenomas. Novel Potential Therapy for Functional Pituitary Tumors. J. Clin. Investig. 1997, 100, 2386–2392. [Google Scholar] [CrossRef]
- Jaquet, P.; Ouafik, L.; Saveanu, A.; Gunz, G.; Fina, F.; Dufour, H.; Culler, M.D.; Moreau, J.P.; Enjalbert, A. Quantitative and Functional Expression of Somatostatin Receptor Subtypes in Human Prolactinomas. J. Clin. Endocrinol. Metab. 1999, 84, 3268–3276. [Google Scholar] [CrossRef]
- Coopmans, E.C.; van Meyel, S.W.F.; Pieterman, K.J.; van Ipenburg, J.A.; Hofland, L.J.; Donga, E.; Daly, A.F.; Beckers, A.; van der Lely, A.-J.; Neggers, S.J.C.M.M. Excellent Response to Pasireotide Therapy in an Aggressive and Dopamine-Resistant Prolactinoma. Eur. J. Endocrinol. 2019, 181, K21–K27. [Google Scholar] [CrossRef]
- Lasolle, H.; Vasiljevic, A.; Borson-Chazot, F.; Raverot, G. Pasireotide: A Potential Therapeutic Alternative for Resistant Prolactinoma. Ann. Endocrinol. 2019, 80, 84–88. [Google Scholar] [CrossRef]
- Marques, P.; Caimari, F.; Hernández-Ramírez, L.C.; Collier, D.; Iacovazzo, D.; Ronaldson, A.; Magid, K.; Lim, C.T.; Stals, K.; Ellard, S.; et al. Significant Benefits of AIP Testing and Clinical Screening in Familial Isolated and Young-Onset Pituitary Tumors. J. Clin. Endocrinol. Metab. 2020, 105, dgaa040. [Google Scholar] [CrossRef] [Green Version]
- Dénes, J.; Korbonits, M. The Clinical Aspects of Pituitary Tumour Genetics. Endocrine 2021, 71, 663–674. [Google Scholar] [CrossRef]
- Seltzer, J.; Scotton, T.C.; Kang, K.; Zada, G.; Carmichael, J.D. Gene Expression in Prolactinomas: A Systematic Review. Pituitary 2016, 19, 93–104. [Google Scholar] [CrossRef]
- De Sousa, S.M.C.; Wang, P.P.S.; Santoreneos, S.; Shen, A.; Yates, C.J.; Babic, M.; Eshraghi, L.; Feng, J.; Koszyca, B.; Roberts-Thomson, S.; et al. The Genomic Landscape of Sporadic Prolactinomas. Endocr. Pathol. 2019, 30, 318–328. [Google Scholar] [CrossRef]
- Li, C.; Xie, W.; Rosenblum, J.S.; Zhou, J.; Guo, J.; Miao, Y.; Shen, Y.; Wang, H.; Gong, L.; Li, M.; et al. Somatic SF3B1 Hotspot Mutation in Prolactinomas. Nat. Commun. 2020, 11, 2506. [Google Scholar] [CrossRef]
- Fusco, A.; Fedele, M. Roles of HMGA Proteins in Cancer. Nat. Rev. Cancer 2007, 7, 899–910. [Google Scholar] [CrossRef]
- Fedele, M.; Pentimalli, F.; Baldassarre, G.; Battista, S.; Klein-Szanto, A.J.P.; Kenyon, L.; Visone, R.; De Martino, I.; Ciarmiello, A.; Arra, C.; et al. Transgenic Mice Overexpressing the Wild-Type Form of the HMGA1 Gene Develop Mixed Growth Hormone/Prolactin Cell Pituitary Adenomas and Natural Killer Cell Lymphomas. Oncogene 2005, 24, 3427–3435. [Google Scholar] [CrossRef] [Green Version]
- Fedele, M.; Battista, S.; Kenyon, L.; Baldassarre, G.; Fidanza, V.; Klein-Szanto, A.J.P.; Parlow, A.F.; Visone, R.; Pierantoni, G.M.; Outwater, E.; et al. Overexpression of the HMGA2 Gene in Transgenic Mice Leads to the Onset of Pituitary Adenomas. Oncogene 2002, 21, 3190–3198. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, O.M.; Lagro, J.; Burman, P.; Jørgensen, J.O.; Romijn, J.A.; Pereira, A.M. Recurrence of Hyperprolactinemia after Withdrawal of Dopamine Agonists: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2010, 95, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Hage, C.; Salvatori, R. Predictors of the Response to Dopaminergic Therapy in Patients With Prolactinoma. J. Clin. Endocrinol. Metab. 2020, 105, e4558–e4566. [Google Scholar] [CrossRef]
- Ma, Q.; Su, J.; Li, Y.; Wang, J.; Long, W.; Luo, M.; Liu, Q. The Chance of Permanent Cure for Micro- and Macroprolactinomas, Medication or Surgery? A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Jethwa, P.R.; Patel, T.D.; Hajart, A.F.; Eloy, J.A.; Couldwell, W.T.; Liu, J.K. Cost-Effectiveness Analysis of Microscopic and Endoscopic Transsphenoidal Surgery Versus Medical Therapy in the Management of Microprolactinoma in the United States. World Neurosurg. 2016, 87, 65–76. [Google Scholar] [CrossRef]
- Colao, A.; Di Sarno, A.; Landi, M.L.; Scavuzzo, F.; Cappabianca, P.; Pivonello, R.; Volpe, R.; Di Salle, F.; Cirillo, S.; Annunziato, L.; et al. Macroprolactinoma Shrinkage during Cabergoline Treatment Is Greater in Naive Patients than in Patients Pretreated with Other Dopamine Agonists: A Prospective Study in 110 Patients. J. Clin. Endocrinol. Metab. 2000, 85, 2247–2252. [Google Scholar] [CrossRef]
- Vale, F.L.; Deukmedjian, A.R.; Hann, S.; Shah, V.; Morrison, A.D. Medically Treated Prolactin-Secreting Pituitary Adenomas: When Should We Operate? Br. J. Neurosurg. 2013, 27, 56–62. [Google Scholar] [CrossRef]
- Kim, D.; Ku, C.R.; Kim, K.; Jung, H.; Lee, E.J. Prolactin ≤1 Ng/ML Predicts Macroprolactinoma Reduction after Cabergoline Therapy. Eur. J. Endocrinol. 2020, 182, 177–183. [Google Scholar] [CrossRef]
- Ono, M.; Miki, N.; Kawamata, T.; Makino, R.; Amano, K.; Seki, T.; Kubo, O.; Hori, T.; Takano, K. Prospective Study of High-Dose Cabergoline Treatment of Prolactinomas in 150 Patients. J. Clin. Endocrinol. Metab. 2008, 93, 4721–4727. [Google Scholar] [CrossRef]
- Molitch, M.E. Pharmacologic Resistance in Prolactinoma Patients. Pituitary 2005, 8, 43–52. [Google Scholar] [CrossRef]
- Lee, Y.; Ku, C.R.; Kim, E.-H.; Hong, J.W.; Lee, E.J.; Kim, S.H. Early Prediction of Long-Term Response to Cabergoline in Patients with Macroprolactinomas. Endocrinol. Metab. 2014, 29, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Biagetti, B.; Sarria-Estrada, S.; Ng-Wong, Y.K.; Martinez-Saez, E.; Casteràs, A.; Cordero Asanza, E.; Hernandez, I.; Giralt-Arnaiz, M.; Simó, R. Shrinkage by the Third Month Predicts Long-Term Response of Macroprolactinoma after Cabergoline. Eur. J. Endocrinol. 2021, EJE-21-0561.R2. [Google Scholar] [CrossRef]
- Coker-Gurkan, A.; Ayhan-Sahin, B.; Keceloglu, G.; Obakan-Yerlikaya, P.; Arisan, E.-D.; Palavan-Unsal, N. Atiprimod Induce Apoptosis in Pituitary Adenoma: Endoplasmic Reticulum Stress and Autophagy Pathways. J. Cell. Biochem. 2019, 120, 19749–19763. [Google Scholar] [CrossRef]
- Aydin, B.; Arslan, S.; Bayraklı, F.; Karademir, B.; Arga, K.Y. MiRNA-Mediated Drug Repurposing Unveiled Potential Candidate Drugs for Prolactinoma Treatment. Neuroendocrinology 2021. [Google Scholar] [CrossRef]
- Zhang, D.; Way, J.S.; Zhang, X.; Sergey, M.; Bergsneider, M.; Wang, M.B.; Yong, W.H.; Heaney, A.P. Effect of Everolimus in Treatment of Aggressive Prolactin-Secreting Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 1929–1936. [Google Scholar] [CrossRef]
- Nakano-Tateno, T.; Lau, K.J.; Wang, J.; McMahon, C.; Kawakami, Y.; Tateno, T.; Araki, T. Multimodal Non-Surgical Treatments of Aggressive Pituitary Tumors. Front. Endocrinol. 2021, 12, 51. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Y.; Ma, L.; Chen, Y.; Wu, J.; Zhang, H.; Wang, X. Inhibiting MAPK14 Showed Anti-Prolactinoma Effect. BMC Endocr. Disord. 2020, 20, 138. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Le Marchand-Brustel, Y.; Tanti, J.-F.; Bost, F. Metformin in Cancer Therapy: A New Perspective for an Old Antidiabetic Drug? Mol. Cancer Ther. 2010, 9, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Liu, Y.; Han, G.; Deng, K.; Liu, X.; Bao, X.; Feng, M.; Yao, Y.; Lian, W.; Xing, B.; et al. Metformin Inhibits Growth and Prolactin Secretion of Pituitary Prolactinoma Cells and Xenografts. J. Cell. Mol. Med. 2018, 22, 6368–6379. [Google Scholar] [CrossRef]
- Portari, L.H.C.; Correa-Silva, S.R.; Abucham, J. PROLACTIN RESPONSE TO METFORMIN IN CABERGOLINE-RESISTANT PROLACTINOMAS: A PILOT STUDY. Neuroendocrinology 2021. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Ishida, M.; Izawa, M.; Arita, J. Differences between Rat Strains in the Development of PRL-Secreting Pituitary Tumors with Long-Term Estrogen Treatment: In Vitro Insulin-like Growth Factor-1-Induced Lactotroph Proliferation and Gene Expression Are Affected in Wistar-Kyoto Rats with Low Estrogen-Susceptibility. Endocr. J. 2013, 60, 1251–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nota, N.M.; Wiepjes, C.M.; de Blok, C.J.M.; Gooren, L.J.G.; Peerdeman, S.M.; Kreukels, B.P.C.; den Heijer, M. The Occurrence of Benign Brain Tumours in Transgender Individuals during Cross-Sex Hormone Treatment. Brain J. Neurol. 2018, 141, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.R.; Chan, K.J.; Safer, J.D. PROLACTIN LEVELS DO NOT RISE AMONG TRANSGENDER WOMEN TREATED WITH ESTRADIOL AND SPIRONOLACTONE. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2018, 24, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Hamrahian, A.H.; Bena, J.F.; Recinos, P.; Kennedy, L.; Dobri, G. THE EFFECT OF RALOXIFENE ON SERUM PROLACTIN LEVEL IN PATIENTS WITH PROLACTINOMA. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2019, 25, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Turchini, J.; Sioson, L.; Clarkson, A.; Sheen, A.; Gill, A.J. PD-L1 Is Preferentially Expressed in PIT-1 Positive Pituitary Neuroendocrine Tumours. Endocr. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Diagnosis and Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagetti, B.; Simò, R. Molecular Pathways in Prolactinomas: Translational and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 11247. https://doi.org/10.3390/ijms222011247
Biagetti B, Simò R. Molecular Pathways in Prolactinomas: Translational and Therapeutic Implications. International Journal of Molecular Sciences. 2021; 22(20):11247. https://doi.org/10.3390/ijms222011247
Chicago/Turabian StyleBiagetti, Betina, and Rafael Simò. 2021. "Molecular Pathways in Prolactinomas: Translational and Therapeutic Implications" International Journal of Molecular Sciences 22, no. 20: 11247. https://doi.org/10.3390/ijms222011247
APA StyleBiagetti, B., & Simò, R. (2021). Molecular Pathways in Prolactinomas: Translational and Therapeutic Implications. International Journal of Molecular Sciences, 22(20), 11247. https://doi.org/10.3390/ijms222011247







