ROS-Scavengers, Osmoprotectants and Violaxanthin De-Epoxidation in Salt-Stressed Arabidopsis thaliana with Different Tocopherol Composition
Abstract
:1. Introduction
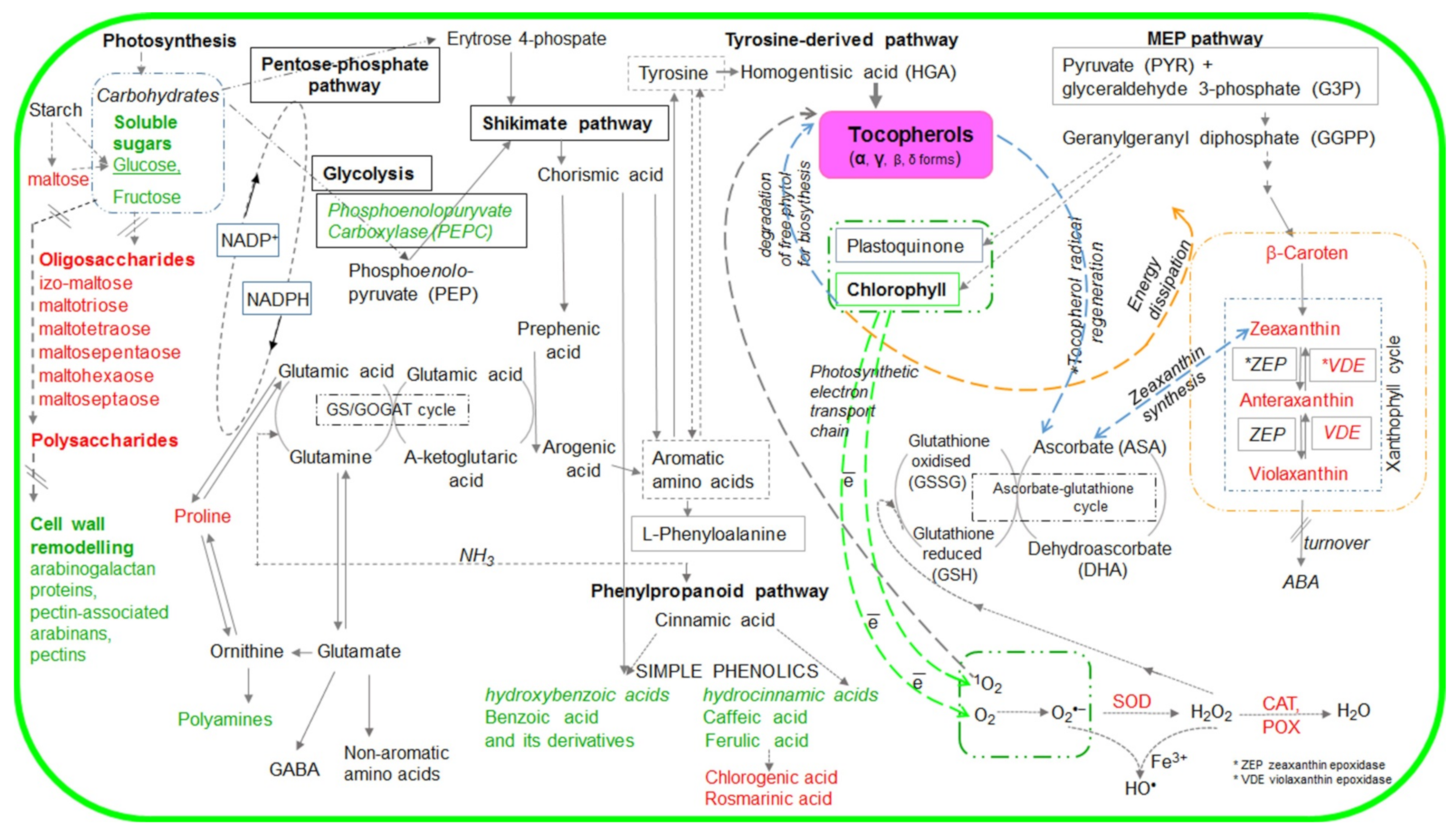
2. Results
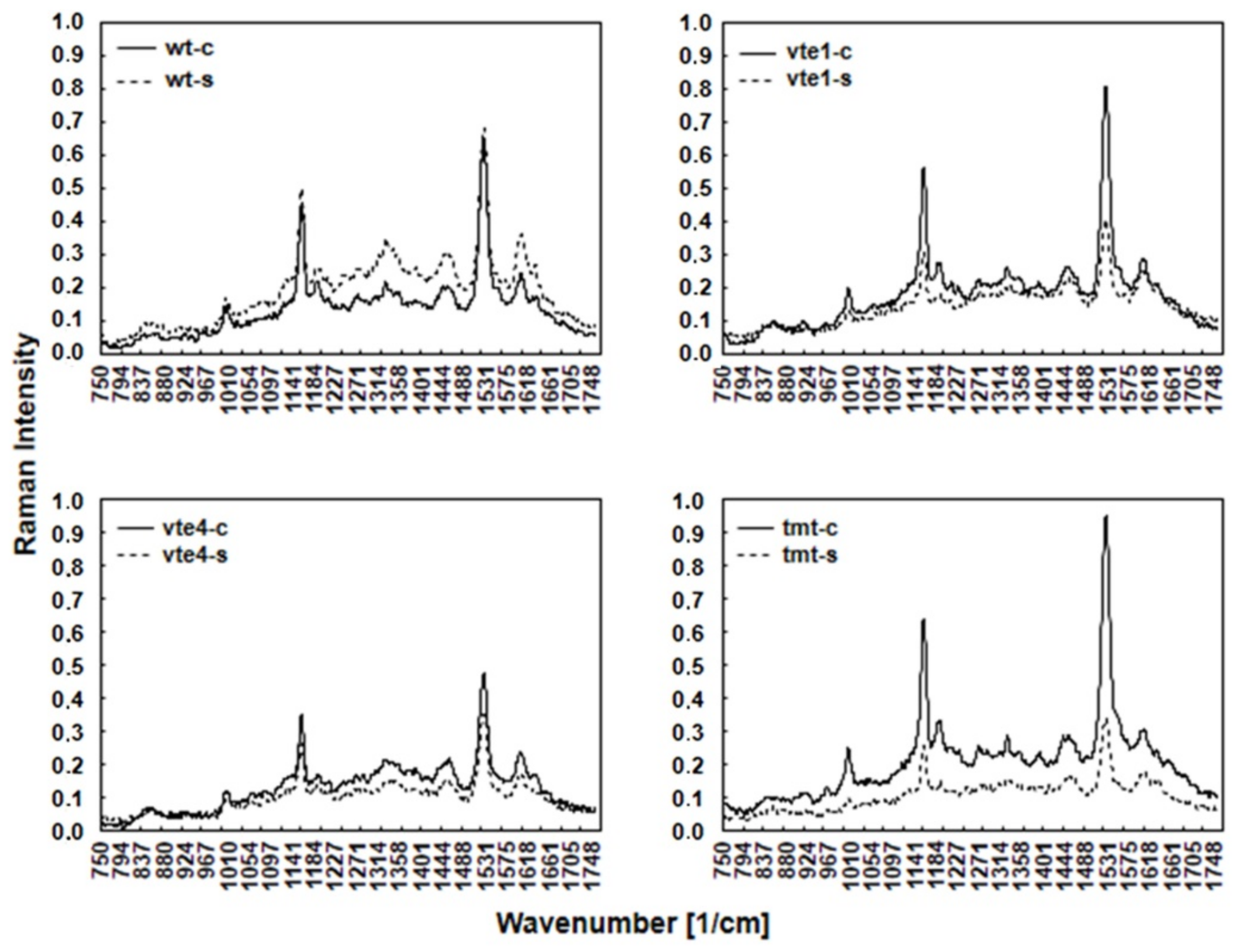
2.1. Leaf Metabolites with Antioxidant and/or Osmotic Properties Detected by FT-Raman Spectroscopy
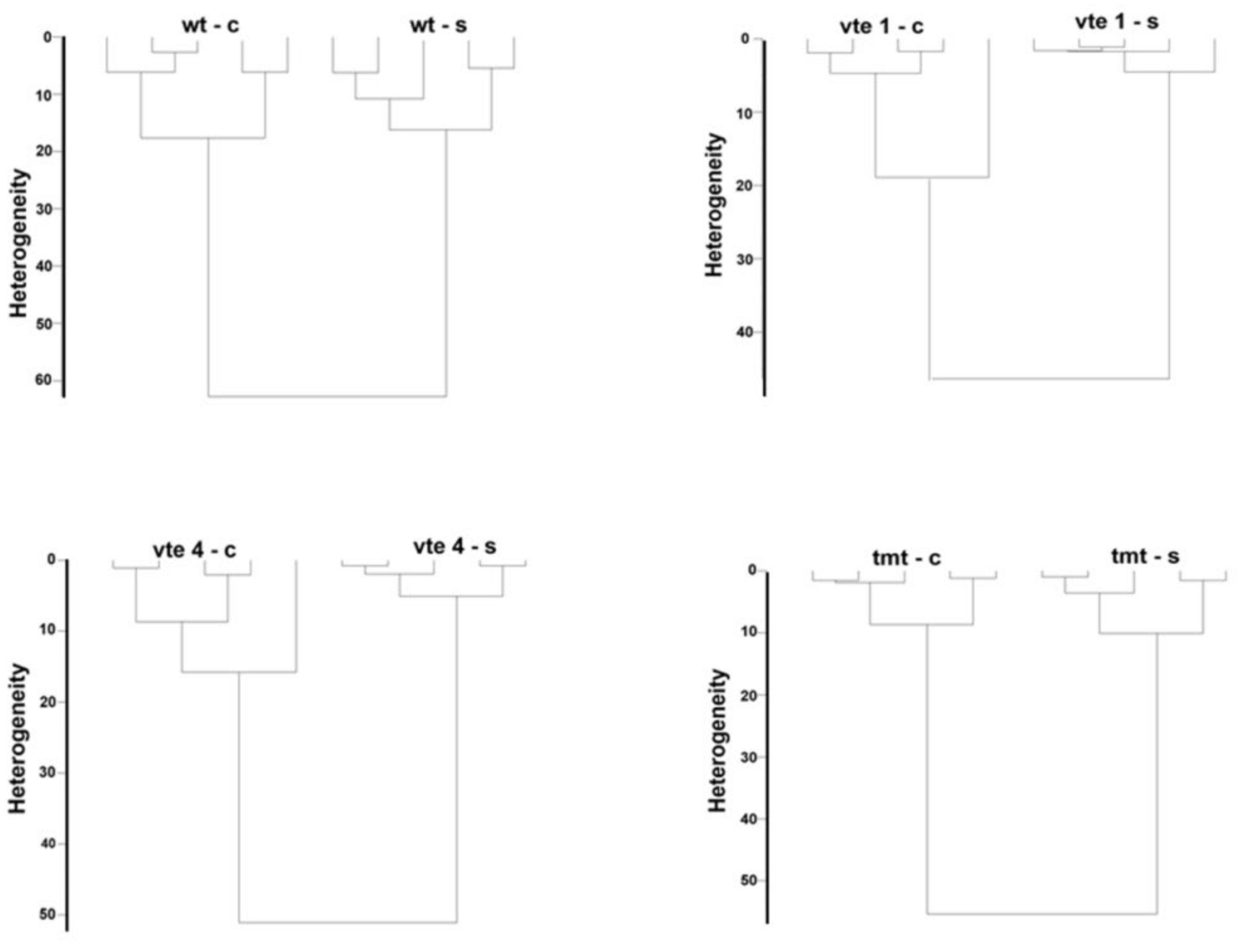
2.2. Chemometrics–Cluster Analysis
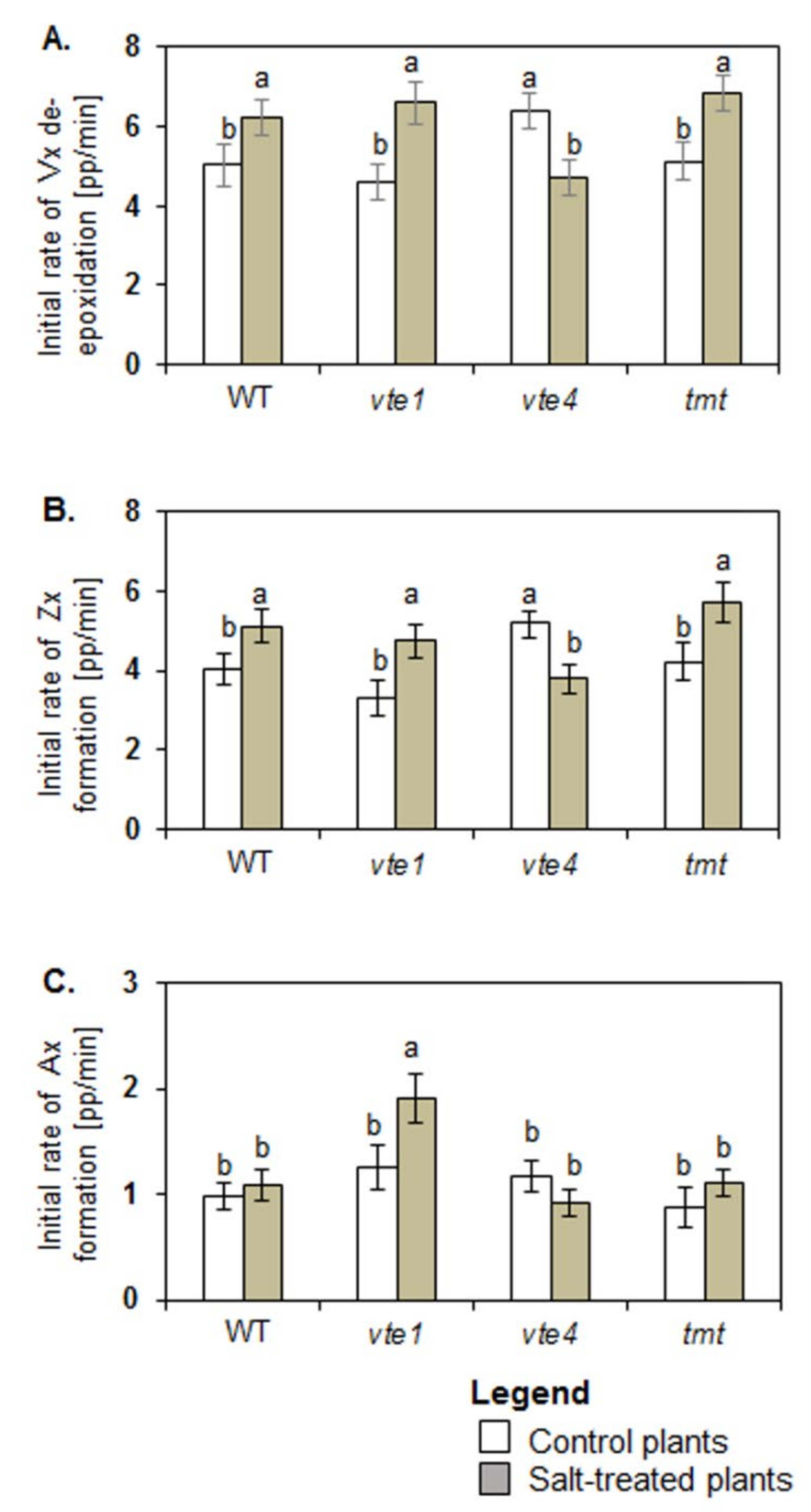
2.3. Vx De-Epaoxidation
2.4. Phenolic Acids
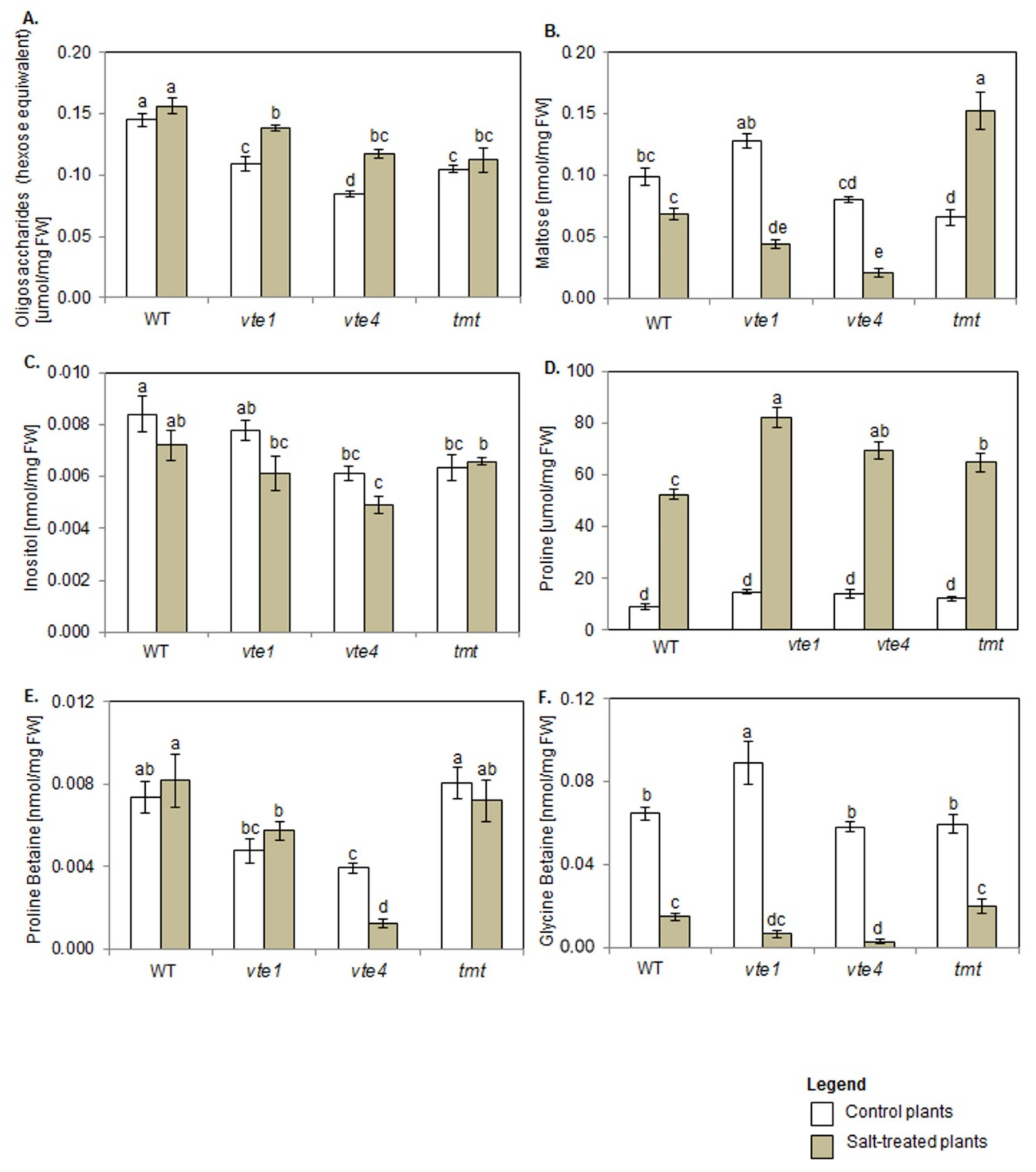
2.5. Carbohydrates and Polyols
2.6. Proline and Betaines
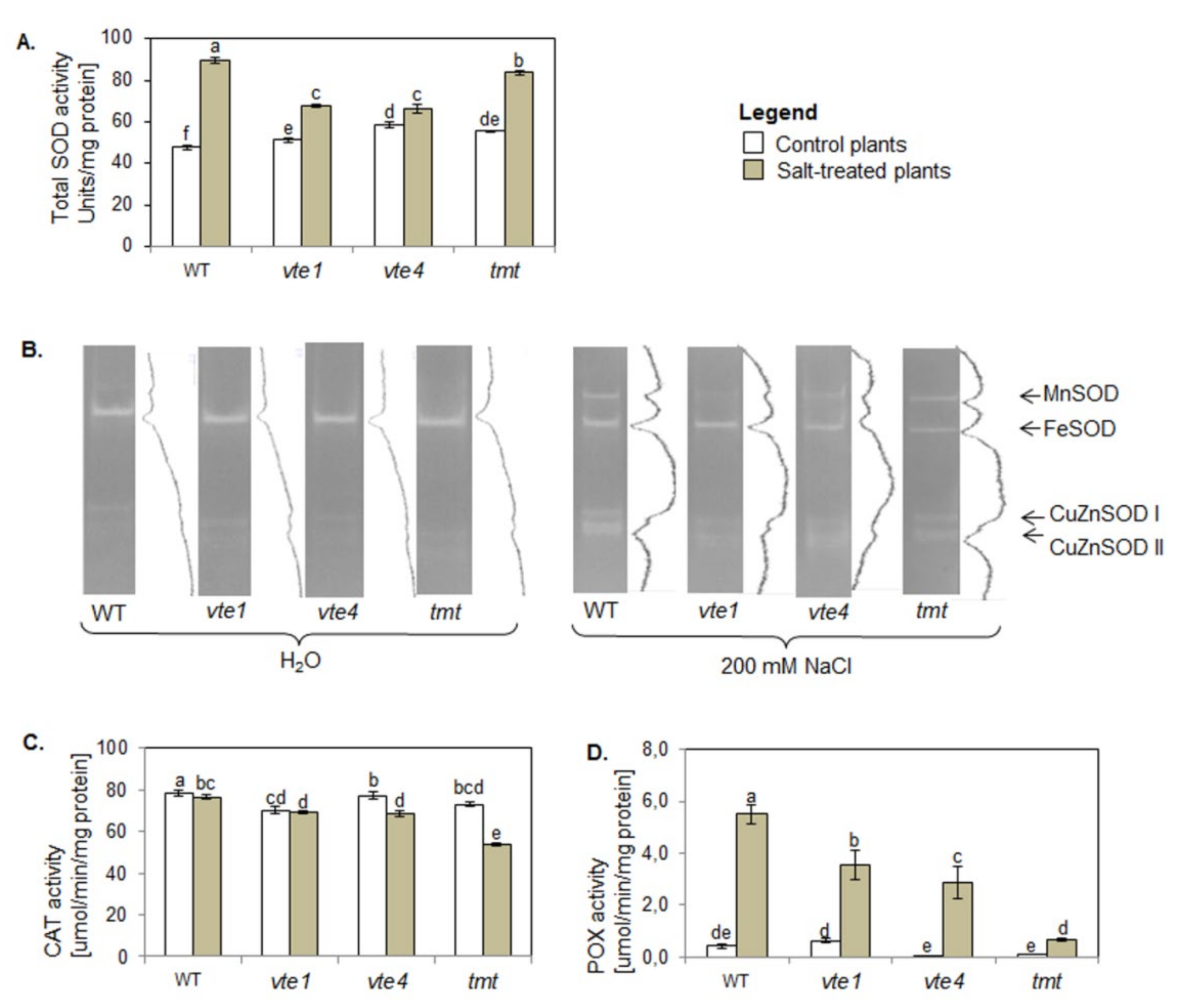
2.7. Activity of Antioxidant Enzymes
3. Discussion
3.1. Tocopherol Composition Modulates the Level of Photosynthetic Pigments (Chlorophylls, Carotenoids) and Intensity of Vx De-Epoxidation
3.2. Tocopherols Affected Energy Use and Dissipation
3.3. Tocopherol Composition modulates the Pool of Primary and Secondary Metabolites under Salt Stress
4. Materials and Methods
4.1. Plant Material
4.2. Fourier Transform Raman Spectroscopy Measurements and Chemometrics
4.3. Violaxanthin De-Epoxidation
4.4. Assays for ROS Scavengers and Osmoprotectants
4.4.1. Analysis of Phenolic Compounds
4.4.2. Carbohydrates and Sugar Alcohols
4.4.3. Proline and Betaine Estimation
- Proline
- Betaines
4.5. Protein Extraction and Determination of Antioxidant Enzyme Activity
4.6. Analysis of SOD by Native PAGE
4.7. Statistical Analysis
5. Conclusions and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| Ax | antheraxanthin |
| BSA | bovine serum albumin |
| CAR | carotenoids |
| Car | carotenes |
| CAT | catalase (EC 1.11.1.6) |
| CGA | chlorogenic acid |
| D-ACC | [2H4]1-amino-1-cyclopropanecarboxylic acid |
| DTT | dithiothreitol |
| EDTA | ethylenediaminetetraacetic acid |
| EGTA | ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid |
| ESI | electrospray ionization |
| FLD | fluorescence detector |
| FT-Raman | (Fourier-transformation Raman) spectroscopy |
| GB | glycine betaine |
| GGPP | geranylgeranyl pyrophosphate |
| Glu | (glutamate)-Pro-Arg-PAs-GABA (γ-aminobutyric acid) pathway |
| HGA | homogentisic acid/chorismate |
| HILIC | hydrophilic interaction liquid chromatography |
| HPLC | high performance liquid chromatography |
| MeOH/HCOOH | methanol/formic acid |
| MEP | 2-C-Methyl-D-erythritol 4-phosphate pathway |
| MS/MS | mass spectrometer |
| NBT | nitro blue tetrazolium |
| PAGE | polyacrylamide gel electrophoresis |
| PAs | polyamines |
| POX | non-specific peroxidase |
| Pro | proline |
| PAR | photosynthetically active radiation |
| pPD | p-phenylenediamine |
| RA | rosmarinic acid |
| RH | relative humidity |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase (EC 1.15.1.1) |
| TC | tocopherols |
| TCA | tricarboxylic acid cycle |
| TEMED | N,N,N′;N-tetramethylethylenediamine |
| TRICINE | N-tris[hydroxymethyl]methylglycine |
| TRIS | tris(hydroxymethyl)aminomethane |
| [U] | a unit of enzyme activity |
| VDE | violaxanthin de-epoxidase |
| Vx | violaxanthin |
| vte1 | A. thaliana mutant deficient in tocopherol cyclase, totally devoid of TCs |
| vte4 | A. thaliana homozygous mutant deficient in γ-tocopherol methyltransferase (γ-TMT, catalyses the conversion of γ-TC to α-TC) gene, accumulating γ-TC instead of α-TC |
| WT | wild type Arabidopsis thaliana (Columbia ecotype, Col-0); |
| γ-TMT | A. thaliana transgenic line (tmt) overexpressing γ-TMT, overproducing α-TC |
| Zx | zeaxanthin |
References
- Gorji, T.; Yildirim, A.; Sertel, E.; Tanik, A. Remote sensing approaches and mapping methods for monitoring soil salinity under different climate regimes. Int. J. Geoinform. 2019, 6, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Surówka, E.; Rapacz, M.; Janowiak, F. Climate change influences the interactive effects of simultaneous impact of abiotic and biotic stresses on plants. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives; Hasanuzzuman, M., Ed.; Springer: Singapore, 2020; pp. 1–50. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Millar, A.H.; Taylor, N.L. Connecting salt stress signalling pathways with salinity-induced changes in mitochondrial metabolic processes in C3 plants. Plant Cell Environ. 2017, 40, 2875–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suo, J.; Zhao, Q.; David, L.; Chen, S.; Dai, S. Salinity response in chloroplasts: Insights from gene characterization. Int. J. Mol. Sci. 2017, 18, 1011. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Corpas, F.J.; del Rio, L.A.; Palma, J.M. Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J. Integr. Plant Biol. 2019, 61, 803–816. [Google Scholar] [PubMed] [Green Version]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, S.; Wang, X.; Jung, C. Recent advances in our understanding of tocopherol biosynthesis in plants: An overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grudziński, W.; Nierzwicki, L.; Welc, R.; Reszczyńska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, P.; Munne-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahao, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Misra, A.N.; Latowski, D.; Strzałka, K. The xanthophyll cycle activity in kidney bean and cabbage leaves under salinity stress. Russ. J. Plant Physiol. 2006, 53, 102–109. [Google Scholar] [CrossRef]
- Surówka, E.; Kornaś, A.; Miszalski, Z. On the role of vitamin E in Arabidopsis thaliana seedlings growing at low light intensity. In Proceedings of the SFRR-E Meeting “Free Radicals, Health and Lifestyle: From Cell Signalling to Disease Prevention—Satellite Symposium on Vitamin E”, Rome, Italy, 26 August 2009; pp. 131–136. [Google Scholar]
- Farouk, S. Ascorbic acid and α-tocopherol minimize salt-induced wheat leaf senescence. J. Stress Physiol. Biochem. 2011, 7, 58–79. [Google Scholar]
- Agarwal, P.K.; Shukla, P.S.; Gupta, K.; Jha, B. Bioengineering for salinity tolerance in plants: State of the art. Mol. Biotechnol. 2013, 54, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Szymańska, R.; Cela, J.; Munne-Bosch, S. Plastochromanol-8: Fifty years of research. Phytochemistry 2014, 108, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Yadav, D.K.; Szymańska, R.; Kruk, J.; Sedlarova, M.; Pospisil, P. Singlet oxygen scavenging activity of tocopherol and plastochromanol in Arabidopsis thaliana: Relevance to photooxidative stress. Plant Cell Environ. 2014, 37, 392–401. [Google Scholar] [CrossRef]
- Surówka, E.; Potocka, I.; Dziurka, M.; Wróbel-Marek, J.; Kurczynska, E.; Żur, I.; Maksymowicz, A.; Gajewska, E.; Miszalski, Z. Tocopherols mutual balance is a key player for maintaining Arabidopsis thaliana growth under salt stress. Plant Physiol. Biochem. 2020, 156, 369–383. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol. 2003, 133, 930–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzaniga, S.; Bressan, M.; Carbonera, D.; Agostini, A.; Dall′Osto, L. Differential roles of carotenes and xanthophylls in photosystem i photoprotection. Biochemistry 2016, 55, 3636–3649. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. BBA Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trocsanyi, E.; Gyorgy, Z.; Zamborine-Nemeth, E. New insights into rosmarinic acid biosynthesis based on molecular studies. Curr. Plant Biol. 2020, 23, 100162. [Google Scholar] [CrossRef]
- Kaplan, F.; Sung, D.Y.; Guy, C.L. Roles of β-amylase and starch breakdown during temperatures stress. Physiol. Plant. 2006, 126, 120–128. [Google Scholar] [CrossRef]
- Purdy, S.J.; Bussell, J.D.; Nunn, C.P.; Smith, S.M. Leaves of the Arabidopsis maltose exporter1 mutant exhibit a metabolic profile with features of cold acclimation in the warm. PLoS ONE 2013, 8, e79412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorelli, S. The fermentation analogy: A point of view for understanding the intriguing role of proline accumulation in stressed plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikilitas, M.; Simsek, E.; Roychoudhury, A. Role of proline and glycine betaine in overcoming abiotic stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives, Roychoudhury; Tripathi, D.K.A., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2020; pp. 1–23. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Ali, E.F. Glycinebetaine in saline conditions: An assessment of the current state of knowledge. Acta Physiol. Plant. 2017, 39, 56. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell′Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.-R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of α-and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cela, J.; Chang, C.; Munne-Bosch, S. Accumulation of γ- rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1389–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Muller, M.; Abdelly, C.; Munne-Bosch, S. Increased sensitivity to salt stress in tocopherol-deficient Arabidopsis mutants growing in a hydroponic system. Plant Signal. Behav. 2013, 8, e23136. [Google Scholar] [CrossRef] [Green Version]
- Kanwischer, M.; Porfirova, S.; Bergmuller, E.; Dormann, P. Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol. 2005, 137, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Asensi-Fabado, M.A.; Ammon, A.; Sonnewald, U.; Munne-Bosch, S.; Voll, L.M. Tocopherol deficiency reduces sucrose export from salt-stressed potato leaves independently of oxidative stress and symplastic obstruction by callose. J. Exp. Bot. 2015, 66, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Surówka, E.; Kornaś, A.; Kuźniak, E. Nitrogen metabolism-related enzymes in Mesembryanthemum crystallinum after Botrytis cinerea infection. Biol. Plant. 2018, 62, 579–587. [Google Scholar] [CrossRef]
- Naliwajski, M.R.; Skłodowska, M. The relationship between carbon and nitrogen metabolism in cucumber leaves acclimated to salt stress. PeerJ 2018, 6, e6043. [Google Scholar] [CrossRef] [Green Version]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Mitsuya, S.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Light dependency of salinity-induced chloroplast degradation. Plant Prod. Sci. 2003, 6, 219–223. [Google Scholar] [CrossRef]
- Skłodowska, M.; Gapińska, M.; Gajewska, E.; Gabara, B. Tocopherol content and enzymatic antioxidant activities in chloroplasts from NaCl-stressed tomato plants. Acta Physiol. Plant. 2009, 31, 393–400. [Google Scholar] [CrossRef]
- Ischebeck, T.; Zbierzak, A.M.; Kanwischer, M.; Dormann, P. A salvage pathway for phytol metabolism in Arabidopsis. J. Biol. Chem. 2006, 281, 2470–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormann, P. Functional diversity of tocochromanols in plants. Planta 2007, 225, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Latowski, D.; Szymańska, R.; Strzałka, K. Carotenoids Involved in Antioxidant System of Chloroplasts. In Oxidative Damage to Plants; Parvaiz, A., Ed.; Academic Press: Cambrigde, MA, USA, 2014; pp. 289–319. [Google Scholar]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Latowski, D.; Surówka, E.; Strzałka, K. Regulatory Role of Components of Ascorbate–Glutathione Pathway in Plant Stress Tolerance. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N.A., Chan, M.-T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–53. [Google Scholar]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. BBA Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Lombard, J.; Moreira, D. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Mol. Biol. Evol. 2011, 28, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Kreszies, V. ABA-Dependent and -Independent Regulation of Tocopherol (Vitamin E) Biosynthesis in Response to Abiotic Stress in Arabidopsis; Rheinische Friedrich-Wilhelms-Universität Bonn: Bonn, Germany, 2019. [Google Scholar]
- Lallemand, L.A.; Zubieta, C.; Lee, S.G.; Wang, Y.; Acajjaoui, S.; Timmins, J.; McSweeney, S.; Jez, J.M.; McCarthy, J.G.; McCarthy, A.A. A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol. 2012, 160, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Akazawa, T.; Uritani, I. Respiratory oxidation and oxidative phosphorylation by cytoplasmic particles of sweet potato. J. Biochem. 1954, 41, 631–638. [Google Scholar] [CrossRef]
- Makovec, P.; Sindelar, L. The effect of phenolic compounds on the activity of respiratory chain enzymes and on respiration and phosphorylation activities of potato tuber mitochondria. Biol. Plant. 1984, 26, 415–422. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteille, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, F.; Guy, C.L. β-amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, S.P.; Gillaspy, G.E.; Perera, I.Y. Biosynthesis and possible functions of inositol pyrophosphates in plants. Front. Plant Sci. 2015, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.; Rigo, G.; Szekely, G.; Nagy, R.; Koncz, C.; Szabados, L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol. Biol. 2003, 51, 363–372. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Kumari, P.H.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef] [Green Version]
- Roychoudhury, A.; Banerjee, A.; Lahiri, V. Metabolic and molecular-genetic regulation of proline signaling and itscross-talk with major effectors mediates abiotic stress tolerance in plants. Turk. J. Botany 2015, 39, 887–910. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuschle, K.; Funck, D.; Forlani, G.; Stransky, H.; Biehl, A.; Leister, D.; van der Graaff, E.; Kunzee, R.; Frommer, W.B. The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell 2004, 16, 3413–3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahikainen, M.; Alegre, S.; Trotta, A.; Pascual, J.; Kangasjarvi, S. Trans-methylation reactions in plants: Focus on the activated methyl cycle. Physiol. Plant. 2018, 162, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Beena, A.S.; Awana, M.; Singh, A. Salt-induced tissue-specific cytosine methylation downregulates expression of HKT genes in contrasting wheat (Triticum aestivum L.) genotypes. DNA Cell Biol. 2017, 36, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Karan, R.; DeLeon, T.; Biradar, H.; Subudhi, P.K. Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE 2012, 7, e40203. [Google Scholar] [CrossRef] [PubMed]
- Toth, V.R.; Meszaros, I.; Veres, S.; Nagy, J. Effects of the available nitrogen on the photosynthetic activity and xanthophyll cycle pool of maize in field. J. Plant Physiol. 2002, 159, 627–634. [Google Scholar] [CrossRef]
- Cheng, L.L. Xanthophyll cycle pool size and composition in relation to the nitrogen content of apple leaves. J. Exp. Bot. 2003, 54, 385–393. [Google Scholar] [CrossRef]
- Kruk, J.; Szymańska, R.; Nowicka, B.; Dłużewska, J. Function of isoprenoid quinones and chromanols during oxidative stress in plants. New Biotechnol. 2016, 33, 636–643. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.M.; Hebelstrup, K.H.; Gupta, K.J. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 2013, 5, pls052. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S.; Weiler, E.W.; Alegre, L.; Muller, M.; Duechting, P.; Falk, J. α-tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 2007, 225, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Desel, C.; Hubbermann, E.M.; Schwarz, K.; Krupińska, K. Nitration of gamma-tocopherol in plant tissues. Planta 2007, 226, 1311–1322. [Google Scholar] [CrossRef]
- Porfirova, S.; Bergmuller, E.; Tropf, S.; Lemke, R.; Dormann, P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12495–12500. [Google Scholar] [CrossRef] [Green Version]
- Bergmuller, E.; Porfirova, S.; Dormann, P. Characterization of an Arabidopsis mutant deficient in gamma-tocopherol methyltransferase. Plant Mol. Biol. 2003, 52, 1181–1190. [Google Scholar] [CrossRef]
- Shintani, D.; DellaPenna, D. Elevating the vitamin E content of plants through metabolic engineering. Science 1998, 282, 2098–2100. [Google Scholar] [CrossRef]
- Rosso, M.G.; Li, Y.; Strizhov, N.; Reiss, B.; Dekker, K.; Weisshaar, B. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 2003, 53, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Surówka, E.; Dziurka, M.; Kocurek, M.; Goraj, S.; Rapacz, M.; Miszalski, Z. Effects of exogenously applied hydrogen peroxide on antioxidant and osmoprotectant profiles and the C-3-CAM shift in the halophyte Mesembryanthemum crystallinum L. J. Plant Physiol. 2016, 200, 102–110. [Google Scholar] [CrossRef]
- Skoczowski, A.; Troć, M. Isothermal Calorimetry and Raman Spectroscopy to Study Response of Plants to Abiotic and Biotic Stresses. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 263–288. [Google Scholar] [CrossRef]
- Ryś, M.; Juhasz, C.; Surówka, E.; Janeczko, A.; Saja, D.; Tobias, I.; Skoczowski, A.; Barna, B.; Gullner, G. Comparison of a compatible and an incompatible pepper-tobamovirus interaction by biochemical and non-invasive techniques: Chlorophyll a fluorescence, isothermal calorimetry and FT-Raman spectroscopy. Plant Physiol. Biochem. 2014, 83, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latowski, D.; Kruk, J.; Strzałka, K. Inhibition of zeaxanthin epoxidase activity by cadmium ions in higher plants. J. Inorg. Biochem. 2005, 99, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Dziurka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. Different allocation of carbohydrates and phenolics in dehydrated leaves of triticale. J. Plant Physiol. 2016, 202, 1–9. [Google Scholar] [CrossRef]
- Gołębiowska-Pikania, G.; Dziurka, M.; Wąsek, I.; Wajdzik, K.; Dyda, M.; Wędzony, M. Changes in phenolic acid abundance involved in low temperature and Microdochium nivale (Samuels and Hallett) cross-tolerance in winter triticale (x Triticosecale Wittmack). Acta Physiol. Plant 2019, 41, 38. [Google Scholar] [CrossRef] [Green Version]
- Wiszniewska, A.; Kosmińska, A.; Hanus-Fajerska, E.; Dziurka, M.; Dziurka, K. Insight into mechanisms of multiple stresses tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019, 180, 12–22. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitationof microgram quantities of protein utilizing the principle ofprotein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Minami, M.; Yoshikawa, H. A simplified assay method at superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar] [PubMed]
- McCord, J.M.; Fridovic, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6050. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. In Methods in Enzymology. Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Luck, H. Peroxidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press Inc.: London, UK, 1963; pp. 895–897. [Google Scholar]
- Allgood, G.S.; Perry, J.J. Oxygen defense systems in obligately thermophilic bacteria. Can. J. Microbiol. 1985, 31, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during as-sembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Miszalski, Z.; Ślesak, I.; Niewiadomska, E.; Bączek-Kwinta, R.; Lüttge, U.; Ratajczak, R. Subcellular localization and stress responses of superoxide dismutase isoforms from leaves in the C-3-CAM intermediate halophyte Mesembryanthemum crystallinum L. Plant Cell Environ. 1998, 21, 169–179. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovic, I. Superoxide dismutase: Improved assays and an assay implicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]


| A. thaliana with Changed TC Composition /Treatment | α-TC μg/g FW | Standard Deviation (SD) | γ-TC μg/g FW | Standard Deviation (SD) |
|---|---|---|---|---|
| WT H2O | 72.6 | 3.67 | 4.1 | 0.2 |
| WT NaCl | 83.5 | 6.05 | 6.5 | 0.8 |
| vte1 H2O | ND * | ND | ND | ND |
| vte1 NaCl | ND | ND | ND | ND |
| vte4 H2O | ND | ND | 58.0 | 3.7 |
| vte4 NaCl | ND | ND | 72.3 | 5.9 |
| tmt H2O | 115.5 | 7.51 | ND | ND |
| tmt NaCl | 134.7 | 11.68 | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surówka, E.; Latowski, D.; Dziurka, M.; Rys, M.; Maksymowicz, A.; Żur, I.; Olchawa-Pajor, M.; Desel, C.; Krzewska, M.; Miszalski, Z. ROS-Scavengers, Osmoprotectants and Violaxanthin De-Epoxidation in Salt-Stressed Arabidopsis thaliana with Different Tocopherol Composition. Int. J. Mol. Sci. 2021, 22, 11370. https://doi.org/10.3390/ijms222111370
Surówka E, Latowski D, Dziurka M, Rys M, Maksymowicz A, Żur I, Olchawa-Pajor M, Desel C, Krzewska M, Miszalski Z. ROS-Scavengers, Osmoprotectants and Violaxanthin De-Epoxidation in Salt-Stressed Arabidopsis thaliana with Different Tocopherol Composition. International Journal of Molecular Sciences. 2021; 22(21):11370. https://doi.org/10.3390/ijms222111370
Chicago/Turabian StyleSurówka, Ewa, Dariusz Latowski, Michał Dziurka, Magdalena Rys, Anna Maksymowicz, Iwona Żur, Monika Olchawa-Pajor, Christine Desel, Monika Krzewska, and Zbigniew Miszalski. 2021. "ROS-Scavengers, Osmoprotectants and Violaxanthin De-Epoxidation in Salt-Stressed Arabidopsis thaliana with Different Tocopherol Composition" International Journal of Molecular Sciences 22, no. 21: 11370. https://doi.org/10.3390/ijms222111370
APA StyleSurówka, E., Latowski, D., Dziurka, M., Rys, M., Maksymowicz, A., Żur, I., Olchawa-Pajor, M., Desel, C., Krzewska, M., & Miszalski, Z. (2021). ROS-Scavengers, Osmoprotectants and Violaxanthin De-Epoxidation in Salt-Stressed Arabidopsis thaliana with Different Tocopherol Composition. International Journal of Molecular Sciences, 22(21), 11370. https://doi.org/10.3390/ijms222111370










