The Potential Role of Electronegative High-Density Lipoprotein H5 Subfraction in RA-Related Atherosclerosis
Abstract
:1. Introduction
2. Results
2.1. Demographic Data and Clinical Characteristics of RA Patients
2.2. Comparison of Lipid Profiles, QRISK-2 Scores, and Subclinical Atherosclerosis between RA Patients with High H5% and Normal H5%
2.3. Increased Plasma H5% in RA Patients
2.4. Logistic Regression Analyses and Receiver Operating Characteristic (ROC) Curve Analysis for Predicting the Presence of Subclinical Atherosclerosis
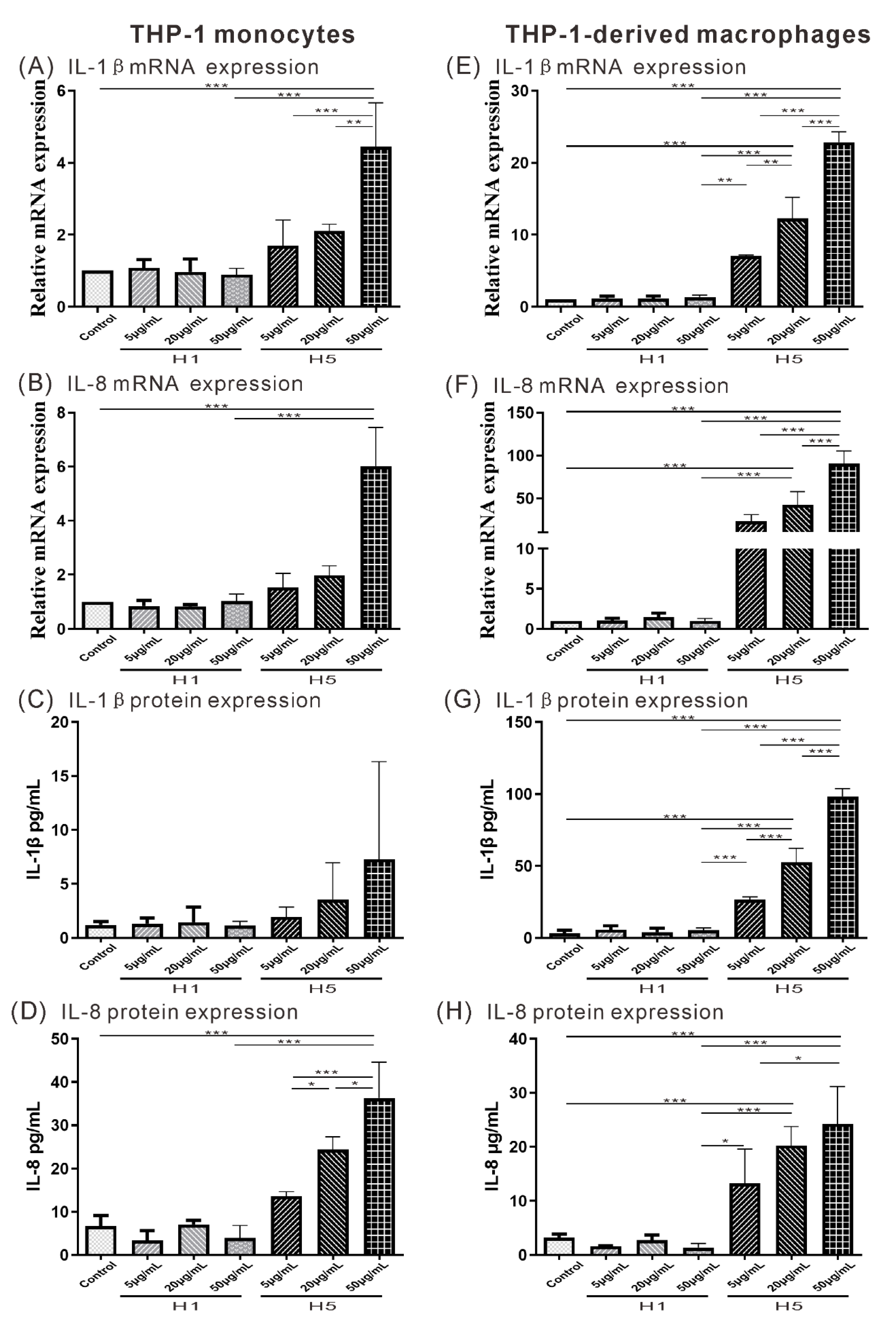
2.5. The Effects of H5 on the Expression of Atherogenesis-Related Cytokine and Chemokine on THP-1 Monocytes or THP-1-Derived Macrophages
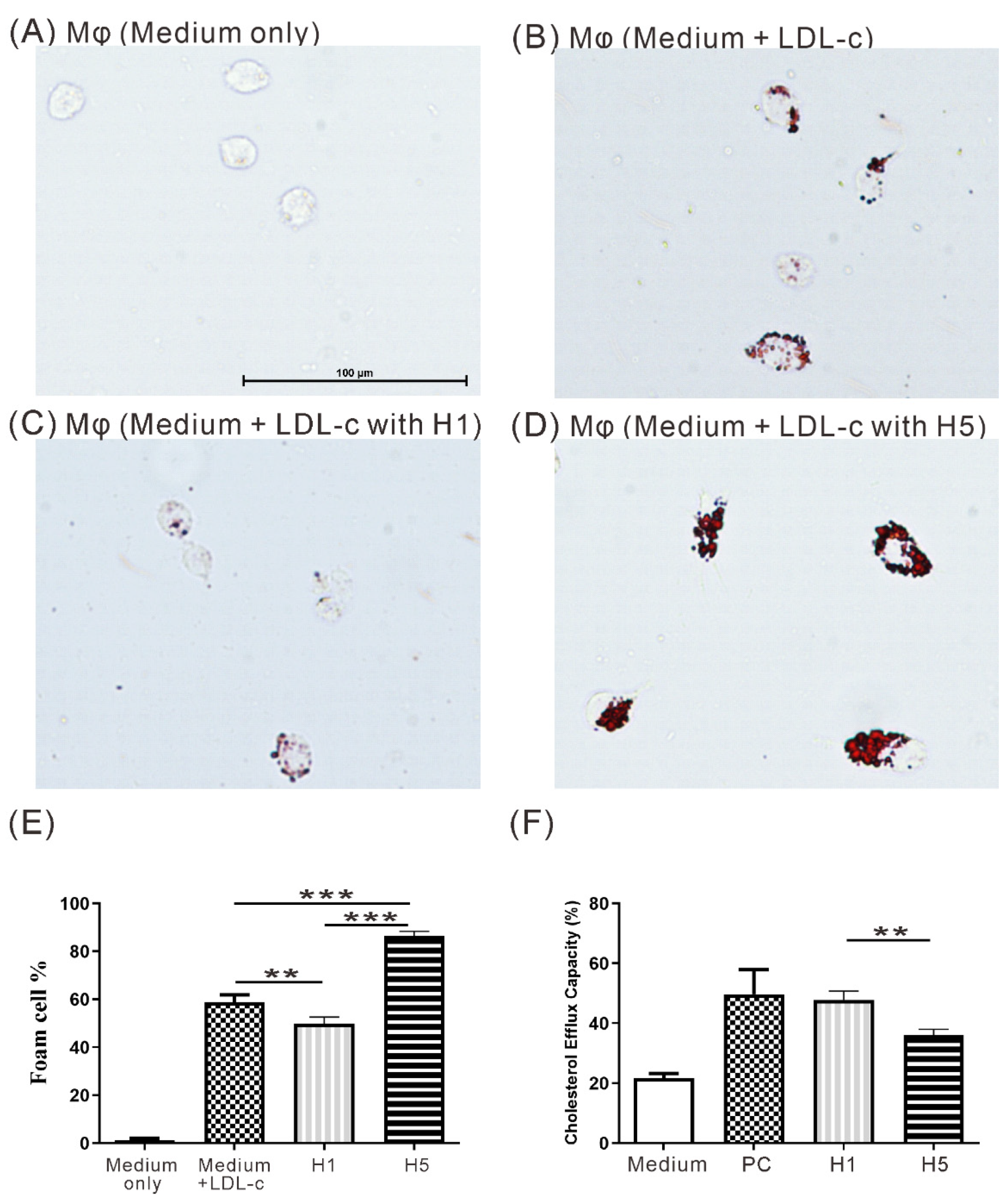
2.6. The Effects of H5 on Macrophage Foam Cell Formation
2.7. The Effects of H5 on Cholesterol Efflux Capacity
2.8. Identification of Discriminative Compositional Proteins of HDL-c between RA Patients with High H5% and Normal H5%
2.9. Comparison of Lp(a) Levels among Patients with High H5%, Patients with Normal H5%, and HC
2.10. Linear Regression Analysis for H5%
2.11. The Potential Role of H5 with RA-Related Atherogenesis in Clinical and In Vitro
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Cell Culture
4.3. Determination of Plasma Lipid Profiles and Atherogenic Index (AI)
4.4. Measurement of 10-Year Risk of Cardiovascular Disease (QRISK-2 Score)
4.5. Isolation and Fractionation of HDL-c
4.6. Isolation of HDL-c Subfractions with Anion-Exchange Column
4.7. Agarose Gel Electrophoresis of HDL-c Subfractions
4.8. IL-1β and IL-8 mRNA Expression in THP-1 Cells Treated with H1 or H5
4.9. Enzyme-linked Immunosorbent Assay (ELISA)
4.10. Examination of Foam Cells Formation in THP-1-Derived Macrophages
4.11. Cholesterol Efflux Capacity Assay
4.12. NanoLC-MS/MS Analysis of HDL-c Composition
4.13. Protein Database Search and Label-Free Quantification
4.14. Ultrasound Vascular Imaging of Carotid Arteries
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACPA | Anticitrullinated peptide antibodies |
| CRP | C-reactive protein |
| AF | Atrial fibrillation |
| AI | Atherogenic index |
| Apo | Apolipoprotein |
| ASCVD | Atherosclerotic cardiovascular disease |
| BMI | Body mass index |
| BSA | Bovine serum albumin |
| csDMARD | Conventional synthetic disease-modifying antirheumatic drug |
| DAS28 | Disease activity score for 28 joints |
| DM | Diabetes mellitus |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ESR | Erythrocyte sedimentation rate |
| FPLC | Fast protein liquid chromatography |
| H5% | The percentage of the most electronegative subfraction of HDL-c |
| HC | Healthy controls |
| HDL | High-density lipoprotein |
| HDL-c | High-density lipoprotein cholesterol |
| IL | Interleukin |
| JAKi | Janus kinase inhibitors |
| LC/MS | Liquid chromatography/mass spectrometry |
| LDL | Low-density lipoprotein |
| LDL-c | Low-density lipoprotein cholesterol |
| Lp(a) | Lipoprotein a |
| RA | Rheumatoid arthritis |
| RF | Rheumatoid factor |
| TC | Total cholesterol |
| TCZ | Tocilizumab |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor-α |
References
- Libby, P. Inflammation in atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [Green Version]
- Kologrivova, I.; Suslova, T.; Koshelskaya, O.; Trubacheva, O.; Haritonova, O.; Vinnitskaya, I. Frequency of monocyte subsets is linked to the severity of atherosclerosis in patients with ischemic heart disease: A case-control study. Biomedicine 2020, 10, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Furst, D.E.; Emery, P. Rheumatoid arthritis pathophysiology: Update on emerging cytokine and cytokine-associated cell targets. Rheumatology 2014, 53, 1560–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.H.; Chung, C.P.; Oeser, A.; Solus, J.; Asanuma, Y.; Sokka, T.; Pincus, T.; Raggi, P.; Gebretsadik, T.; Shintani, A.; et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009, 61, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Boyer, J.F.; Gourraud, P.A.; Cantagrel, A.; Davignon, J.L.; Constantin, A. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Jt. Bone Spine 2011, 78, 179–183. [Google Scholar] [CrossRef]
- Im, C.H.; Kim, N.R.; Kang, J.W.; Kim, J.H.; Kang, J.Y.; Bae, G.B.; Nam, E.J.; Kang, Y.M. Inflammatory burden interacts with conventional cardiovascular risk factors for carotid plaque formation in rheumatoid arthritis. Rheumatology 2015, 54, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Negre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arter. Thromb. Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Aikawa, M. High-density lipoproteins: From function to therapy. J. Am. Coll. Cardiol. 2012, 60, 2380–2383. [Google Scholar] [CrossRef] [Green Version]
- Rosso, L.G.; Lhomme, M.; Merono, T.; Sorroche, P.; Catoggio, L.; Soriano, E.; Saucedo, C.; Malah, V.; Dauteuille, C.; Boero, L.; et al. Altered lipidome and antioxidative activity of small, dense HDL in normolipidemic rheumatoid arthritis: Relevance of inflammation. Atherosclerosis 2014, 237, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Ganeshalingam, K.; Semb, A.G.; Szekanecz, Z.; Nurmohamed, M. Cardiovascular risk in rheumatoid arthritis: Recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014, 53, 2143–2154. [Google Scholar] [CrossRef] [Green Version]
- Giraud, C.; Tournadre, A.; Pereira, B.; Dutheil, F.; Soubrier, M.; Lhomme, M.; Kontush, A.; Sebedio, J.L.; Capel, F. Alterations of HDL particle phospholipid composition and role of inflammation in rheumatoid arthritis. J. Physiol. Biochem. 2019, 75, 453–462. [Google Scholar] [CrossRef]
- Berg, K. A New Serum Type System in Man--the Lp System. Acta Pathol. Microbiol. Scand. 1963, 59, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Gaubatz, J.W.; Chari, M.V.; Nava, M.L.; Guyton, J.R.; Morrisett, J.D. Isolation and characterization of the two major apoproteins in human lipoprotein [a]. J. Lipid Res. 1987, 28, 69–79. [Google Scholar] [CrossRef]
- Govindan, K.P.; Basha, S.; Ramesh, V.; Kumar, C.N.; Swathi, S. A comparative study on serum lipoprotein (a) and lipid profile between rheumatoid arthritis patients and normal subjects. J. Pharm. Bioallied Sci. 2015, 7, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Segura, B.; Macia-Diaz, M.; Machado, J.D.; de Vera-Gonzalez, A.; Garcia-Dopico, J.A.; Olmos, J.M.; Hernandez, J.L.; Diaz-Gonzalez, F.; Gonzalez-Gay, M.A.; Ferraz-Amaro, I. HDL cholesterol efflux capacity in rheumatoid arthritis patients: Contributing factors and relationship with subclinical atherosclerosis. Arthritis Res. Ther. 2017, 19, 113. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Merchant, J.; Fleming, S.E. Lp(a)-cholesterol is associated with HDL-cholesterol in overweight and obese African American children and is not an independent risk factor for CVD. Cardiovasc. Diabetol. 2012, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Dursunoglu, D.; Evrengul, H.; Polat, B.; Tanriverdi, H.; Cobankara, V.; Kaftan, A.; Kilic, M. Lp(a) lipoprotein and lipids in patients with rheumatoid arthritis: Serum levels and relationship to inflammation. Rheumatol. Int. 2005, 25, 241–245. [Google Scholar] [CrossRef]
- Chang, C.T.; Shen, M.Y.; Hsieh, J.Y.; Chang, C.M.; Liao, H.Y.; Chen, F.Y.; Hsu, C.Y.; Yang, C.Y.; Chen, C.J. Increased electronegativity of high-density lipoprotein in uremia patients impairs its functional properties and is associated with the risk of coronary artery disease. Atherosclerosis 2018, 278, 147–155. [Google Scholar] [CrossRef]
- Liu, W.; Yin, Y.; Zhou, Z.; He, M.; Dai, Y. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm. Res. 2014, 63, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Dechkhajorn, W.; Maneerat, Y.; Prasongsukarn, K.; Kanchanaphum, P.; Kumsiri, R. Interleukin-8 in Hyperlipidemia and Coronary Heart Disease in Thai Patients Taking Statin Cholesterol-Lowering Medication While Undergoing Coronary Artery Bypass Grafting Treatment. Scientifica 2020, 2020, 5843958. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Anti-Inflammatory Therapy for Atherosclerosis: Focusing on Cytokines. Int. J. Mol. Sci. 2021, 22, 7061. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaisar, T.; Mayer, P.; Nilsson, E.; Zhao, X.Q.; Knopp, R.; Prazen, B.J. HDL in humans with cardiovascular disease exhibits a proteomic signature. Clin. Chim. Acta 2010, 411, 972–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onat, A.; Can, G.; Murat, S.; Cicek, G.; Ornek, E.; Yuksel, H. Aggregation of lipoprotein(a) to apolipoprotein A-I underlying HDL dysfunction as a major coronary risk factor. Anadolu Kardiyol. Derg. 2013, 13, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Jia, C.; Anderson, J.L.C.; Gruppen, E.G.; Lei, Y.; Bakker, S.J.L.; Dullaart, R.P.F.; Tietge, U.J.F. High-Density Lipoprotein Anti-Inflammatory Capacity and Incident Cardiovascular Events. Circulation 2021, 143, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Grossman, J.; FitzGerald, J.; Dahlin-Lee, E.; Wallace, D.J.; Thong, B.Y.; Badsha, H.; Kalunian, K.; Charles, C.; Navab, M.; et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2541–2549. [Google Scholar] [CrossRef]
- Dessein, P.H.; Solomon, A.; Hollan, I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pr. Res. Clin. Rheumatol. 2016, 30, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, G.; Nurmohamed, M.T.; Gonzalez-Gay, M.A.; Seres, I.; Paragh, G.; Kardos, Z.; Barath, Z.; Tamasi, L.; Soltesz, P.; Szekanecz, Z. Rheumatoid arthritis and metabolic syndrome. Nat. Rev. Rheumatol. 2014, 10, 691–696. [Google Scholar] [CrossRef]
- Martinelli, A.E.M.; Maranhao, R.C.; Carvalho, P.O.; Freitas, F.R.; Silva, B.M.O.; Curiati, M.N.C.; Kalil Filho, R.; Pereira-Barretto, A.C. Cholesteryl ester transfer protein (CETP), HDL capacity of receiving cholesterol and status of inflammatory cytokines in patients with severe heart failure. Lipids Health Dis. 2018, 17, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Tsuchida, Y.; Diab, R.; Xu, C.; Yermalitsky, V.; Davies, S.S.; Ikizler, T.A.; Hung, A.M.; Kon, V.; Flynn, C.R. Pro-inflammatory HDL in women with obesity and nonalcoholic steatohepatitis. Obes. Res. Clin. Pr. 2020, 14, 333–338. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.A.; Tas, S.W.; Wolfs, I.M.J.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-kappaB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Lutgens, E.; Atzler, D.; Doring, Y.; Duchene, J.; Steffens, S.; Weber, C. Immunotherapy for cardiovascular disease. Eur. Heart J. 2019, 40, 3937–3946. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; Thuren, T.; Glynn, R.J.; Group, C.T. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet 2018, 391, 319–328. [Google Scholar] [CrossRef]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef]
- Asanuma, Y.; Chung, C.P.; Oeser, A.; Shintani, A.; Stanley, E.; Raggi, P.; Stein, C.M. Increased concentration of proatherogenic inflammatory cytokines in systemic lupus erythematosus: Relationship to cardiovascular risk factors. J. Rheumatol. 2006, 33, 539–545. [Google Scholar]
- Boisvert, W.A.; Curtiss, L.K.; Terkeltaub, R.A. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunol. Res. 2000, 21, 129–137. [Google Scholar] [CrossRef]
- Tang, X.E.; Li, H.; Chen, L.Y.; Xia, X.D.; Zhao, Z.W.; Zheng, X.L.; Zhao, G.J.; Tang, C.K. IL-8 negatively regulates ABCA1 expression and cholesterol efflux via upregulating miR-183 in THP-1 macrophage-derived foam cells. Cytokine 2019, 122, 154385. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Chang, C.T.; Huang, M.T.; Chang, C.M.; Chen, C.Y.; Shen, M.Y.; Liao, H.Y.; Wang, G.J.; Chen, C.H.; Chen, C.J.; et al. Biochemical and functional characterization of charge-defined subfractions of high-density lipoprotein from normal adults. Anal. Chem. 2013, 85, 11440–11448. [Google Scholar] [CrossRef] [Green Version]
- Hippe, D.S.; Phan, B.A.P.; Sun, J.; Isquith, D.A.; O’Brien, K.D.; Crouse, J.R.; Anderson, T.; Huston, J.; Marcovina, S.M.; Hatsukami, T.S.; et al. Lp(a) (Lipoprotein(a)) Levels Predict Progression of Carotid Atherosclerosis in Subjects With Atherosclerotic Cardiovascular Disease on Intensive Lipid Therapy: An Analysis of the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes) Carotid Magnetic Resonance Imaging Substudy-Brief Report. Arter. Thromb. Vasc. Biol. 2018, 38, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.H.; Cao, Y.X.; Jin, J.L.; Zhang, H.W.; Hua, Q.; Li, Y.F.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Xu, R.X.; et al. Predicting Cardiovascular Outcomes by Baseline Lipoprotein(a) Concentrations: A Large Cohort and Long-Term Follow-up Study on Real-World Patients Receiving Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2020, 9, e014581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, L.; Jia, X.; Pang, X.; Xiang, Q.; Zhao, X.; Ma, L.; Liu, Z.; Hu, K.; Wang, Z.; et al. Comparative Lipid-Lowering/Increasing Efficacy of 7 Statins in Patients with Dyslipidemia, Cardiovascular Diseases, or Diabetes Mellitus: Systematic Review and Network Meta-Analyses of 50 Randomized Controlled Trials. Cardiovasc. Ther. 2020, 2020, 3987065. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; Van ‘t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Hippisley-Cox, J.; Coupland, C.; Vinogradova, Y.; Robson, J.; Minhas, R.; Sheikh, A.; Brindle, P. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ 2008, 336, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Yang, J.H.; Burns, A.R.; Chen, H.H.; Tang, D.; Walterscheid, J.P.; Suzuki, S.; Yang, C.Y.; Sawamura, T.; Chen, C.H. Mediation of electronegative low-density lipoprotein signaling by LOX-1: A possible mechanism of endothelial apoptosis. Circ. Res. 2009, 104, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Huang, Y.; Xie, Y.; Lan, T.; Le, K.; Chen, J.; Chen, S.; Gao, S.; Xu, X.; Shen, X.; et al. Evaluation of foam cell formation in cultured macrophages: An improved method with Oil Red O staining and DiI-oxLDL uptake. Cytotechnology 2010, 62, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Chao, Y.M.; Lin, H.F.; Chen, C.J.; Chen, C.S.; Yang, J.L.; Chan, J.Y.H.; Juo, S.H. miR-195 reduces age-related blood-brain barrier leakage caused by thrombospondin-1-mediated selective autophagy. Aging Cell 2020, 19, e13236. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Liao, W.L.; Chang, C.T.; Liao, H.Y.; Tsai, F.J. Urine proteome analysis by C18 plate-matrix-assisted laser desorption/ionization time-of-flight mass spectrometry allows noninvasive differential diagnosis and prediction of diabetic nephropathy. PLoS ONE 2018, 13, e0200945. [Google Scholar] [CrossRef] [Green Version]
- Corrales, A.; Gonzalez-Juanatey, C.; Peiro, M.E.; Blanco, R.; Llorca, J.; Gonzalez-Gay, M.A. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann. Rheum. Dis. 2014, 73, 722–727. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [Green Version]




| RA with High H5% (n = 38) | RA with Normal H5% (n = 31) | Healthy Controls (n = 11) | |
|---|---|---|---|
| Age at study entry, years | 59.6 ± 12.4 | 55.5 ± 13.6 | 40.9 ± 9.9 |
| Women proportion | 29 (76.3%) | 23 (74.2%) | 7 (64%) |
| RA duration, months | 70.5 ± 29.1 | 70.7 ± 26.5 | NA |
| BMI, kg/m2 | 24.5 ± 4.4 | 23.0 ± 2.3 | 23.3 ± 2.3 |
| RF positivity | 28 (73.7%) | 22 (71.0%) | NA |
| ACPA positivity | 27 (71.1%) | 20 (64.5%) | NA |
| ESR at study entry, mm/1st hour | 32.5 ± 25.1 | 23.0 ± 17.5 | NA |
| CRP at study entry, mg/dl | 2.80 ± 5.03 | 1.61 ± 2.47 | NA |
| DAS28 at study entry | 4.66 ± 1.72 | 4.18 ± 1.73 | NA |
| csDMARDs alone at study entry | 16 (42.1%) | 14 (45.2%) | NA |
| Biologics used at study entry | |||
| Tofacitinib | 10 (26.3%) | 8 (25.8%) | NA |
| TNF-α inhibitors | 6 (15.8%) | 5 (16.1%) | NA |
| IL-6R inhibitor | 5 (13.2%) | 3 (9.7%) | NA |
| Abatacept | 1 (2.6%) | 1 (3.2%) | NA |
| Comorbidities | |||
| Hypertension | 23 (60.5%) | 17 (54.8%) | 0 (0.0%) |
| Diabetes mellitus | 3 (7.9%) | 4 (12.9%) | 0 (0.0%) |
| Current smoker | 3 (7.9%) | 3 (9.7%) | 0 (0.0%) |
| Coronary artery disease | 3 (7.9%) | 2 (6.5%) | 0 (0.0%) |
| Chronic kidney disease | 3 (7.9%) | 1 (3.2%) | 0 (0.0%) |
| Lipid profile at study entry | |||
| TC, mg/dL | 192 (166–215) | 196 (158–227) | 172 (146–215) |
| HDL-c, mg/dL | 57 (47–66) | 54 (44–69) | 41 (43–59) |
| Triglyceride, mg/dL | 89 (53–116) | 102 (74–135) | 73 (33–122) |
| LDL-c, mg/dL | 111 (95–124) | 124 (85–146) | 111 (92–141) |
| Atherogenic index | 3.3 (2.7–4.1) | 3.3 (3.0–4.3) | 3.1 (2.8–4.2) |
| QRISK-2 score, % | 6.9 (3.5–13.7) | 4.5 (2.5–13.6) | 0.4 (0.3–1.0) |
| ccIMT, mm | 1.28 (1.16–1.40) | 1.14 (1.06–1.26) | NA |
| Carotid plaque | 11 (28.9%) | 5 (16.1%) | NA |
| Subclinical atherosclerosis | 25 (65.8%) b | 9 (29.0%) | NA |
| Risk Factor | p-Value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|
| Univariate | |||
| Gender (Female) | 0.367 | 0.6 | 0.198–1.819 |
| Age | 0.003 | 1.081 | 1.028–1.138 |
| TC | 0.717 | 0.998 | 0.987–1.009 |
| TG | 0.537 | 1.002 | 0.995–1.009 |
| HDL-c | 0.331 | 0.983 | 0.95–1.017 |
| LDL-c | 0.879 | 0.999 | 0.987–1.012 |
| TC/HDL-c | 0.986 | 1.003 | 0.689–1.461 |
| BMI | 0.572 | 0.966 | 0.858–1.088 |
| DM | 0.97 | 1.032 | 0.193–5.51 |
| AF | 0.976 | 1.031 | 0.137–7.769 |
| H5% | 0.007 | 1.265 | 1.066–1.501 |
| Smoking | 0.687 | 1.336 | 0.327–5.467 |
| Hypertension | 0.888 | 1.071 | 0.412–2.788 |
| Multivariable | |||
| Age | 0.005 | 1.077 | 1.023–1.134 |
| H5% | 0.014 | 1.255 | 1.047–1.506 |
| Factor | p-Value | β-Value | 95% Confidence Interval |
|---|---|---|---|
| Univariate | |||
| Age | 0.259 | 0.138 | −0.104–0.379 |
| Gender (Female) | 0.439 | −0.095 | −0.337–0.148 |
| LDL-c | 0.452 | −0.095 | −0.346–0.156 |
| HDL-c | 0.865 | −0.021 | −0.275–0.231 |
| TC/HDL-c | 0.712 | −0.047 | −0.304–0.208 |
| DAS28 | 0.678 | −0.051 | −0.294–0.193 |
| ESR | 0.917 | 0.013 | −0.231–0.257 |
| CRP | 0.894 | −0.016 | −0.260–0.227 |
| Lp(a) | 0.064 | 0.225 | −0.013–0.462 |
| Multivariable | |||
| Age | 0.264 | 0.175 | −0.138–0.493 |
| Gender (Female) | 0.685 | −0.058 | −0.337–0.223 |
| LDL-c | 0.249 | −0.311 | −0.852–0.226 |
| HDL-c | 0.880 | −0.037 | −0.528–0.454 |
| TC/HDL-c | 0.727 | 0.123 | −0.576–0.821 |
| DAS28 | 0.944 | −0.020 | −0.607–0.565 |
| ESR | 0.538 | −0.210 | −0.960–0.507 |
| CRP | 0.982 | −0.004 | −0.368–0.360 |
| Lp(a) | <0.05 (0.028) | 0.299 | 0.032–0.551 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-K.; Cheng, W.-C.; Ma, W.-L.; Chen, P.-K.; Chen, C.-H.; Shen, P.-C.; Chen, C.-C.; Chang, S.-H.; Lai, Y.-H.; Chen, D.-Y. The Potential Role of Electronegative High-Density Lipoprotein H5 Subfraction in RA-Related Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 11419. https://doi.org/10.3390/ijms222111419
Chang C-K, Cheng W-C, Ma W-L, Chen P-K, Chen C-H, Shen P-C, Chen C-C, Chang S-H, Lai Y-H, Chen D-Y. The Potential Role of Electronegative High-Density Lipoprotein H5 Subfraction in RA-Related Atherosclerosis. International Journal of Molecular Sciences. 2021; 22(21):11419. https://doi.org/10.3390/ijms222111419
Chicago/Turabian StyleChang, Ching-Kun, Wei-Chung Cheng, Wen-Lung Ma, Po-Ku Chen, Chu-Huang Chen, Pei-Chun Shen, Chia-Ching Chen, Shih-Hsin Chang, Yi-Hua Lai, and Der-Yuan Chen. 2021. "The Potential Role of Electronegative High-Density Lipoprotein H5 Subfraction in RA-Related Atherosclerosis" International Journal of Molecular Sciences 22, no. 21: 11419. https://doi.org/10.3390/ijms222111419







