Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease
Abstract
1. Introduction
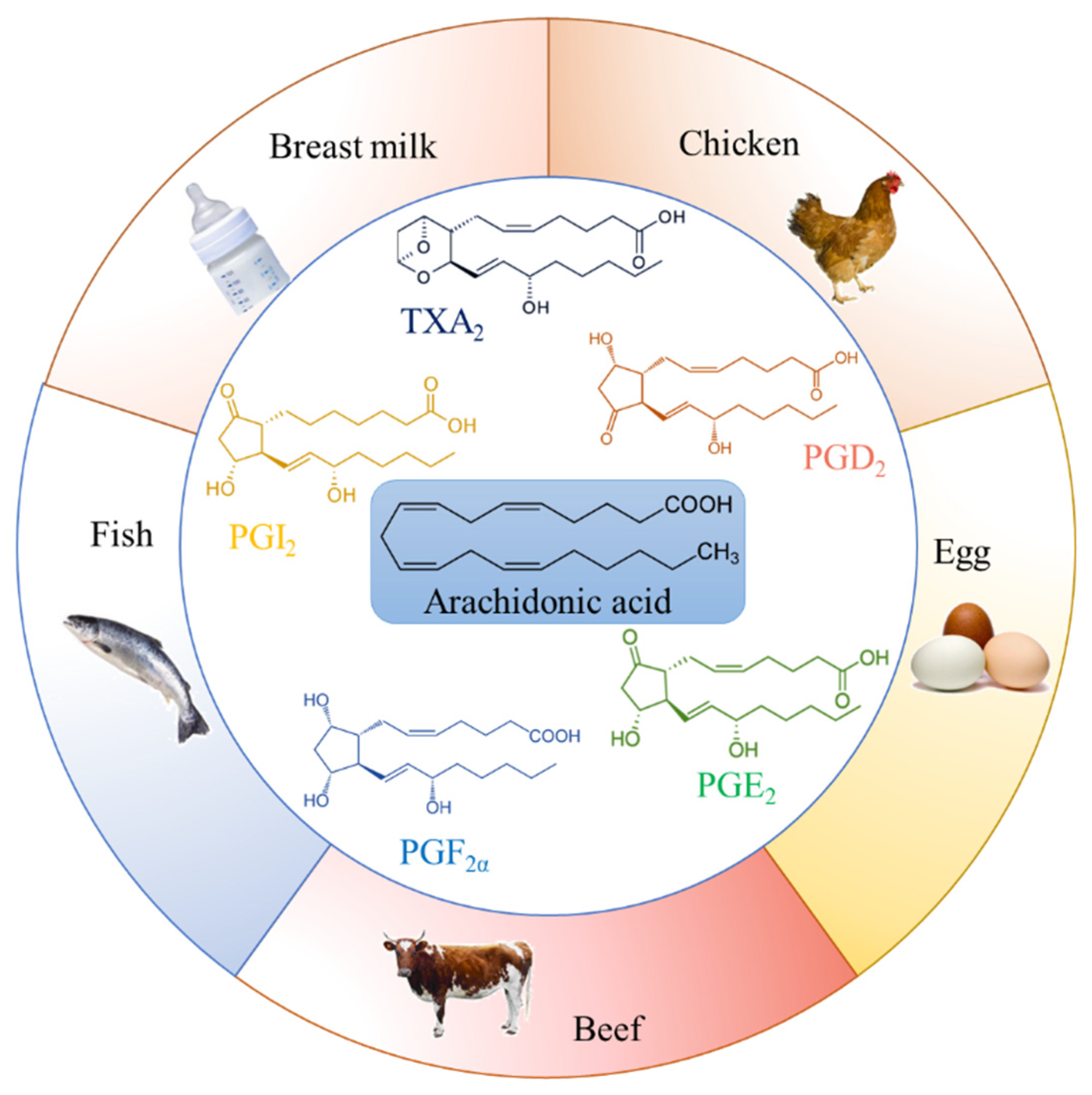
2. Food Sources of Arachidonic Acid
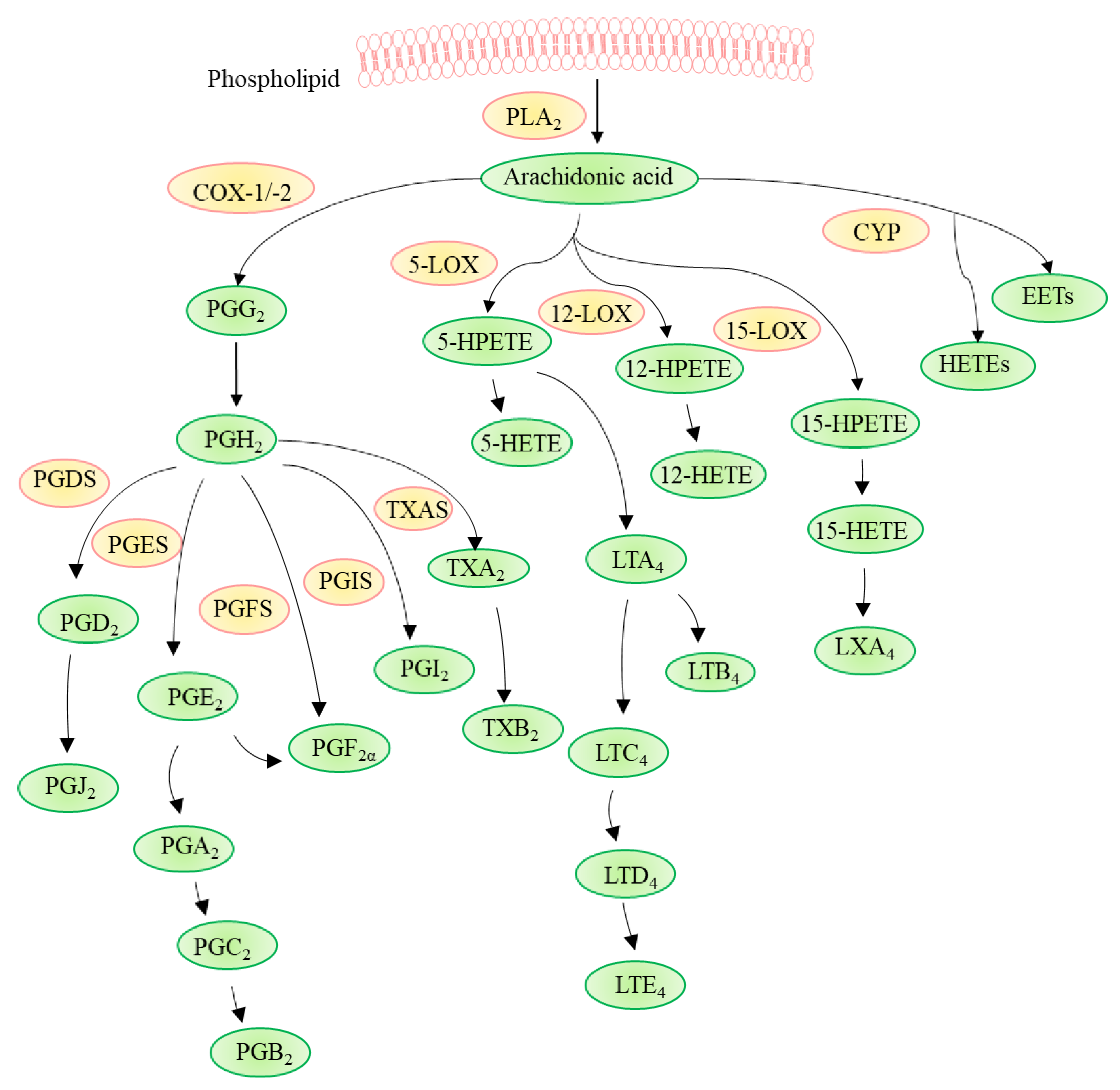
3. Pathways Involved in Arachidonic Acid Metabolism
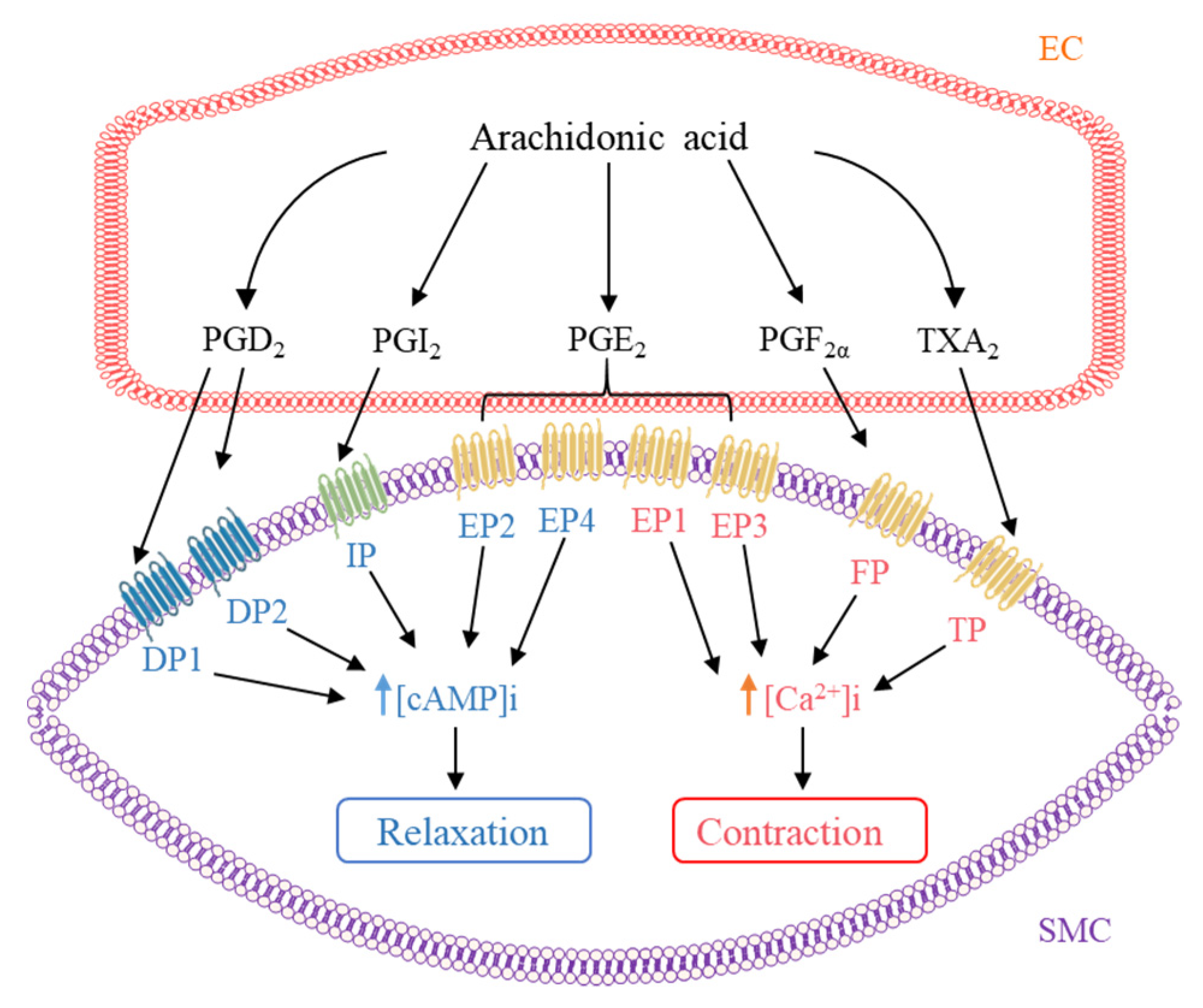
4. Contribution of AA Metabolites to the Regulation of Vascular Tone
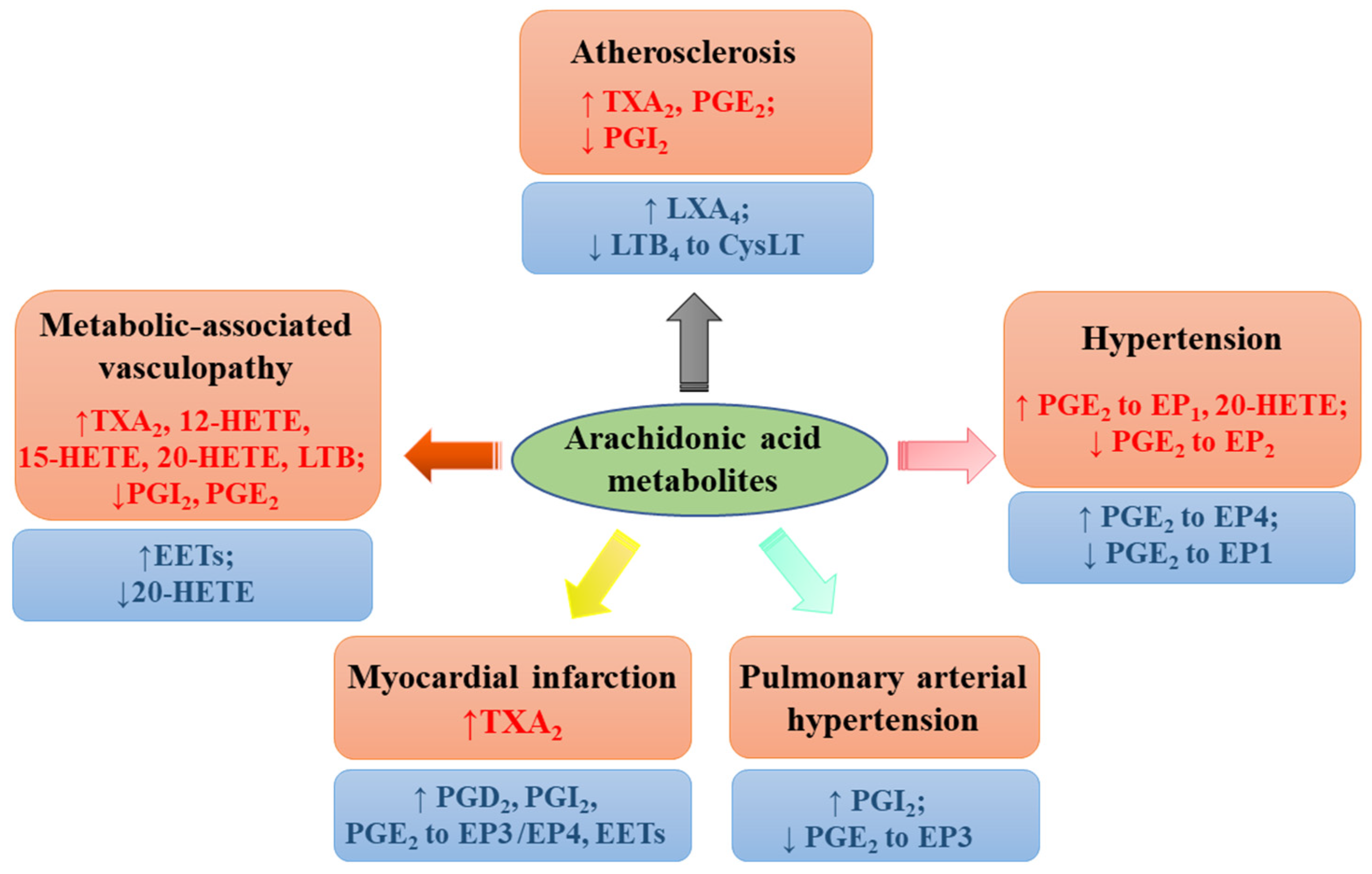
5. Preventing and Managing Vascular Complications in Metabolic Disorders
6. Regulation of Blood Pressure
7. Antiatherosclerosis Effect
8. Modulating Heart Function and Protecting against Myocardial Infarction
9. Clinical Significance of AA Metabolites
10. Potential Health Concerns of Dietary AA
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Picard, F.; Steg, P.G. Cardiovascular Disease Risk Reduction in Mild-Moderate Hypertriglyceridemia: Integrating Prescription of Omega-3 with Standard Treatment. Curr. Atheroscler. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.O.; et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD012345. [Google Scholar] [CrossRef]
- Yuan, S.; Back, M.; Bruzelius, M.; Mason, A.M.; Burgess, S.; Larsson, S. Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study. Nutrients 2019, 11, 3001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.H.; Si, S.C.; Li, Y.X.; Li, W.C.; Chen, X.L.; Liu, C.C.; Li, J.Q.; Wang, B.J.; Hou, L.; Liu, Y.X.; et al. Roles for circulating polyunsaturated fatty acids in ischemic stroke and modifiable factors: A Mendelian randomization study. Nutr. J. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Hikita, H.; Shigeta, T.; Kimura, S.; Takahashi, A.; Isobe, M. Coronary Artery Disease Severity and Cardiovascular Biomarkers in Patients with Peripheral Artery Disease. Int. J. Angiol. 2015, 24, 278–282. [Google Scholar] [CrossRef]
- Narverud, I.; Bogsrud, M.P.; Ril, L.; Ulven, S.M.; Retterstøl, K.; Ueland, T.; Mulder, M.; van Lennep, J.R.; Halvorsen, B.; Aukrust, P.; et al. Lipoprotein (a) concentration is associated with plasma arachidonic acid in subjects with familial hypercholesterolemia. Br. J. Nutr. 2019, 122, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The Key Contribution Of Platelet And Vascular Arachidonic Acid Metabolism To The Pathophysiology Of Atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Hsiao, G.; Al-Shabrawey, M. Eicosanoids and Oxidative Stress in Diabetic Retinopathy. Antioxidants 2020, 9, 520. [Google Scholar] [CrossRef]
- Imig, J.D. Eicosanoid blood vessel regulation in physiological and pathological states. Clin. Sci. 2020, 134, 2707–2727. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Satoh, Y.; Ogasawara, Y.; Maruyama, C.; Hamano, Y.; Ujihara, T.; Dairi, T. Control Mechanism for cis Double-Bond Formation by Polyunsaturated Fatty-Acid Synthases. Angew. Chem. Int. Ed. 2019, 58, 2326–2330. [Google Scholar] [CrossRef]
- Kratzsch, S.; Drossler, K.; Sprinz, H.; Brede, O. Thiyl radicals in biosystems: Inhibition of the prostaglandin metabolism by the cis-trans-isomerization of arachidonic acid double bonds. Arch. Biochem. Biophys. 2003, 416, 238–248. [Google Scholar] [CrossRef]
- Wang, T.Q.; Fu, X.J.; Chen, Q.F.; Patra, J.K.; Wang, D.D.; Wang, Z.G.; Gai, Z.B. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.J.; Chen, J.; Dong, L.L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Tar. 2021, 6, 94. [Google Scholar] [CrossRef]
- Zhang, T.; Yeung, S.L.A.; Schooling, C.M. Associations of Arachidonic Acid Synthesis with Cardiovascular Risk Factors and Relation to Ischemic Heart Disease and Stroke: A Univariable and Multivariable Mendelian Randomization Study. Nutrients 2021, 13, 1489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, J.V.; Schooling, C.M. The associations of plasma phospholipid arachidonic acid with cardiovascular diseases: A Mendelian randomization study. EBioMedicine 2021, 63, 103189. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Liu, J.; Yin, P.; Wang, L.; Qi, J.; You, J.; Lin, L.; Meng, S.; Wang, F.; et al. Mortality and years of life lost of cardiovascular diseases in China, 2005–2020: Empirical evidence from national mortality surveillance system. Int. J. Cardiol. 2021, 340, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M. Recent updates on novel therapeutic targets of cardiovascular diseases. Mol. Cell. Biochem. 2021, 476, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Kaneko, H.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Morita, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Metabolically Healthy Obesity and the Risk of Cardiovascular Disease in the General Population-Analysis of a Nationwide Epidemiological Database. Circ. J. 2021, 85, 914–920. [Google Scholar] [CrossRef]
- Groenland, E.H.; Bots, M.L.; Asselbergs, F.W.; de Borst, G.J.; Kappelle, L.J.; Visseren, F.L.J.; Spiering, W.; Grp, U.-S.S. Apparent treatment resistant hypertension and the risk of recurrent cardiovascular events and mortality in patients with established vascular disease. Int. J. Cardiol. 2021, 334, 135–141. [Google Scholar] [CrossRef]
- Taber, L.; Chiu, C.H.; Whelan, J. Assessment of the arachidonic acid content in foods commonly consumed in the American diet. Lipids 1998, 33, 1151–1157. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. Intake ratio and major food sources of n-3 and n-6 fatty acids in Korea: A study based on the sixth Korea national health and nutrition examination survey (2013–2014). Asia Pac. J. Clin. Nutr. 2018, 27, 433–440. [Google Scholar] [CrossRef]
- Tani, S.; Matsuo, R.; Matsumoto, N. A longitudinal study of the association of the eicosapentaenoic acid/arachidonic acid ratio derived from fish consumption with the serum lipid levels: A pilot study. Heart Vessel. 2019, 34, 189–196. [Google Scholar] [CrossRef]
- Asil, S.M.; Kenari, A.A.; Mianji, G.R.; Van der Kraak, G. Estimation of Arachidonic Acid Requirement for Improvement of Pre-maturation Growth and Egg and Larval Quality in the Female Blue Gourami (Trichopodus trichopterus; Pallas, 1770): A Model for the Anabantidae Family. J. World Aquacult Soc. 2019, 50, 359–373. [Google Scholar] [CrossRef]
- Arts, M.T.; Ackman, R.G.; Holub, B.J. “Essential fatty acids” in aquatic ecosystems: A crucial link between diet and human health and evolution. Can. J. Fish Aquat. Sci. 2001, 58, 122–137. [Google Scholar] [CrossRef]
- Seah, J.Y.; Gay, G.M.; Su, J.; Tai, E.S.; Yuan, J.M.; Koh, W.P.; Ong, C.N.; van Dam, R.M. Consumption of Red Meat, but Not Cooking Oils High in Polyunsaturated Fat, Is Associated with Higher Arachidonic Acid Status in Singapore Chinese Adults. Nutrients 2017, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36. [Google Scholar] [CrossRef]
- Markworth, J.F.; Mitchell, C.J.; D’Souza, R.F.; Aasen, K.M.M.; Durainayagam, B.R.; Mitchell, S.M.; Chan, A.H.C.; Sinclair, A.J.; Garg, M.; Cameron-Smith, D. Arachidonic acid supplementation modulates blood and skeletal muscle lipid profile with no effect on basal inflammation in resistance exercise trained men. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 74–86. [Google Scholar] [CrossRef]
- Bermudez Menendez de la Granda, M.; Sinclair, A.J. Fatty acids and obesity. Curr. Pharm. Des. 2009, 15, 4117–4125. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.L.; Nakamura, M.T.; Ma, D.W.L. Differentiating the biological effects of linoleic acid from arachidonic acid in health and disease. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 1–4. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.T.; Anthony, J.C.; Diersen-Schade, D.A.; Rumsey, S.C.; Lawrence, P.; Li, C.; Nathanielsz, P.W.; Brenna, J.T. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr. Res. 2007, 61, 537–545. [Google Scholar] [CrossRef]
- Tyburczy, C.; Brenna, M.E.; DeMari, J.A.; Kothapalli, K.S.D.; Blank, B.S.; Valentine, H.; McDonough, S.P.; Banavara, D.; Diersen-Schade, D.A.; Brenna, J.T. Evaluation of bioequivalency and toxicological effects of three sources of arachidonic acid (ARA) in domestic piglets. Food Chem. Toxicol. 2011, 49, 2320–2327. [Google Scholar] [CrossRef]
- Steinberg, D.; Parthasarathy, S.; Ca Rew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef]
- FAO/WHO. Fats and oils in human nutrition. Report of a joint expert consultation. FAO Food Nutr. Pap. 1994, i–xix, 1–147. [Google Scholar]
- Huang, M.C.; Brenna, J.T.; Chao, A.C.; Tschanz, C.; Diersen-Schade, D.A.; Hung, H.C. Differential tissue dose responses of (n-3) and (n-6) PUFA in neonatal piglets fed docosahexaenoate and arachidonoate. J. Nutr. 2007, 137, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- AFSSA. Opinion of the French Food Safety Agency on the Update of French Population Reference Intakes (ANCs) for Fatty Acids; AFSSA: Maisons-Alfort, France, 2010; Available online: http://www.anses.fr/en/content/opinion-french-food-safety-agency-update-french-population-reference-intakes-ancs-fatty (accessed on 8 October 2021).
- Tunctan, B.; Senol, S.P.; Temiz-Resitoglu, M.; Guden, D.S.; Sahan-Firat, S.; Falck, J.R.; Malik, K.U. Eicosanoids derived from cytochrome P450 pathway of arachidonic acid and inflammatory shock. Prostaglandins Other Lipid Mediat. 2019, 145, 106377. [Google Scholar] [CrossRef] [PubMed]
- Trostchansky, A.; Moore-Carrasco, R.; Fuentes, E. Oxidative pathways of arachidonic acid as targets for regulation of platelet activation. Prostaglandins Other Lipid Mediat. 2019, 145, 106382. [Google Scholar] [CrossRef]
- Marnett, L.J.; Rowlinson, S.W.; Goodwin, D.C.; Kalgutkar, A.S.; Lanzo, C.A. Arachidonic acid oxygenation by COX-1 and COX-2-Mechanisms of catalysis and inhibition. J. Biol. Chem. 1999, 274, 22903–22906. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Hayek, M.G.; Meydani, S.N. Vitamin E and macrophage cyclooxygenase regulation in the aged. J. Nutr. 2001, 131, 382S–388S. [Google Scholar] [CrossRef]
- Tirsan, T. Analysis of prostaglandins induced by spontaneous platelet aggregation in patients with primary thrombocythemia. Jpn. J. Thromb. Hemost. 2010, 16, 169–171. [Google Scholar] [CrossRef]
- Moreira, V.; Gutierrez, J.M.; Lomonte, B.; Vinolo, M.A.R.; Curi, R.; Lambeau, G.; Teixeira, C. 12-HETE is a regulator of PGE(2) production via COX-2 expression induced by a snake venom group IIA phospholipase A(2) in isolated peritoneal macrophages. Chem.-Biol. Interact. 2020, 317, 108903. [Google Scholar] [CrossRef]
- Dichlberger, A.; Schlager, S.; Kovanen, P.T.; Schneider, W.J. Lipid droplets in activated mast cells-a significant source of triglyceride-derived arachidonic acid for eicosanoid production. Eur. J. Pharmacol. 2016, 785, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Oda, H.; Shiina, Y.; Shikata, K.; Tsushima, M.; Nakano, S.; Maruyama, T.; Kyotani, S.; Eguchi, N.; Urade, Y.; et al. Association of serum lipocalin-type prostaglandin D synthase levels with subclinical atherosclerosis in untreated asymptomatic subjects. Hypertens Res. 2008, 31, 1931–1939. [Google Scholar] [CrossRef][Green Version]
- Jansen, C.; Hofheinz, K.; Vogel, R.; Roffeis, J.; Anton, M.; Reddanna, P.; Kuhn, H.; Walther, M. Stereocontrol of arachidonic acid oxygenation by vertebrate lipoxygenases: Newly cloned zebrafish lipoxygenase 1 does not follow the Ala-versus-Gly concept. J. Biol. Chem. 2011, 286, 37804–37812. [Google Scholar] [CrossRef]
- Reddy, K.K.; Vidya Rajan, V.K.; Gupta, A.; Aparoy, P.; Reddanna, P. Exploration of binding site pattern in arachidonic acid metabolizing enzymes, Cyclooxygenases and Lipoxygenases. BMC Res. Notes 2015, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, J.; Hersberger, M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 67–77. [Google Scholar] [CrossRef]
- Mittal, M.; Kumar, R.B.; Balagunaseelan, N.; Hamberg, M.; Jegerschold, C.; Radmark, O.; Haeggstrom, J.Z.; Rinaldo-Matthis, A. Kinetic investigation of human 5-lipoxygenase with arachidonic acid. Bioorg Med. Chem. Lett. 2016, 26, 3547–3551. [Google Scholar] [CrossRef]
- Lotzer, K.; Funk, C.D.; Habenicht, A.J.R. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. BBA-Mol. Cell Biol. Lipids 2005, 1736, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bender, G.; Schexnaydre, E.E.; Murphy, R.C.; Uhlson, C.; Newcomer, M.E. Membrane-dependent Activities of Human 15-LOX-2 and Its Murine Counterpart Implications for Murine Models of Atherosclerosis. J. Biol. Chem. 2016, 291, 19413–19424. [Google Scholar] [CrossRef]
- Suardiaz, R.; Jambrina, P.G.; Masgrau, L.; Gonzalez-Lafont, A.; Rosta, E.; Lluch, J.M. Understanding the Mechanism of the Hydrogen Abstraction from Arachidonic Acid Catalyzed by the Human Enzyme 15-Lipoxygenase-2. A Quantum Mechanics/Molecular Mechanics Free Energy Simulation. J. Chem. Theory Comput. 2016, 12, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Watkins, G.; Douglas-Jones, A.; Mansel, R.E. Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 235–245. [Google Scholar] [CrossRef]
- Gervasini, G.; Garcia-Cerrada, M.; Vergara, E.; Garcia-Pino, G.; Alvarado, R.; Fernandez-Cavada, M.J.; Barroso, S.; Doblare, E.; Cubero, J.J. Polymorphisms in CYP-mediated arachidonic acid routes affect the outcome of renal transplantation. Eur. J. Clin. Investig. 2015, 45, 1060–1068. [Google Scholar] [CrossRef]
- Capdevila, J.H.; Falck, J.R. The CYP P450 arachidonic acid monooxygenases: From cell signaling to blood pressure regulation. Biochem. Biophys. Res. Commun. 2001, 285, 571–576. [Google Scholar] [CrossRef]
- Gu, R.M.; Yang, L.; Zhang, Y.; Wang, L.; Kong, S.; Zhang, C.; Zhai, Y.; Wang, M.; Wu, P.; Liu, L.; et al. CYP-omega-hydroxylation-dependent metabolites of arachidonic acid inhibit the basolateral 10 pS chloride channel in the rat thick ascending limb. Kidney Int. 2009, 76, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Colombero, C.; Cardenas, S.; Venara, M.; Martin, A.; Pennisi, P.; Barontini, M.; Nowicki, S. Cytochrome 450 metabolites of arachidonic acid (20-HETE, 11,12-EET and 14,15-EET) promote pheochromocytoma cell growth and tumor associated angiogenesis. Biochimie 2020, 171–172, 147–157. [Google Scholar] [CrossRef]
- Al-Lawati, H.; Vakili, M.R.; Lavasanifar, A.; Ahmed, S.; Jamali, F. Reduced Heart Exposure of Diclofenac by Its Polymeric Micellar Formulation Normalizes CYP-Mediated Metabolism of Arachidonic Acid Imbalance in An Adjuvant Arthritis Rat Model: Implications in Reduced Cardiovascular Side Effects of Diclofenac by Nanodrug Delivery. Mol. Pharm. 2020, 17, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Ulven, T.; Kostenis, E. Targeting the Prostaglandin D-2 Receptors DP1 and CRTH2 for Treatment of Inflammation. Front. Med. Chem. 2010, 5, 350–380. [Google Scholar] [CrossRef]
- Mawhin, M.A.; Tilly, P.; Fabre, J.E. The receptor EP3 to PGE2: A rational target to prevent atherothrombosis without inducing bleeding. Prostaglandins Other Lipid Mediat. 2015, 121, 4–16. [Google Scholar] [CrossRef]
- Leng, X.; Jiang, H. Effects of arachidonic acid and its major prostaglandin derivatives on bovine myoblast proliferation, differentiation, and fusion. Domest. Anim. Endocrin. 2019, 67, 28–36. [Google Scholar] [CrossRef]
- Siangjong, L.; Goldman, D.H.; Kriska, T.; Gauthier, K.M.; Smyth, E.M.; Puli, N.; Kumar, G.; Falck, J.R.; Campbell, W.B. Vascular hepoxilin and trioxilins mediate vasorelaxation through TP receptor inhibition in mouse arteries. Acta Physiol. 2017, 219, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, K.; Nishihashi, T.; Trandafir, C.C.; Wang, A.M.; Murakami, S.; Ji, X. Diversity of endothelium-derived vasocontracting factors-arachidonic acid metabolites. Acta Pharmacol. Sin. 2003, 24, 1065–1069. [Google Scholar] [CrossRef]
- Trostchansky, A.; Wood, I.; Rubbo, H. Regulation of arachidonic acid oxidation and metabolism by lipid electrophiles. Prostaglandins Other Lipid Mediat. 2021, 152, 106482. [Google Scholar] [CrossRef]
- Bauer, J.; Ripperger, A.; Frantz, S.; Ergun, S.; Schwedhelm, E.; Benndorf, R.A. Pathophysiology of isoprostanes in the cardiovascular system: Implications of isoprostane-mediated thromboxane A2 receptor activation. Br. J. Pharmacol. 2014, 171, 3115–3131. [Google Scholar] [CrossRef] [PubMed]
- Trostchansky, A.; Rubbo, H. Anti-inflammatory signaling actions of electrophilic nitro-arachidonic acid in vascular cells and astrocytes. Arch. Biochem. Biophys. 2017, 617, 155–161. [Google Scholar] [CrossRef]
- Yokomizo, T. Two distinct leukotriene B4 receptors, BLT1 and BLT2. J. Biochem. 2015, 157, 65–71. [Google Scholar] [CrossRef]
- Duah, E.; Adapala, R.K.; Al-Azzam, N.; Kondeti, V.; Gombedza, F.; Thodeti, C.K.; Paruchuri, S. Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT(2) and CysLT(1) receptors. Sci. Rep.-UK 2013, 3, 3274. [Google Scholar] [CrossRef]
- Nunes, V.S.; Rogerio, A.P.; Abrahao, O. Insights into the Activation Mechanism of the ALX/FPR2 Receptor. J. Phys. Chem. Lett. 2020, 11, 8952–8957. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pan, J.; Wang, L.; Zhu, H.; Yu, R.; Zou, Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis 2006, 184, 425–430. [Google Scholar] [CrossRef]
- Delannoy, E.; Courtois, A.; Freund-Michel, V.; Leblais, V.; Marthan, R.; Muller, B. Hypoxia-induced hyperreactivity of pulmonary arteries: Role of cyclooxygenase-2, isoprostanes, and thromboxane receptors. Cardiovasc. Res. 2010, 85, 582–592. [Google Scholar] [CrossRef]
- Feletou, M.; Huang, Y.; Vanhoutte, P.M. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol. 2011, 164, 894–912. [Google Scholar] [CrossRef] [PubMed]
- Ozen, G.; Gomez, I.; Daci, A.; Deschildre, C.; Boubaya, L.; Teskin, O.; Uydes-Dogan, B.S.; Jakobsson, P.J.; Longrois, D.; Topal, G.; et al. Inhibition of microsomal PGE synthase-1 reduces human vascular tone by increasing PGI2: A safer alternative to COX-2 inhibition. Br. J. Pharmacol. 2017, 174, 4087–4098. [Google Scholar] [CrossRef]
- Tang, X.; Edwards, E.M.; Holmes, B.B.; Falck, J.R.; Campbell, W.B. Role of phospholipase C and diacylglyceride lipase pathway in arachidonic acid release and acetylcholine-induced vascular relaxation in rabbit aorta. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H37–H45. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Liu, B.; Luo, W.; Li, H.; Zhou, Y. Role of E-type prostaglandin receptor EP3 in the vasoconstrictor activity evoked by prostacyclin in thromboxane-prostanoid receptor deficient mice. Sci. Rep. 2017, 7, 42167. [Google Scholar] [CrossRef]
- Yu, Y.; Ricciotti, E.; Scalia, R.; Tang, S.Y.; Grant, G.; Yu, Z.; Landesberg, G.; Crichton, I.; Wu, W.; Pure, E.; et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci. Transl. Med. 2012, 4, 132ra154. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Price, D.T.; Gokce, N.; Eberhardt, R.T.; Duffy, S.J.; Holbrook, M.; Maxwell, C.; Palmisano, J.; Keaney, J.F., Jr.; Morrow, J.D.; et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 2003, 42, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Chenevard, R.; Hurlimann, D.; Bechir, M.; Enseleit, F.; Spieker, L.; Hermann, M.; Riesen, W.; Gay, S.; Gay, R.E.; Neidhart, M.; et al. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation 2003, 107, 405–409. [Google Scholar] [CrossRef]
- Howes, L.G.; Krum, H. Selective cyclo-oxygenase-2 inhibitors and myocardial infarction-How strong is the link? Drug Safety 2002, 25, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Jiang, B.Y.; McNeill, K.; Farish, S.; Kirkham, B.; Chowienczyk, P. Effects of selective and non-selective cyclo-oxygenase inhibition on endothelial function in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2007, 36, 265–269. [Google Scholar] [CrossRef]
- Coxib and Traditional NSAID Trialists’ (CNT) Collaboration; Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 2013, 382, 769–779. [Google Scholar] [CrossRef]
- Ross, S.J.; Elgendy, I.Y.; Bavry, A.A. Cardiovascular Safety and Bleeding Risk Associated with Nonsteroidal Anti-Inflammatory Medications in Patients with Cardiovascular Disease. Curr. Cardiol. Rep. 2017, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid Res. 2009, 50, S423–S428. [Google Scholar] [CrossRef] [PubMed]
- Gluais, P.; Lonchampt, M.; Morrow, J.D.; Vanhoutte, P.M.; Feletou, M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: The Janus face of prostacyclin. Br. J. Pharmacol. 2005, 146, 834–845. [Google Scholar] [CrossRef]
- Legler, D.F.; Bruckner, M.; Uetz-von Allmen, E.; Krause, P. Prostaglandin E-2 at new glance: Novel insights in functional diversity offer therapeutic chances. Int. J. Biochem. Cell B 2010, 42, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Schweda, F.; Klar, J.; Narumiya, S.; Nusing, R.M.; Kurtz, A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am. J. Physiol. Renal. Physiol. 2004, 287, F427–F433. [Google Scholar] [CrossRef]
- Davis, R.J.; Murdoch, C.E.; Ali, M.; Purbrick, S.; Ravid, R.; Baxter, G.S.; Tilford, N.; Sheldrick, R.L.; Clark, K.L.; Coleman, R.A. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br. J. Pharmacol. 2004, 141, 580–585. [Google Scholar] [CrossRef]
- Foudi, N.; Kotelevets, L.; Louedec, L.; Leseche, G.; Henin, D.; Chastre, E.; Norel, X. Vasorelaxation induced by prostaglandin E2 in human pulmonary vein: Role of the EP4 receptor subtype. Br. J. Pharmacol. 2008, 154, 1631–1639. [Google Scholar] [CrossRef]
- Kobayashi, K.; Murata, T.; Hori, M.; Ozaki, H. Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur. J. Pharmacol. 2011, 660, 375–380. [Google Scholar] [CrossRef]
- Foudi, N.; Kotelevets, L.; Gomez, I.; Louedec, L.; Longrois, D.; Chastre, E.; Norel, X. Differential reactivity of human mammary artery and saphenous vein to prostaglandin E(2): Implication for cardiovascular grafts. Br. J. Pharmacol. 2011, 163, 826–834. [Google Scholar] [CrossRef]
- Jadhav, V.; Jabre, A.; Lin, S.Z.; Lee, T.J. EP1- and EP3-receptors mediate prostaglandin E2-induced constriction of porcine large cerebral arteries. J. Cereb. Blood Flow Metab. 2004, 24, 1305–1316. [Google Scholar] [CrossRef]
- Pan, X.F.; Grigoryeva, L.; Seyrantepe, V.; Peng, J.Z.; Kollmann, K.; Tremblay, J.; Lavoie, J.L.; Hinek, A.; Lubke, T.; Pshezhetsky, A.V. Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1. PLoS Genet. 2014, 10, e1004146. [Google Scholar] [CrossRef]
- Yu, Y.; Lucitt, M.B.; Stubbe, J.; Cheng, Y.; Friis, U.G.; Hansen, P.B.; Jensen, B.L.; Smyth, E.M.; FitzGerald, G.A. Prostaglandin F2alpha elevates blood pressure and promotes atherosclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 7985–7990. [Google Scholar] [CrossRef]
- Wong, S.L.; Leung, F.P.; Lau, C.W.; Au, C.L.; Yung, L.M.; Yao, X.; Chen, Z.Y.; Vanhoutte, P.M.; Gollasch, M.; Huang, Y. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ. Res. 2009, 104, 228–235. [Google Scholar] [CrossRef]
- Feletou, M.; Vanhoutte, P.M.; Verbeuren, T.J. The thromboxane/endoperoxide receptor (TP): The common villain. J. Cardiovasc. Pharmacol. 2010, 55, 317–332. [Google Scholar] [CrossRef]
- Chawengsub, Y.; Gauthier, K.M.; Campbell, W.B. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H495–H507. [Google Scholar] [CrossRef] [PubMed]
- Randriamboavonjy, V.; Busse, R.; Fleming, I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension 2003, 41, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Miyata, N.; Roman, R.J. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J. Smooth Muscle Res. 2005, 41, 175–193. [Google Scholar] [CrossRef]
- Chen, Y.; Medhora, M.; Falck, J.R.; Pritchard, K.A., Jr.; Jacobs, E.R. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L378–L385. [Google Scholar] [CrossRef]
- Campbell, W.B.; Falck, J.R.; Gauthier, K. Role of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor in bovine coronary arteries. Med. Sci. Monit. 2001, 7, 578–584. [Google Scholar] [PubMed]
- Larsen, B.T.; Miura, H.; Hatoum, O.A.; Campbell, W.B.; Hammock, B.D.; Zeldin, D.C.; Falck, J.R.; Gutterman, D.D. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: Implications for soluble epoxide hydrolase inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H491–H499. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Jaggi, A.S. TRPV4 channels: Physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 2015, 110, 54. [Google Scholar] [CrossRef] [PubMed]
- Peredo, H.A.; Rodriguez, R.; Susemihl, M.C.; Villarreal, I.; Filinger, E. Long-term streptozotocin-induced diabetes alters prostanoid production in rat aorta and mesenteric bed. Auton. Autacoid Pharmacol. 2006, 26, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Zuccollo, A.; Shi, C.; Mastroianni, R.; Maitland-Toolan, K.A.; Weisbrod, R.M.; Zang, M.; Xu, S.; Jiang, B.; Oliver-Krasinski, J.M.; Cayatte, A.J.; et al. The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation 2005, 112, 3001–3008. [Google Scholar] [CrossRef]
- Chan, P.C.; Liao, M.T.; Hsieh, P.S. The Dualistic Effect of COX-2-Mediated Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 3115. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, H.; Liu, J.; Lau, C.W.; Liu, P.; Chen, Z.Y.; Lee, H.K.; Tipoe, G.L.; Ho, H.M.; Yao, X.; et al. Cyclooxygenase-2-dependent oxidative stress mediates palmitate-induced impairment of endothelium-dependent relaxations in mouse arteries. Biochem. Pharmacol. 2014, 91, 474–482. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Qu, D.; Wang, L.; Luo, J.Y.; Lau, C.W.; Liu, P.; Gao, Z.; Tipoe, G.L.; Lee, H.K.; et al. Inhibition of miR-200c Restores Endothelial Function in Diabetic Mice Through Suppression of COX-2. Diabetes 2016, 65, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wong, S.L.; Lau, C.W.; Liu, J.; Wang, Y.X.; Dan He, Z.; Fai Ng, C.; Yu Chen, Z.; Yao, X.; Xu, A.; et al. Calcitriol restores renovascular function in estrogen-deficient rats through downregulation of cyclooxygenase-2 and the thromboxane-prostanoid receptor. Kidney Int. 2013, 84, 54–63. [Google Scholar] [CrossRef]
- Kim, D.H.; Puri, N.; Sodhi, K.; Falck, J.R.; Abraham, N.G.; Shapiro, J.; Schwartzman, M.L. Cyclooxygenase-2 dependent metabolism of 20-HETE increases adiposity and adipocyte enlargement in mesenchymal stem cell-derived adipocytes. J. Lipid Res. 2013, 54, 786–793. [Google Scholar] [CrossRef]
- Peterson, L.R. To Lose Weight or Not to Lose Weight, That Is the Big Question--in Obesity-Related Heart Failure. Diabetes 2015, 64, 1509–1510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, A.S.; Elshafey, S.; Sellak, H.; Hussein, K.A.; El-Sherbiny, M.; Abdelsaid, M.; Rizk, N.; Beasley, S.; Tawfik, A.M.; Smith, S.B.; et al. A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase. J. Lipid Res. 2015, 56, 599–611. [Google Scholar] [CrossRef]
- Issan, Y.; Hochhauser, E.; Guo, A.; Gotlinger, K.H.; Kornowski, R.; Leshem-Lev, D.; Lev, E.; Porat, E.; Snir, E.; Thompson, C.I.; et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 2013, 100–101, 15–21. [Google Scholar] [CrossRef]
- Yousif, M.H.; Benter, I.F.; Roman, R.J. Cytochrome P450 metabolites of arachidonic acid play a role in the enhanced cardiac dysfunction in diabetic rats following ischaemic reperfusion injury. Auton. Autacoid Pharmacol. 2009, 29, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; McClung, J.A.; Bellner, L.; Cao, J.; Waldman, M.; Schragenheim, J.; Arad, M.; Hochhauser, E.; Falck, J.R.; Weingarten, J.A.; et al. CYP-450 Epoxygenase Derived Epoxyeicosatrienoic Acid Contribute To Reversal of Heart Failure in Obesity-Induced Diabetic Cardiomyopathy via PGC-1 alpha Activation. Cardiovasc. Pharm. Open Access. 2018, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Du, Y. Distinct roles of central and peripheral prostaglandin E2 and EP subtypes in blood pressure regulation. Am. J. Hypertens 2012, 25, 1042–1049. [Google Scholar] [CrossRef]
- Nasrallah, R.; Hassouneh, R.; Hebert, R.L. PGE2, Kidney Disease, and Cardiovascular Risk: Beyond Hypertension and Diabetes. J. Am. Soc. Nephrol. 2016, 27, 666–676. [Google Scholar] [CrossRef]
- Rutkai, I.; Feher, A.; Erdei, N.; Henrion, D.; Papp, Z.; Edes, I.; Koller, A.; Kaley, G.; Bagi, Z. Activation of prostaglandin E2 EP1 receptor increases arteriolar tone and blood pressure in mice with type 2 diabetes. Cardiovasc. Res. 2009, 83, 148–154. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y.; Wu, J.; Qi, Z.; Yang, G.; Dou, D.; Gao, Y.; Chen, L.; Zhang, X.; Davis, L.S.; et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J. Clin. Investig. 2007, 117, 2496–2505. [Google Scholar] [CrossRef]
- Lu, A.; Zuo, C.; He, Y.; Chen, G.; Piao, L.; Zhang, J.; Xiao, B.; Shen, Y.; Tang, J.; Kong, D.; et al. EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-beta1 signaling. J. Clin. Investig. 2015, 125, 1228–1242. [Google Scholar] [CrossRef]
- Kennedy, C.R.; Zhang, Y.; Brandon, S.; Guan, Y.; Coffee, K.; Funk, C.D.; Magnuson, M.A.; Oates, J.A.; Breyer, M.D.; Breyer, R.M. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat. Med. 1999, 5, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Hristovska, A.M.; Rasmussen, L.E.; Hansen, P.B.; Nielsen, S.S.; Nusing, R.M.; Narumiya, S.; Vanhoutte, P.; Skott, O.; Jensen, B.L. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension 2007, 50, 525–530. [Google Scholar] [CrossRef]
- Abe, S.; Ishida, K.; Masuda, M.; Ueda, H.; Kohno, H.; Matsuura, K.; Tamura, Y.; Watanabe, M.; Matsumiya, G. A prospective, randomized study of inhaled prostacyclin versus nitric oxide in patients with residual pulmonary hypertension after pulmonary endarterectomy. Gen. Thorac. Cardiovasc. Surg. 2016, 65, 153–159. [Google Scholar] [CrossRef]
- Jacobs, W.; Vonk-Noordegraaf, A. Epoprostenol in pulmonary arterial hypertension. Expert Opin. Drug Metab. Toxicol. 2009, 5, 83–90. [Google Scholar] [CrossRef]
- Menon, A.A.; Sahay, S.; Braverman, L.E.; Farber, H.W. Thyroid Dysfunction in Patients with Pulmonary Artery Hypertension (PAH): The Effect of Therapies Affecting the Prostanoid Pathway. Lung 2019, 197, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Wong, W.T.; Leung, F.P.; Zhang, Y.; Wang, Y.X.; Lee, H.K.; Ng, C.F.; Chen, Z.Y.; Yao, X.; Au, C.L.; et al. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin f(2alpha) impairs endothelial function in renovascular hypertensive rats. Antioxid. Redox Signal. 2012, 16, 363–373. [Google Scholar] [CrossRef]
- Wong, W.T.; Tian, X.Y.; Chen, Y.; Leung, F.P.; Liu, L.; Lee, H.K.; Ng, C.F.; Xu, A.; Yao, X.; Vanhoutte, P.M.; et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: Implications on hypertension. Circ. Res. 2010, 107, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Tian, X.Y.; Wong, W.T.; Lau, C.W.; Xu, A.; Xu, G.; Ng, C.F.; Yao, X.; Gao, Y.; et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid. Redox Signal. 2014, 21, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Cheng, J.; Deng, H.; Kemp, R.; Ishizuka, T.; Nasjletti, A.; Schwartzman, M.L. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 2007, 50, 123–129. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, C.C.; Gotlinger, K.H.; Zhang, F.; Falck, J.R.; Narsimhaswamy, D.; Schwartzman, M.L. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. J. Pharmacol. Exp. Ther. 2010, 332, 57–65. [Google Scholar] [CrossRef]
- Inoue, K.; Sodhi, K.; Puri, N.; Gotlinger, K.H.; Cao, J.; Rezzani, R.; Falck, J.R.; Abraham, N.G.; Laniado-Schwartzman, M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am. J. Physiol. Renal. Physiol. 2009, 297, F875–F884. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, A.; Watanabe, J.; Lin, J.A.; Hama, S.; Langenbach, R.; Navab, M.; Fogelman, A.M.; Reddy, S.T. A novel anti-atherogenic role for COX-2--potential mechanism for the cardiovascular side effects of COX-2 inhibitors. Prostaglandins Other Lipid Mediat. 2007, 84, 24–33. [Google Scholar] [CrossRef]
- Yu, Z.; Crichton, I.; Tang, S.Y.; Hui, Y.; Ricciotti, E.; Levin, M.D.; Lawson, J.A.; Pure, E.; FitzGerald, G.A. Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to COX-2 deletion in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 6727–6732. [Google Scholar] [CrossRef]
- Yuen, C.Y.; Wong, S.L.; Lau, C.W.; Tsang, S.Y.; Xu, A.; Zhu, Z.; Ng, C.F.; Yao, X.; Kong, S.K.; Lee, H.K.; et al. From skeleton to cytoskeleton: Osteocalcin transforms vascular fibroblasts to myofibroblasts via angiotensin II and Toll-like receptor 4. Circ. Res. 2012, 111, e55–e66. [Google Scholar] [CrossRef]
- Wong, S.L.; Lau, C.W.; Wong, W.T.; Xu, A.; Au, C.L.; Ng, C.F.; Ng, S.S.; Gollasch, M.; Yao, X.; Huang, Y. Pivotal role of protein kinase Cdelta in angiotensin II-induced endothelial cyclooxygenase-2 expression: A link to vascular inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 1169–1176. [Google Scholar] [CrossRef]
- Patrono, C. Low-dose aspirin in primary prevention: Cardioprotection, chemoprevention, both, or neither? Eur. Heart J. 2013, 34, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhan, M.; Gao, C.; Wu, G.; Zhang, H. I4, a new synthetic sulfonylurea compound, inhibits the action of TXA2 in vivo and in vitro on platelets and aorta vascular smooth muscle. Thromb. Res. 2012, 130, e209–e215. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, G.; Yan, J.; Liu, W.; Feng, W.; Ma, B.; Yang, L.; Wang, J.A.; Tu, L.; Wang, D.W. CYP2J2 overexpression increases EETs and protects against angiotensin II-induced abdominal aortic aneurysm in mice. J. Lipid Res. 2013, 54, 1448–1456. [Google Scholar] [CrossRef]
- Cipollone, F.; Fazia, M.L.; Iezzi, A.; Cuccurullo, C.; De Cesare, D.; Ucchino, S.; Spigonardo, F.; Marchetti, A.; Buttitta, F.; Paloscia, L.; et al. Association between prostaglandin E receptor subtype EP4 overexpression and unstable phenotype in atherosclerotic plaques in human. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; St Amand, T.; Li, X.; Yoon, S.H.; Wang, C.P.; Song, H.; Maruyama, T.; Brown, P.M.; Zelt, D.T.; Funk, C.D. Prostaglandin receptor EP4 in abdominal aortic aneurysms. Am. J. Pathol. 2012, 181, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Dilme, J.; Sola-Villa, D.; Rodriguez, C.; Bellmunt, S.; Siguero, L.; Alcolea, S.; Romero, J.M.; Escudero, J.R.; Martinez-Gonzalez, J.; et al. Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm. J. Lipid Res. 2013, 54, 3506–3515. [Google Scholar] [CrossRef]
- Tang, E.H.; Shimizu, K.; Christen, T.; Rocha, V.Z.; Shvartz, E.; Tesmenitsky, Y.; Sukhova, G.; Shi, G.P.; Libby, P. Lack of EP4 receptors on bone marrow-derived cells enhances inflammation in atherosclerotic lesions. Cardiovasc. Res. 2011, 89, 234–243. [Google Scholar] [CrossRef]
- Tang, E.H.; Shvartz, E.; Shimizu, K.; Rocha, V.Z.; Zheng, C.; Fukuda, D.; Shi, G.P.; Sukhova, G.; Libby, P. Deletion of EP4 on bone marrow-derived cells enhances inflammation and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mouillesseaux, K.P.; Montoya, D.; Cruz, D.; Gharavi, N.; Dun, M.; Koroniak, L.; Berliner, J.A. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ. Res. 2006, 98, 642–650. [Google Scholar] [CrossRef]
- Zhu, S.; Xue, R.; Zhao, P.; Fan, F.L.; Kong, X.; Zheng, S.; Han, Q.; Zhu, Y.; Wang, N.; Yang, J.; et al. Targeted disruption of the prostaglandin E2 E-prostanoid 2 receptor exacerbates vascular neointimal formation in mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1739–1747. [Google Scholar] [CrossRef]
- Gross, S.; Tilly, P.; Hentsch, D.; Vonesch, J.L.; Fabre, J.E. Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J. Exp. Med. 2007, 204, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hara, A.; Xiao, C.Y.; Okada, Y.; Takahata, O.; Nakaya, K.; Sugimoto, Y.; Ichikawa, A.; Narumiya, S.; Ushikubi, F. Increased bleeding tendency and decreased susceptibility to thromboembolism in mice lacking the prostaglandin E receptor subtype EP(3). Circulation 2001, 104, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, F.; Tang, J.; Zhang, Q.; Gong, Y.; Wang, Q.; Shen, Y.; Xiong, L.; Breyer, R.M.; Lazarus, M.; et al. Cyclooxygenase-2-derived prostaglandin E(2) promotes injury-induced vascular neointimal hyperplasia through the E-prostanoid 3 receptor. Circ. Res. 2013, 113, 104–114. [Google Scholar] [CrossRef]
- Gomez-Hernandez, A.; Sanchez-Galan, E.; Martin-Ventura, J.L.; Vidal, C.; Blanco-Colio, L.M.; Ortego, M.; Vega, M.; Serrano, J.; Ortega, L.; Hernandez, G.; et al. Atorvastatin reduces the expression of prostaglandin E2 receptors in human carotid atherosclerotic plaques and monocytic cells: Potential implications for plaque stabilization. J. Cardiovasc. Pharmacol. 2006, 47, 60–69. [Google Scholar] [CrossRef]
- Mawhin, M.A.; Tilly, P.; Zirka, G.; Charles, A.L.; Slimani, F.; Vonesch, J.L.; Michel, J.B.; Back, M.; Norel, X.; Fabre, J.E. Neutrophils recruited by leukotriene B4 induce features of plaque destabilization during endotoxaemia. Cardiovasc. Res. 2018, 114, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hall, S.R.; Moos, M.P.; Cao, R.Y.; Ishii, S.; Ogunyankin, K.O.; Melo, L.G.; Funk, C.D. Endothelial cysteinyl leukotriene 2 receptor expression mediates myocardial ischemia-reperfusion injury. Am. J. Pathol. 2008, 172, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Gabrielsen, A.; Agardh, H.E.; Wan, M.; Wetterholm, A.; Wong, C.H.; Hedin, U.; Swedenborg, J.; Hansson, G.K.; Samuelsson, B.; et al. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc. Natl. Acad. Sci. USA 2006, 103, 8161–8166. [Google Scholar] [CrossRef]
- Hoxha, M.; Capra, V.; Malaj, V.; Sala, A.; Rovati, G. The Role of Montelukast in Cardiovascular Events. Atherosclerosis 2017, 263, E150. [Google Scholar] [CrossRef]
- Leedom, A.J.; Sullivan, A.B.; Dong, B.; Lau, D.; Gronert, K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 2010, 176, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.X.; Ye, Y.; Xu, H.R.; Xiang, S.Y.; Yang, Q.; Ma, H.Y.; Jin, S.W.; Wang, Q. LXA4 Inhibits Lipopolysaccharide-Induced Inflammatory Cell Accumulation by Resident Macrophages in Mice. J. Inflamm. Res. 2021, 14, 1375–1385. [Google Scholar] [CrossRef]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Bioactive Lipids as Mediators of the Beneficial Actions of Statins. J. Cardiovasc. Pharm. 2019, 74, 4–8. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Schmidt, E.B.; Stegger, J.; Gorst-Rasmussen, A.; Tjonneland, A.; Overvad, K. Adipose tissue arachidonic acid content is associated with the risk of myocardial infarction: A Danish case-cohort study. Atherosclerosis 2013, 227, 386–390. [Google Scholar] [CrossRef]
- Degousee, N.; Fazel, S.; Angoulvant, D.; Stefanski, E.; Pawelzik, S.C.; Korotkova, M.; Arab, S.; Liu, P.; Lindsay, T.F.; Zhuo, S.; et al. Microsomal prostaglandin E2 synthase-1 deletion leads to adverse left ventricular remodeling after myocardial infarction. Circulation 2008, 117, 1701–1710. [Google Scholar] [CrossRef]
- Martin, M.; Meyer-Kirchrath, J.; Kaber, G.; Jacoby, C.; Flogel, U.; Schrader, J.; Ruther, U.; Schror, K.; Hohlfeld, T. Cardiospecific overexpression of the prostaglandin EP3 receptor attenuates ischemia-induced myocardial injury. Circulation 2005, 112, 400–406. [Google Scholar] [CrossRef][Green Version]
- Zacharowski, K.; Olbrich, A.; Piper, J.; Hafner, G.; Kondo, K.; Thiemermann, C. Selective activation of the prostanoid EP(3) receptor reduces myocardial infarct size in rodents. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ji, Y.; Yao, J.; Zhao, X.; Xu, H.; Guan, Y.; Breyer, R.M.; Sheng, H.; Zhu, J. Knockout of the Prostaglandin E2 Receptor Subtype 3 Promotes Eccentric Cardiac Hypertrophy and Fibrosis in Mice. J. Cardiovasc. Pharmacol. Ther. 2016, 22, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.Y.; Yuhki, K.; Hara, A.; Fujino, T.; Kuriyama, S.; Yamada, T.; Takayama, K.; Takahata, O.; Karibe, H.; Taniguchi, T.; et al. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation 2004, 109, 2462–2468. [Google Scholar] [CrossRef]
- Hishikari, K.; Suzuki, J.; Ogawa, M.; Isobe, K.; Takahashi, T.; Onishi, M.; Takayama, K.; Isobe, M. Pharmacological activation of the prostaglandin E2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2009, 81, 123–132. [Google Scholar] [CrossRef]
- Katsumata, Y.; Shinmura, K.; Sugiura, Y.; Tohyama, S.; Matsuhashi, T.; Ito, H.; Yan, X.; Ito, K.; Yuasa, S.; Ieda, M.; et al. Endogenous prostaglandin D2 and its metabolites protect the heart against ischemia-reperfusion injury by activating Nrf2. Hypertension 2014, 63, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.Y.; Hara, A.; Yuhki, K.; Fujino, T.; Ma, H.; Okada, Y.; Takahata, O.; Yamada, T.; Murata, T.; Narumiya, S.; et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: A study using mice lacking their respective receptors. Circulation 2001, 104, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar]
- Wacker, M.J.; Kosloski, L.M.; Gilbert, W.J.; Touchberry, C.D.; Moore, D.S.; Kelly, J.K.; Brotto, M.; Orr, J.A. Inhibition of thromboxane A2-induced arrhythmias and intracellular calcium changes in cardiac myocytes by blockade of the inositol trisphosphate pathway. J. Pharmacol. Exp. Ther. 2009, 331, 917–924. [Google Scholar] [CrossRef]
- Santos, M.T.; Fuset, M.P.; Ruano, M.; Moscardo, A.; Valles, J. Effect of Atorvastatin on Platelet Thromboxane A(2) Synthesis in Aspirin-Treated Patients with Acute Myocardial Infarction. Am. J. Cardiol. 2009, 104, 1618–1623. [Google Scholar] [CrossRef]
- Yang, L.; Ni, L.; Duan, Q.; Wang, X.; Chen, C.; Chen, S.; Chaugai, S.; Zeldin, D.C.; Tang, J.R.; Wang, D.W. CYP epoxygenase 2J2 prevents cardiac fibrosis by suppression of transmission of pro-inflammation from cardiomyocytes to macrophages. Prostaglandins Other Lipid Mediat. 2015, 116-117, 64–75. [Google Scholar] [CrossRef]
- Batchu, S.N.; Lee, S.B.; Qadhi, R.S.; Chaudhary, K.R.; El-Sikhry, H.; Kodela, R.; Falck, J.R.; Seubert, J.M. Cardioprotective effect of a dual acting epoxyeicosatrienoic acid analogue towards ischaemia reperfusion injury. Br. J. Pharmacol. 2011, 162, 897–907. [Google Scholar] [CrossRef]
- Nithipatikom, K.; Moore, J.M.; Isbell, M.A.; Falck, J.R.; Gross, G.J. Epoxyeicosatrienoic acids in cardioprotection: Ischemic versus reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H537–H542. [Google Scholar] [CrossRef]
- Huang, C.C.; Chang, M.T.; Leu, H.B.; Yin, W.H.; Tseng, W.K.; Wu, Y.W.; Lin, T.H.; Yeh, H.I.; Chang, K.C.; Wang, J.H.; et al. Association of Arachidonic Acid-derived Lipid Mediators with Subsequent Onset of Acute Myocardial Infarction in Patients with Coronary Artery Disease. Sci. Rep. 2020, 10, 8105. [Google Scholar] [CrossRef]
- Wang, C.H.; Ouyang, Q.; Tang, C.W.; Huang, M.H.; Li, X. [Effects of selective and non-selective cyclooxygenase-2 inhibitor on the growth of colon cancer cells]. Sichuan Da Xue Xue Bao Yi Xue Ban 2006, 37, 547–550. [Google Scholar]
- Martin-Garcia, C.; Hinojosa, M.; Berges, P.; Camacho, E.; Garcia-Rodriguez, R.; Alfaya, T. Celecoxib, a highly selective COX-2 inhibitor, is safe in aspirin-induced asthma patients. J. Investig. Allergol. Clin. Immunol. 2003, 13, 20–25. [Google Scholar]
- Stuntz, M.; Bernstein, B. Recent trends in the prevalence of low-dose aspirin use for primary and secondary prevention of cardiovascular disease in the United States, 2012–2015. Prev. Med. Rep. 2017, 5, 183–186. [Google Scholar] [CrossRef]
- Antithrombotic Trialists’ Collaboration; Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [CrossRef]
- Rodondi, N.; Bauer, D.C. Assessing the risk/benefit profile before recommending aspirin for the primary prevention of cardiovascular events. Am. J. Med. 2004, 117, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Grosser, T.; Fries, S.; FitzGerald, G.A. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J. Clin. Investig. 2006, 116, 4–15. [Google Scholar] [CrossRef]
- Sun, S.X.; Lee, K.Y.; Bertram, C.T.; Goldstein, J.L. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: Impact on NSAID and gastroprotective drug prescribing and utilization. Curr. Med. Res. Opin. 2007, 23, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Bolten, W.W.; Reiter, S.; Kommission Pharmakotherapie und des Vorstandes der Deutschen Gesellschaft für Rheumatologie. Cardiovascular risks of Cox-2-antagonists. Opinion on the marketed name rofecoxib, and its market-withdrawn valdecoxib and the actual therapeutic restrictions. Z. Rheumatol. 2005, 64, 286–289. [Google Scholar] [CrossRef]
- Howes, L.G. Selective COX-2 inhibitors, NSAIDs and cardiovascular events-is celecoxib the safest choice? Ther. Clin. Risk. Manag. 2007, 3, 831–845. [Google Scholar] [PubMed]
- Cayatte, A.J.; Du, Y.; Oliver-Krasinski, J.; Lavielle, G.; Verbeuren, T.J.; Cohen, R.A. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice: Evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1724–1728. [Google Scholar] [CrossRef]
- Viles-Gonzalez, J.F.; Fuster, V.; Corti, R.; Valdiviezo, C.; Hutter, R.; Corda, S.; Anand, S.X.; Badimon, J.J. Atherosclerosis regression and TP receptor inhibition: Effect of S18886 on plaque size and composition--a magnetic resonance imaging study. Eur. Heart J. 2005, 26, 1557–1561. [Google Scholar] [CrossRef]
- Bousser, M.G.; Amarenco, P.; Chamorro, A.; Fisher, M.; Ford, I.; Fox, K.M.; Hennerici, M.G.; Mattle, H.P.; Rothwell, P.M.; de Cordoue, A.; et al. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): A randomised, double-blind, parallel-group trial. Lancet 2011, 377, 2013–2022. [Google Scholar] [CrossRef]
- Bots, M.L.; Ford, I.; Lloyd, S.M.; Laurent, S.; Touboul, P.J.; Hennerici, M.G.; Prevention of Cerebrovascular and Cardiovascular Events of Ischemic Origin with Terutroban in Patients with a History of Ischemic Stroke or Transient Ischemic Attack Vascular Ultrasound Study Investigators. Thromboxane prostaglandin receptor antagonist and carotid atherosclerosis progression in patients with cerebrovascular disease of ischemic origin: A randomized controlled trial. Stroke 2014, 45, 2348–2353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakariassen, K.S.; Femia, E.A.; Daray, F.M.; Podda, G.M.; Razzari, C.; Pugliano, M.; Errasti, A.E.; Armesto, A.R.; Nowak, W.; Alberts, P.; et al. EV-077 in vitro inhibits platelet aggregation in type-2 diabetics on aspirin. Thromb. Res. 2012, 130, 746–752. [Google Scholar] [CrossRef]
- McCarberg, B.H.; Cryer, B. Evolving therapeutic strategies to improve nonsteroidal anti-inflammatory drug safety. Am. J. Ther. 2015, 22, e167–e178. [Google Scholar] [CrossRef] [PubMed]
- Yam, D.; Eliraz, A.; Berry, E.M. Diet and disease--the Israeli paradox: Possible dangers of a high omega-6 polyunsaturated fatty acid diet. Isr. J. Med. Sci. 1996, 32, 1134–1143. [Google Scholar]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake with Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Shin, S. Red meat and processed meat consumption and the risk of dyslipidemia in Korean adults: A prospective cohort study based on the Health Examinees (HEXA) study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Wahlqvist, M.L.; Boxall, J.A.; Balazs, N.D. Can linoleic acid contribute to coronary artery disease? Am. J. Clin. Nutr. 1993, 58, 228–234. [Google Scholar] [CrossRef]
- Loeper, J.; Goy, J.; Emerit, J. Lipid peroxidation in human and experimental atherosclerosis. Bull. Acad. Natl. Med. 1984, 168, 91–97. [Google Scholar] [PubMed]
- Loeper, J.; Goy, J.; Emerit, J.; Rozensztajn, L.; Jeny, C.; Bedu, O. Fatty acid and lipid peroxidation in human atherosclerosis. Sem. Hop. 1983, 59, 1657–1660. [Google Scholar] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Effects of dietary fats on blood lipids: A review of direct comparison trials. Open Heart 2018, 5, e000871. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.M. Are diets high in omega-6 polyunsaturated fatty acids unhealthy? Eur. Heart J. Suppl. 2001, 3, D37–D41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 12029. https://doi.org/10.3390/ijms222112029
Zhou Y, Khan H, Xiao J, Cheang WS. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. International Journal of Molecular Sciences. 2021; 22(21):12029. https://doi.org/10.3390/ijms222112029
Chicago/Turabian StyleZhou, Yan, Haroon Khan, Jianbo Xiao, and Wai San Cheang. 2021. "Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease" International Journal of Molecular Sciences 22, no. 21: 12029. https://doi.org/10.3390/ijms222112029
APA StyleZhou, Y., Khan, H., Xiao, J., & Cheang, W. S. (2021). Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. International Journal of Molecular Sciences, 22(21), 12029. https://doi.org/10.3390/ijms222112029








