Immune Synapse Residency of Orai1 Alters Ca2+ Response of T Cells
Abstract
:1. Introduction
2. Results
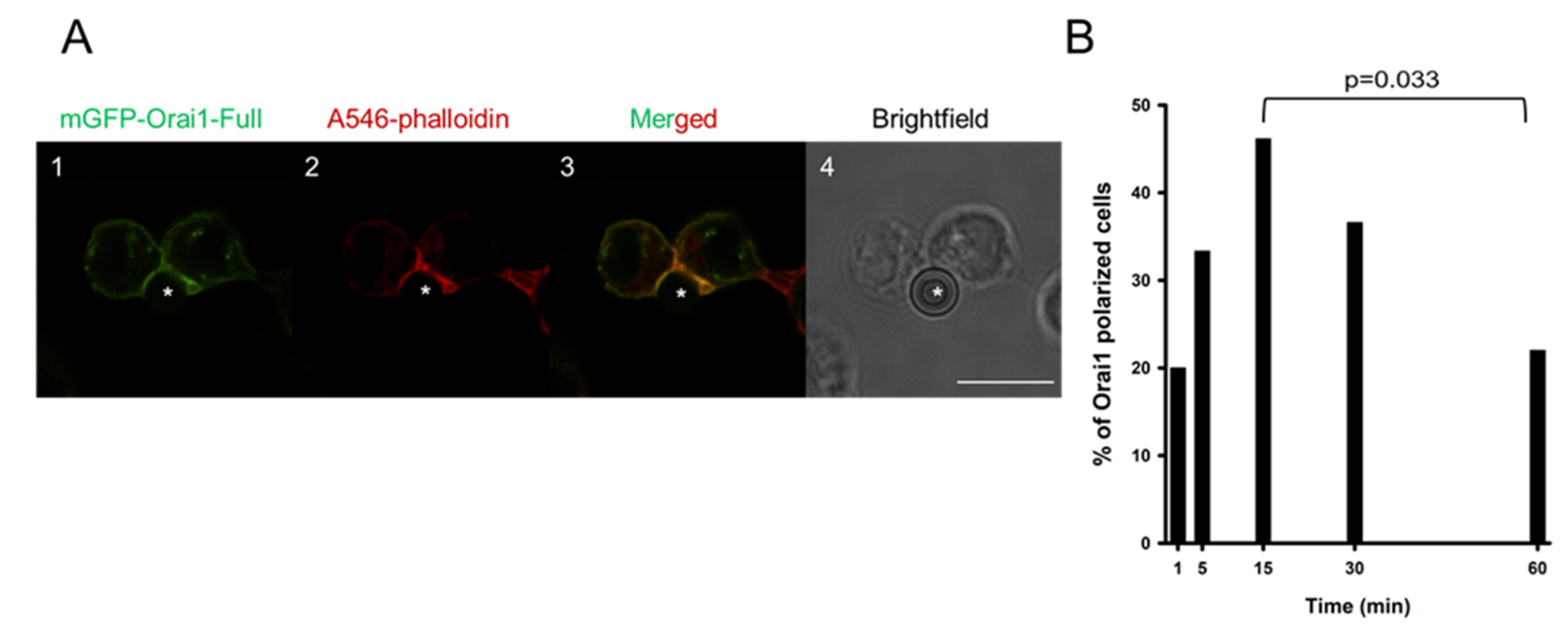
2.1. Kinetics of Orai1 Redistribution into the Immunological Synapse
2.2. STIM1 Controls the IS-Dwelling Time of Orai1
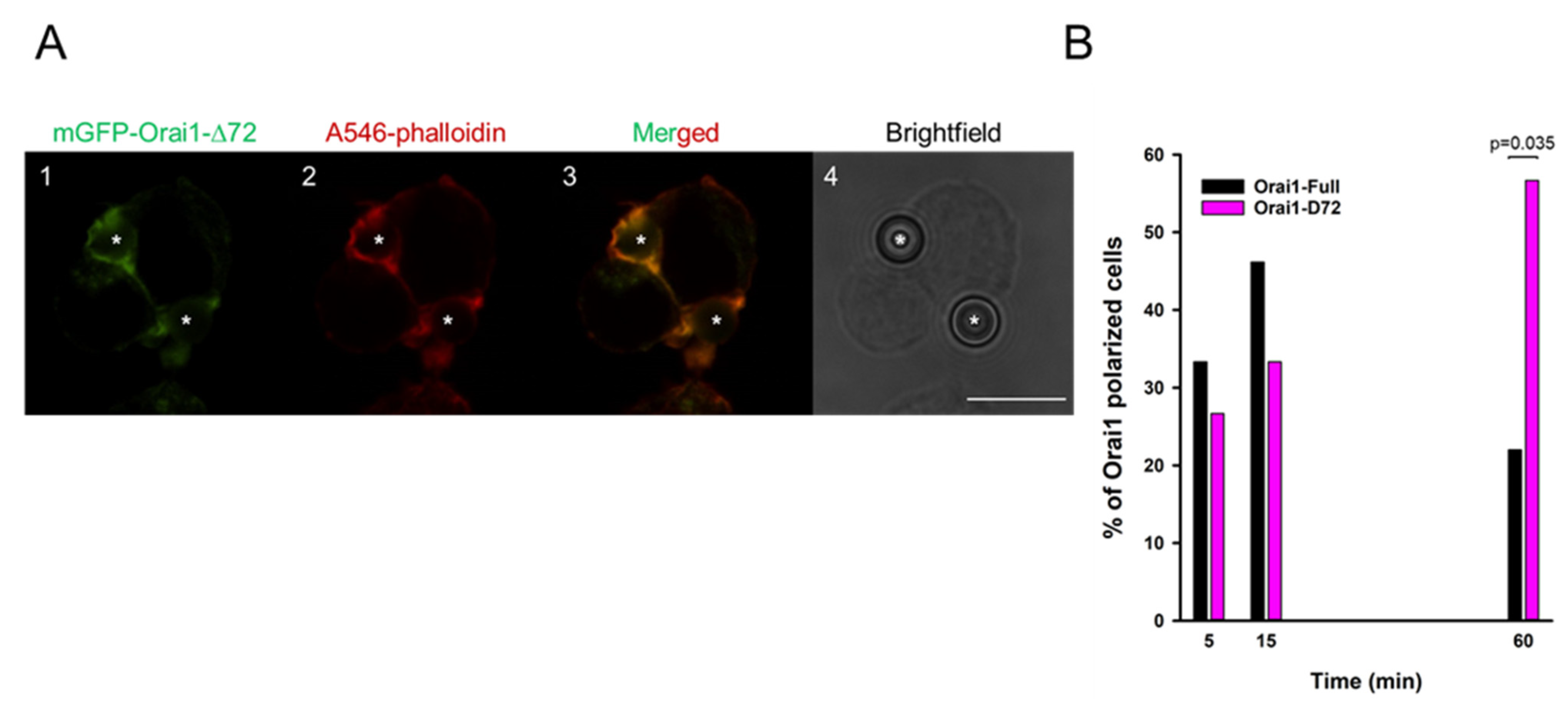
2.3. N-Terminal Truncation of Orai1 Mimics STIM1 Knock-Down
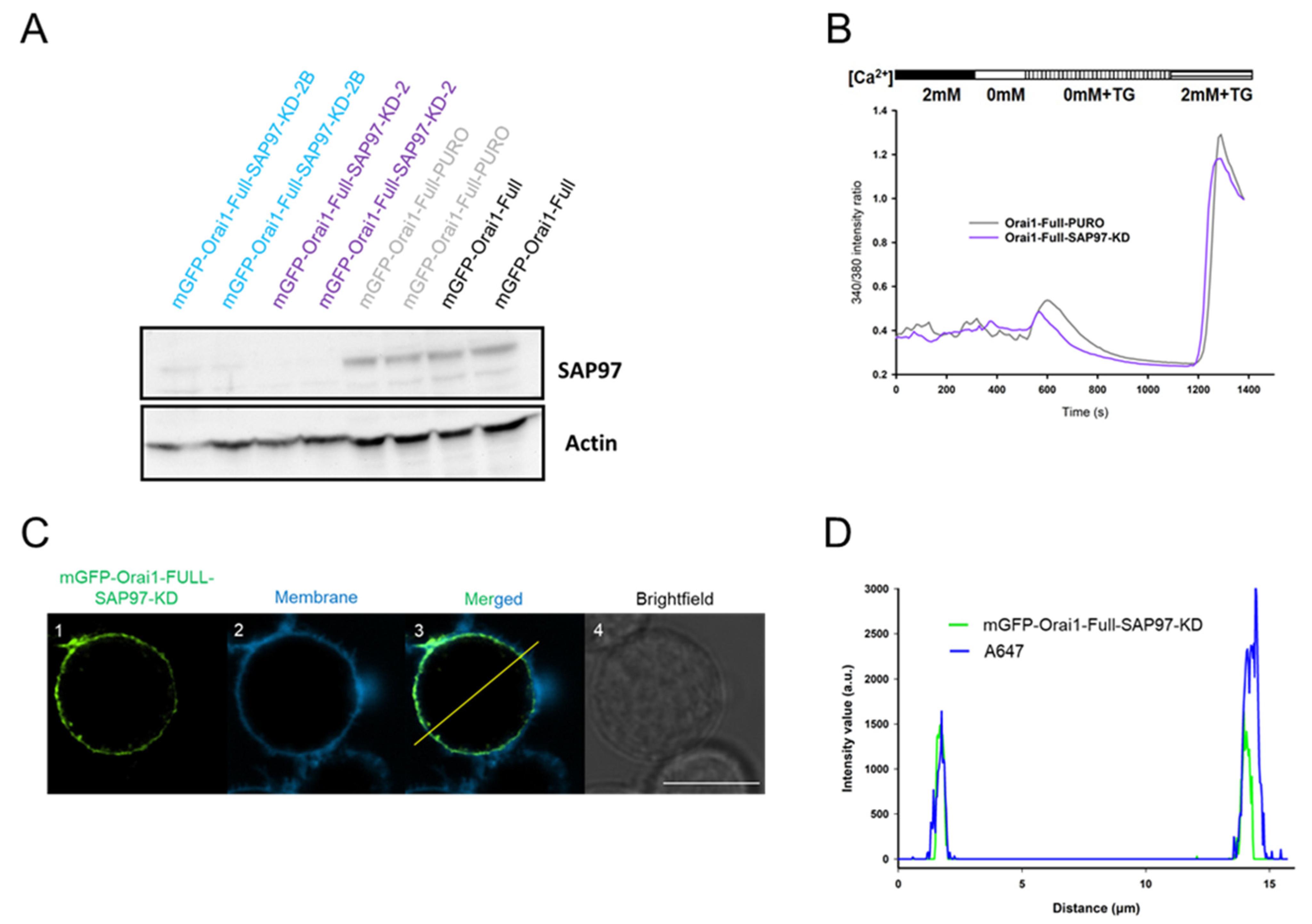
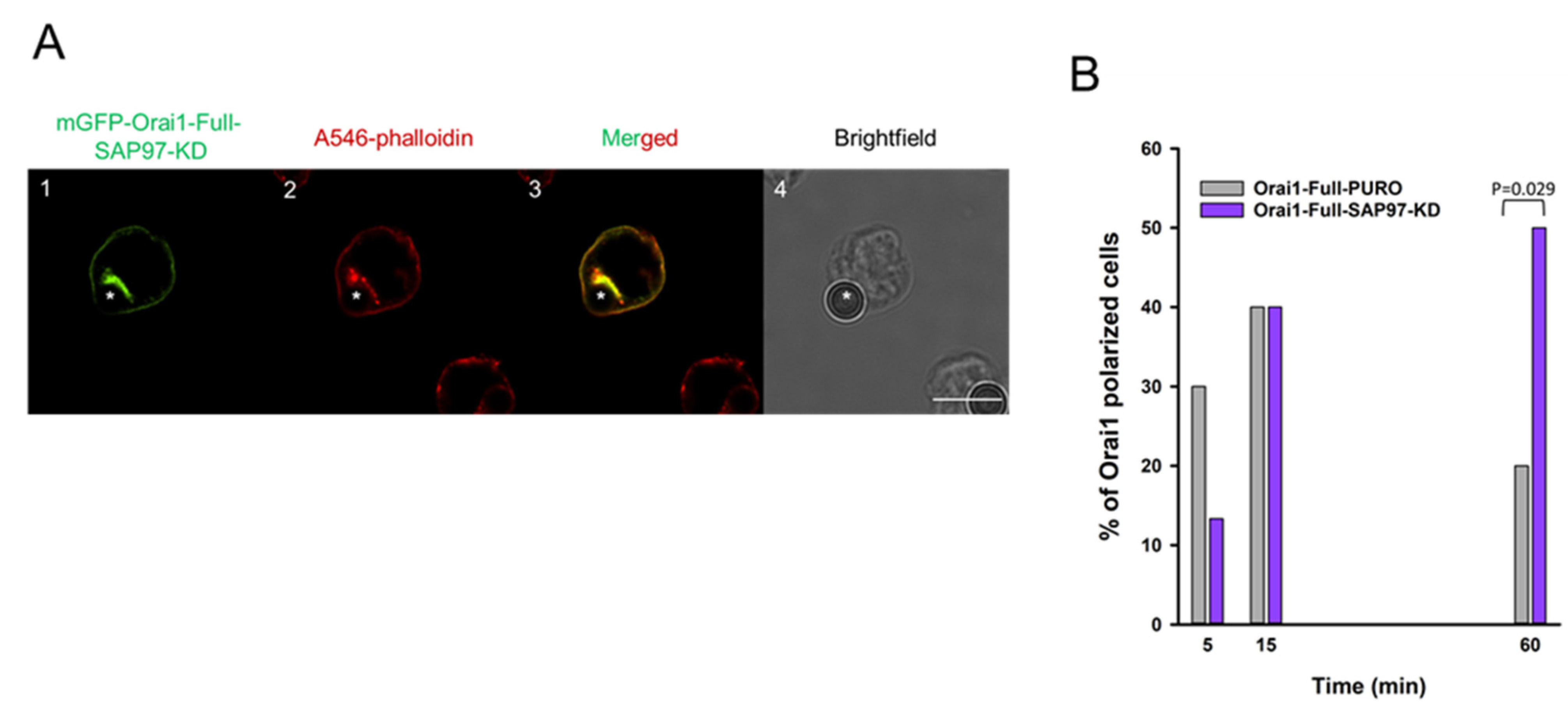
2.4. SAP97 Could Control the Leaving of Orai1 from the Immunological Synapse
2.5. Sustained Orai1 IS-Residence Modifies Ca2+-Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmids and Cloning
4.3. Membrane Targeting
4.4. Western Blotting
4.5. Viral Transduction
4.6. Knockdown of STIM1 and SAP97 with shRNA
4.7. Immunological Synapse Formation and Immunofluorescence
4.8. Confocal Microscopy and Image Analysis
4.9. Intracellular Ca2+ Measurements
4.10. Intracellular Ca2+ Measurements in IS
4.11. Statistical Analysis
4.12. Reagents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cemerski, S.; Shaw, A. Immune synapses in t-cell activation. Curr. Opin. Immunol. 2006, 18, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L. The immunological synapse. Arthritis Res. 2002, 4 (Suppl. 3), S119–S125. [Google Scholar] [CrossRef]
- Krummel, M.F.; Cahalan, M.D. The immunological synapse: A dynamic platform for local signaling. J. Clin. Immunol. 2010, 30, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascoigne, N.R.; Zal, T.; Alam, S.M. T-cell receptor binding kinetics in T-cell development and activation. Expert Rev. Mol. Med. 2001, 2001, 1–17. [Google Scholar] [CrossRef]
- Murphy, K.W.C.J.C. Janeway’s Immunobiology; Taylor and Francis: Abingdon, UK, 2017. [Google Scholar]
- Yeung, P.S.; Yamashita, M.; Prakriya, M. Pore opening mechanism of CRAC channels. Cell Calcium. 2017, 63, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Lunz, V.; Romanin, C.; Frischauf, I. Stim1 activation of orai1. Cell Calcium. 2019, 77, 29–38. [Google Scholar] [CrossRef]
- Shim, A.H.; Tirado-Lee, L.; Prakriya, M. Structural and functional mechanisms of CRAC channel regulation. J. Mol. Biol. 2015, 427, 77–93. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.S.; Boltz, R.C.; Blake, J.T.; Nguyen, M.; Talento, A.; Fischer, P.A.; Springer, M.S.; Sigal, N.H.; Slaughter, R.S.; Garcia, M.L.; et al. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J. Exp. Med. 1993, 177, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Cahalan, M.D.; Chandy, K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009, 231, 59–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rader, R.K.; Kahn, L.E.; Anderson, G.D.; Martin, C.L.; Chinn, K.S.; Gregory, S.A. T cell activation is regulated by voltage-dependent and calcium-activated potassium channels. J. Immunol. 1996, 156, 1425–1430. [Google Scholar]
- Panyi, G.; Vamosi, G.; Bacso, Z.; Bagdany, M.; Bodnar, A.; Varga, Z.; Gaspar, R.; Matyus, L.; Damjanovich, S. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Szilagyi, O.; Boratko, A.; Panyi, G.; Hajdu, P. The role of PSD-95 in the rearrangement of Kv1.3 channels to the immunological synapse. Pflug. Arch. 2013, 465, 1341–1353. [Google Scholar] [CrossRef]
- Lioudyno, M.I.; Kozak, J.A.; Penna, A.; Safrina, O.; Zhang, S.L.; Sen, D.; Roos, J.; Stauderman, K.A.; Cahalan, M.D. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc. Natl. Acad. Sci. USA 2008, 105, 2011–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaou, S.A.; Neumeier, L.; Peng, Y.; Devor, D.C.; Conforti, L. The Ca(2+)-activated K(+) channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes. Am. J. Physiol. Cell Physiol. 2007, 292, C1431–C1439. [Google Scholar] [CrossRef] [Green Version]
- Luik, R.M.; Wu, M.M.; Buchanan, J.; Lewis, R.S. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by stim1 at er-plasma membrane junctions. J. Cell Biol. 2006, 174, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Chen, L.H.; Shen, M.R. The distinct role of stim1 and stim2 in the regulation of store-operated ca(2+) entry and cellular function. J. Cell Physiol. 2019, 234, 8727–8739. [Google Scholar] [CrossRef]
- Yamashita, M.; Yeung, P.S.; Ing, C.E.; McNally, B.A.; Pomes, R.; Prakriya, M. STIM1 activates CRAC channels through rotation of the pore helix to open a hydrophobic gate. Nat. Commun. 2017, 8, 14512. [Google Scholar] [CrossRef] [Green Version]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Derler, I.; Plenk, P.; Fahrner, M.; Muik, M.; Jardin, I.; Schindl, R.; Gruber, H.J.; Groschner, K.; Romanin, C. The extended transmembrane Orai1 n-terminal (eton) region combines binding interface and gate for Orai1 activation by STIM1. J. Biol. Chem. 2013, 288, 29025–29034. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Cai, X.; Nwokonko, R.M.; Loktionova, N.A.; Wang, Y.; Gill, D.L. The STIM-Orai coupling interface and gating of the Orai1 channel. Cell Calcium. 2017, 63, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, V.A.; Bernot, K.M.; Srikanth, S.; Gwack, Y.; Balagopalan, L.; Regan, C.K.; Helman, D.J.; Sommers, C.L.; Oh-Hora, M.; Rao, A.; et al. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: Puncta and distal caps. Mol. Biol. Cell 2008, 19, 2802–2817. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Pasche, M.; Junker, C.; Al-Ansary, D.; Rieger, H.; Kummerow, C.; Nunez, L.; Villalobos, C.; Meraner, P.; Becherer, U.; et al. Calcium microdomains at the immunological synapse: How Orai channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J. 2011, 30, 3895–3912. [Google Scholar] [CrossRef] [Green Version]
- Hartzell, C.A.; Jankowska, K.I.; Burkhardt, J.K.; Lewis, R.S. Calcium influx through CRAC channels controls actin organization and dynamics at the immune synapse. Elife 2016, 5, e14850. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Tomita, T.; Janoshazi, A.; Putney, J.W. Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities. J. Cell Sci. 2012, 125, 4354–4361. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Zhou, Y.; Nwokonko, R.M.; Loktionova, N.A.; Wang, X.; Xin, P.; Trebak, M.; Wang, Y.; Gill, D.L. The Orai1 store-operated calcium channel functions as a hexamer. J. Biol. Chem. 2016, 291, 25764–25775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, M.; Lokteva, L.A.; Lewis, R.S. Functional analysis of Orai1 concatemers supports a hexameric stoichiometry for the CRAC channel. Biophys. J. 2016, 111, 1897–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal structure of the calcium release-activated calcium channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef] [Green Version]
- Feske, S.; Picard, C.; Fischer, A. Immunodeficiency due to mutations in Orai1 and STIM1. Clin. Immunol. 2010, 135, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, S.A.; Szigligeti, P.; Neumeier, L.; Lee, S.M.; Duncan, H.J.; Kant, S.K.; Mongey, A.B.; Filipovich, A.H.; Conforti, L. Altered dynamics of Kv1.3 channel compartmentalization in the immunological synapse in systemic lupus erythematosus. J. Immunol. 2007, 179, 346–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, P.S.; Yamashita, M.; Prakriya, M. Molecular basis of allosteric Orai1 channel activation by STIM1. J. Physiol. 2019, 598, 1707–1723. [Google Scholar] [CrossRef]
- Calloway, N.; Vig, M.; Kinet, J.P.; Holowka, D.; Baird, B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol. Biol. Cell 2009, 20, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodeify, R.; Selvaraj, S.; Wen, J.; Arredouani, A.; Hubrack, S.; Dib, M.; Al-Thani, S.N.; McGraw, T.; Machaca, K. A STIM1-dependent ‘trafficking trap’ mechanism regulates Orai1 plasma membrane residence and Ca(2)(+) influx levels. J. Cell Sci. 2015, 128, 3143–3154. [Google Scholar]
- Numaga-Tomita, T.; Putney, J.W. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J. Cell Sci. 2013, 126, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Jousset, H.; Frieden, M.; Demaurex, N. STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (serca) pumps silently refill the endoplasmic reticulum. J. Biol. Chem. 2007, 282, 11456–11464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.Y.; Hoover, P.J.; Mullins, F.M.; Bachhawat, P.; Covington, E.D.; Raunser, S.; Walz, T.; Garcia, K.C.; Dolmetsch, R.E.; Lewis, R.S. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 2009, 136, 876–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, B.A.; Somasundaram, A.; Jairaman, A.; Yamashita, M.; Prakriya, M. The C- and N-terminal STIM1 binding sites on Orai1 are required for both trapping and gating CRAC channels. J. Physiol. 2013, 591, 2833–2850. [Google Scholar] [CrossRef]
- Abdelazeem, K.N.M.; Droppova, B.; Sukkar, B.; Al-Maghout, T.; Pelzl, L.; Zacharopoulou, N.; Ali Hassan, N.H.; Abdel-Fattah, K.I.; Stournaras, C.; Lang, F. Upregulation of Orai1 and STIM1 expression as well as store-operated Ca(2+) entry in ovary carcinoma cells by placental growth factor. Biochem. Biophys. Res. Commun. 2019, 512, 467–472. [Google Scholar] [CrossRef]
- Butorac, C.; Muik, M.; Derler, I.; Stadlbauer, M.; Lunz, V.; Krizova, A.; Lindinger, S.; Schober, R.; Frischauf, I.; Bhardwaj, R.; et al. A novel STIM1-Orai1 gating interface essential for CRAC channel activation. Cell Calcium. 2019, 79, 57–67. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, M.H.; Hu, C.; Kuo, E.; Peng, X.; Hu, J.; Kuo, L.; Zhang, S.L. Differential roles of the C and N termini of Orai1 protein in interacting with stromal interaction molecule 1 (STIM1) for Ca2+ release-activated Ca2+ (CRAC) channel activation. J. Biol. Chem. 2013, 288, 11263–11272. [Google Scholar] [CrossRef] [Green Version]
- Xavier, R.; Rabizadeh, S.; Ishiguro, K.; Andre, N.; Ortiz, J.B.; Wachtel, H.; Morris, D.G.; Lopez-Ilasaca, M.; Shaw, A.C.; Swat, W.; et al. Discs large (Dlg1) complexes in lymphocyte activation. J. Cell Biol. 2004, 166, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Tiffany, A.M.; Manganas, L.N.; Kim, E.; Hsueh, Y.P.; Sheng, M.; Trimmer, J.S. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J. Cell Biol. 2000, 148, 147–158. [Google Scholar] [CrossRef]
- Cai, C.; Li, H.; Rivera, C.; Keinanen, K. Interaction between SAP97 and PSD-95, two maguk proteins involved in synaptic trafficking of ampa receptors. J. Biol. Chem. 2006, 281, 4267–4273. [Google Scholar] [CrossRef] [Green Version]
- Fourie, C.; Li, D.; Montgomery, J.M. The anchoring protein SAP97 influences the trafficking and localisation of multiple membrane channels. Biochim. Biophys. Acta 2014, 1838, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Woehrle, T.; Yip, L.; Elkhal, A.; Sumi, Y.; Chen, Y.; Yao, Y.; Insel, P.A.; Junger, W.G. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate tTcell activation at the immune synapse. Blood 2010, 116, 3475–3484. [Google Scholar] [CrossRef] [Green Version]
- Castro-Ochoa, K.F.; Guerrero-Fonseca, I.M.; Schnoor, M. Hematopoietic cell-specific Lyn substrate (HCLS1 or HS1): A versatile actin-binding protein in leukocytes. J. Leukoc. Biol. 2019, 105, 881–890. [Google Scholar] [CrossRef]
- Nyakeriga, A.M.; Fichtenbaum, C.J.; Goebel, J.; Nicolaou, S.A.; Conforti, L.; Chougnet, C.A. Engagement of the CD4 receptor affects the redistribution of Lck to the immunological synapse in primary T cells: Implications for T-cell activation during human immunodeficiency virus type 1 infection. J. Virol. 2009, 83, 1193–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Round, J.L.; Tomassian, T.; Zhang, M.; Patel, V.; Schoenberger, S.P.; Miceli, M.C. Dlgh1 coordinates actin polymerization, synaptic t cell receptor and lipid raft aggregation, and effector function in T cells. J. Exp. Med. 2005, 201, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, P.; Lin, Y.P.; Bhardwaj, R.; Tucker, C.J.; Bird, G.S.; Hediger, M.A.; Monico, C.; Amin, N.; Parekh, A.B. The N terminus of Orai1 couples to the AKAP79 signaling complex to drive NFAT1 activation by local Ca(2+) entry. Proc. Natl. Acad. Sci. USA 2021, 118, e2012908118. [Google Scholar] [CrossRef] [PubMed]
- Huppa, J.B.; Davis, M.M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 2003, 3, 973–983. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voros, O.; Panyi, G.; Hajdu, P. Immune Synapse Residency of Orai1 Alters Ca2+ Response of T Cells. Int. J. Mol. Sci. 2021, 22, 11514. https://doi.org/10.3390/ijms222111514
Voros O, Panyi G, Hajdu P. Immune Synapse Residency of Orai1 Alters Ca2+ Response of T Cells. International Journal of Molecular Sciences. 2021; 22(21):11514. https://doi.org/10.3390/ijms222111514
Chicago/Turabian StyleVoros, Orsolya, György Panyi, and Péter Hajdu. 2021. "Immune Synapse Residency of Orai1 Alters Ca2+ Response of T Cells" International Journal of Molecular Sciences 22, no. 21: 11514. https://doi.org/10.3390/ijms222111514





