1. Introduction
Bladder cancer ranks as the tenth most common cancer worldwide [
1]. Accounting for more than 90% of all bladder cancers in industrialized countries, urothelial carcinoma (UC) is by far the most common histological type. The remaining 10% show different histological features, comprising, among others, squamous cell carcinoma, adenocarcinoma (AC), urachal carcinoma (UrC), and small cell neuroendocrine carcinoma (SCC) [
2]. Squamous cell carcinoma is the most common subtype, accounting for 3–5% of all urinary bladder cancers worldwide [
3,
4]. In western countries, this subtype occurs more frequently in women than in men and is associated with infections, chronic irritation from urinary calculi, urinary retention, indwelling catheters, and bladder exstrophy, in addition to smoking [
5]. ACs and UrCs account for 1.5% and are found predominantly in men [
2]. UrCs, arising from remnants of the embryonic urachus, are, in most cases, glandular neoplasms. Patients are, on average, about 10 years younger than patients with conventional AC [
4]. SCCs, which account for 0.7% of all bladder tumors, also occur more frequently in men than in women, are equally associated with smoking, and are very aggressive tumors with a propensity for metastasis. The 5-year overall survival rate is low, ranging from 8% to 25% depending on the study [
6].
So far, bladder cancer has been treated similarly, almost regardless of its histological subtype. Only SCC patients routinely receive chemotherapy with platin and etoposit [
7] and benefit from neoadjuvant chemotherapy [
8]. However, studies have shown that muscle-invasive bladder cancer (MIBC) with squamous differentiation also has poor response to standard chemotherapy (MVAC—methotrexate, vinblastine, doxorubicin, and cisplatin, or GC—gemcitabine and cisplatin) [
9]. Recently, immune checkpoint inhibitors (ICI) have evolved as a new therapeutic concept for several cancers and have now become an integral part of first-line (in platinum-ineligible patients) and second-line therapy of advanced (urothelial) bladder cancer [
10].
Moreover, targeted therapies and personalized treatments are becoming increasingly important in bladder cancer. In the last two years, the pan-FGFR Inhibitor Erdafitinib and the antibody-drug conjugates enfortumab vedotin and sacituzumab govitecan received accelerated approval from the Food and Drug Administration (FDA) [
11]. FGFR-inhibitors are small-molecule inhibitors blocking tyrosine kinase receptor signaling, limiting tumor cell growth and survival. Enfortumab vedotin, a monoclonal anti-Nectin-4 antibody conjugated to the microtubule-disrupting agent monomethyl auristatin E (MMAE), binds to cells that express Nectin-4, a cell adhesion molecule that is highly expressed in several solid tumors, including urothelial carcinomas. Subsequently, the MMAE is internalized and released into the target cells and then impairs the formation of the microtubule network [
12,
13]. The antibody-drug conjugate sacituzumab govitecan targets the transmembrane glycoprotein Trop-2 that is overexpressed on the surface of bladder tumor cells, and incorporates the topoisomerase I inhibitor SN-38, resulting in double-stranded DNA breaks during the mitotic S-phase of affected cells [
11,
14].
As therapeutic options are now changing, the purpose of our study was to investigate special histological subtypes of bladder cancer for therapeutic marker expression in order to evaluate the possible use of targeted therapies in these subtypes.
3. Discussion
Thus far, targeted therapies are an integral part of treatment of various types of cancer; however, the treatment options for histological subtypes of bladder cancer are still limited. For this reason, we evaluated potential treatment approaches for these subtypes by analyzing the protein expression and molecular status of potential targets that are commonly used in other entities, including immune response, ERBB, and hormone receptor pathways. In the following, we discuss the putative relevance of analyzed targets to indicate novel treatment options for nonurothelial cancer subtypes.
Recent milestones in bladder cancer treatment were the implementation of immune checkpoint inhibitor treatment in first- and second-line therapy of metastatic bladder cancer [
11]. As an additional treatment option, especially in platin-ineligible patients, response rates of 24% in metastasized urothelial cancers can be achieved [
23]. Besides harmonization studies on different antibodies and cell types, there are few studies that have reported the evaluation of different bladder cancer variants and subtypes. Studies have shown that squamous differentiated bladder cancers frequently exhibit PD-L1 positivity [
24,
25], and we previously demonstrated that the PD-L1 expression of squamous bladder carcinomas is comparable to that of urothelial carcinomas of the bladder [
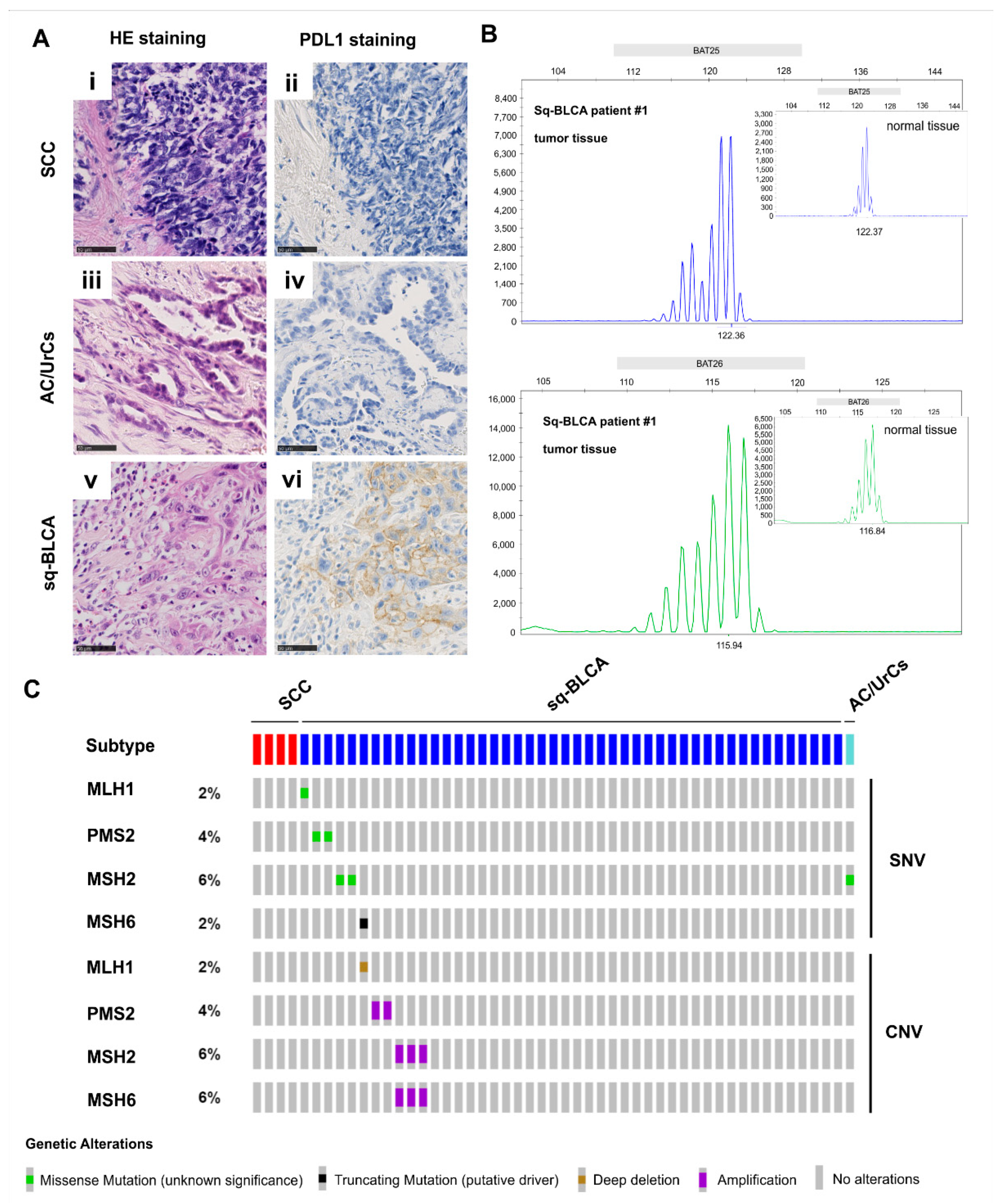
15]. With FDA and EMA approval of Pembrolizumab for the treatment of patients with unresectable or metastatic microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR) solid tumors that have progressed after prior treatment, the patient population that can potentially be treated with an immune checkpoint inhibitor has expanded [
16,
17]. Although microsatellite instability is present only in rare cases in bladder cancer [
26], our data showed that 3% of patients with Sq-BLCA could benefit from immune checkpoint inhibitor therapy. Thus, considering the frequent PD-L1 expression and the rare presence of MSI-high tumors in Sq-BCLA, we recommend its use for these patients.
In hormone-dependent tumors such as prostate carcinoma or hormone receptor-positive breast cancer, anti-hormonal therapies, for example, by inhibiting AR or ER, are already an integral part of therapy [
27,
28]. Recent studies have indicated that bladder cancer is also an endocrine-related tumor, and modulation of the androgen-dependent signaling pathways can alter tumor behavior [
29]. Large prospective studies are still missing to confirm a benefit. Previous clinical trials have shown that the risk of bladder cancer recurrence after transurethral resection of the bladder tumor (TURBT) could be reduced by androgen suppression [
30]. It has been further shown in preclinical experimental settings that blocking the androgen-dependent pathway can enhance the sensitivity of bladder cancer cell lines to radiation and chemotherapy [
30,
31]. Mizushima and Shang used in vivo and in vitro models to show that anti-androgen therapy enhances the efficacy of Bacillus Calmette-Guérin (BCG), an anti-tumor agent for treating noninvasive bladder cancer, to better suppress bladder cancer progression [
32,
33]. Meanwhile, a first phase-I-study (NCT02300610) has been conducted, including patients diagnosed with metastatic bladder cancer showing a response of half of the patients upon enzalutamide therapy. Interestingly, a female patient diagnosed with a strong AR-expressing tumor showed complete remission upon treatment, but all patients received parallel chemotherapy impairing a direct drug-effect correlation [
34]. Thus, data for potential sensitization of tumors by anti-hormone therapy are still required, especially regarding combinations with novel antibody drug conjugates. As, in this study, we revealed 22% of SCCs and 16% of AC/UrCs AR-positive, anti-AR therapy might address a significant proportion of patients, and we are looking forward to future clinical trials also including aberrant differentiated bladder cancers.
Targeting activated tyrosine receptor kinases and subsequent pathways is another possibility of targeted therapy. An effective therapeutic target, for example, in RAS wild-type colorectal cancer and EGFR-mutated non-small-cell lung cancer (NSCLC) [
35,
36], is aberrant activation of ERBB signaling pathways. In recent years, studies have been published suggesting the promising efficacy of anti-EGFR TKIs also in Sq-BLCA of the urinary bladder, representing a wildtype EGFR [
19,
37]. For example, our group showed that Sq-BLCA is dependent on a functioning EGFR signaling pathway and that Sq-BCLA is sensitive to EGFR inhibition [
19]. Overall, 95% of Sq-BLCAs were positive for EGFR, making them potentially suitable for anti-ERBB therapy. Fewer tumors were positive for EGFR in our AC/UrCs and SCC cohorts (i.e., 36% in AC/UrCs and 28% in SCCs); however, the use of anti-ERBB therapy may also be appropriate in rare cases in these patients.
In recent years, the antibody-drug conjugates enfortumab vedotin and sacituzumab govetican received accelerated approval for the treatment of bladder cancer by the FDA regardless of their protein expression level on tumor tissues. Our data showed that both Nectin-4 and Trop-2 are expressed in all of the subtypes of bladder cancer studied here. Thus, 43% of SCCs, 18% of AC/UrCs, and 11% of Sq-BLCAs showed high Nectin-4 expression. Trop-2 was strongly expressed by 67% of Sq-BLCAs, 38% of Ac/UrCs, and 6% of SCCs and was further associated with advanced tumor stages independently of a given subtype. However, it is still unclear whether a high expression of the targets is associated with a better response to therapy as evaluation studies are missing. If this would be the case, evaluation of the expression level prior to the respective targeted therapy would be helpful, as expression differs among the subtypes of bladder cancer. As our study confirmed strong expression values of Nectin-4 and Trop-2, antibody-drug conjugate therapies should also be considered for nonurothelial subtypes of bladder cancer.
Members of the fibroblast growth factor receptor family of kinases (FGFR1–4) are dysregulated in various cancers [
38], increasing cell proliferation and survival of tumor cells [
39,
40,
41,
42]. In bladder cancer, FGFR3 is frequently dysregulated, mainly caused by genetic alterations such as activating mutations or gene fusions [
43,
44]. FGFR3 mutations, which are more frequently found in noninvasive bladder cancer than in muscle-invasive cancer, are associated with favorable features and prognosis compared with bladder cancers, showing FGFR3 overexpression only [
22,
45,
46]. In 2019, the Food and Drug Administration (FDA) approved the Fibroblast Growth Factor Receptor (FGFR)-Inhibitor treatment, which has become the first targeted therapy in advanced muscle-invasive bladder cancer (MIBC) [
11]. Approval by the European Medicines Agency (EMA) is awaited for 2022/2023. However, therapy is restricted to patients with alterations in the FGFR3 (and possibly FGFR2) gene, mainly activating mutations and gene fusions. As hope for the effectiveness of anti-FGFR treatment has been recently placed for patients with squamous bladder cancer, we have now confirmed dysregulation and genetic alterations in a significant subset of squamous differentiated bladder cancers. In turn, both SCCs and AC/UrCs do not seem to be frequently affected by FGFR dysregulation, thus not being eligible for anti-FGFR treatment. As the evolving field of liquid biopsy holds promise to improve noninvasive monitoring and therapy prediction via the detection of, for instance, FGFR3 mutations in urine [
47], future urine-based studies should also consider histological subtypes of bladder cancer. However, only simple analyses of protein, mRNA, or DNA alterations are possible (alteration present/not present) in urine samples. Complex semiquantitative immunohistochemical scorings systems based on staining localization and immune cells, such as PD-L1, preclude the assessment of extracted proteins or single cells derived from urine.