From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release
Abstract
:1. Introduction
2. Materials and Methods
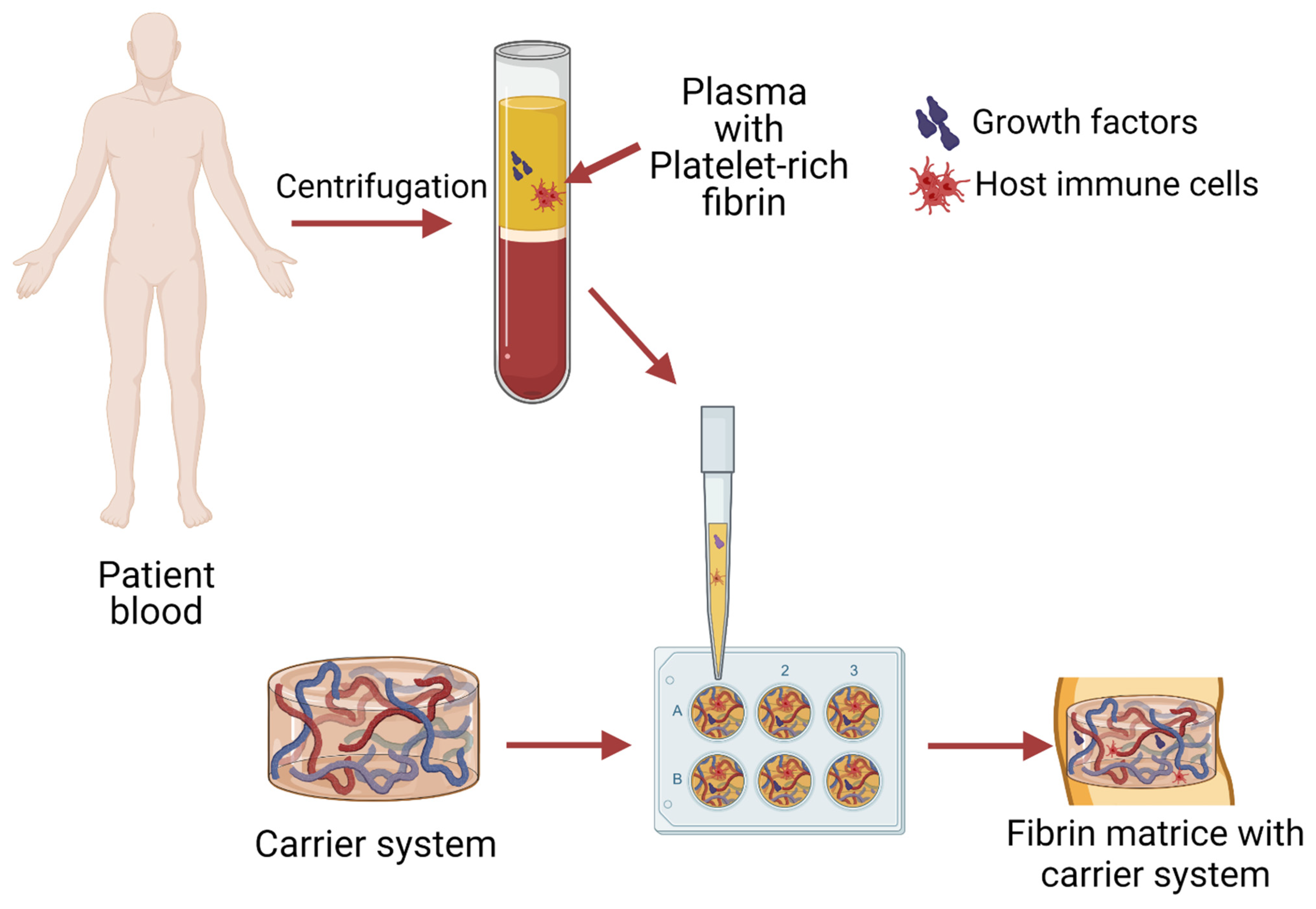
3. From Blood to Injectable or Solid System
4. Therapeutic Enhancement of PRF
4.1. Antibiotics
Lincosamides
4.2. Bisphosphonates
4.3. Statins
4.4. Biguanides
4.5. Non-Steroidal Anti-Inflammatory Drugs
4.6. PRF Combination with Several Drugs
4.7. PRF Combination with Materials and Drugs
5. PRF as a Bioactive Agent in Different Matrices
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garraud, O.; Cognasse, F. Are Platelets Cells? And if Yes, are They Immune Cells? Front. Immunol. 2015, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitua, E.; Sánchez, M.; Nurden, A.T.; Nurden, P.; Orive, G.; Andía, I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006, 24, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Shashank, B.; Bhushan, M. Injectable Platelet-Rich Fibrin (PRF): The newest biomaterial and its use in various dermatological conditions in our practice: A case series. J. Cosmet. Dermatol. 2021, 20, 1421–1426. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Arora, R.; Shukla, S. Injectable-platelet-rich fibrin-smart blood with stem cells for the treatment of alopecia: A report of three patients. Int. J. Trichol. 2019, 11, 128. [Google Scholar] [CrossRef]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Toffler, M.; Toscano, N.; Holtzclaw, D.; Corso, M.D.; Ehrenfest, D.D. Introducing Choukroun’s Platelet Rich Fibrin (PRF) to the Reconstructive Surgery Milieu. J. Implant Adv. Clin. Dent. 2009, 1, 21–32. [Google Scholar]
- Ravi, S.; Santhanakrishnan, M. Mechanical, chemical, structural analysis and comparative release of PDGF-AA from L-PRF, A-PRF and T-PRF—An in vitro study. Biomater. Res. 2020, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Kim, S.-G.; Kim, J.-Y.; Lee, Y.-C.; Choi, J.-Y.; Dragos, R.; Rotaru, H. Restoration of a peri-implant defect by platelet-rich fibrin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 459–463. [Google Scholar] [CrossRef]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.C.; Sabeh Junior, N.; Santos, L.D.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets 2015, 26, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, H.N.; Galanopoulos, T.; Neville-Golden, J.; Kiritsy, C.P.; Lynch, S.E. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc. Natl. Acad. Sci. USA 1991, 88, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, H.N. Human platelet-derived growth factor (PDGF): Purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc. Natl. Acad. Sci. USA 1981, 78, 7314–7317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolidakis, D.; Jansen, J.A. The Biology of Platelet-Rich Plasma and Its Application in Oral Surgery: Literature Review. Tissue Eng. Part B Rev. 2008, 14, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Vinaya Kumar, R.; Shubhashini, N. Platelet rich fibrin: A new paradigm in periodontal regeneration. Cell Tissue Bank. 2013, 14, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Hock, J.M.; Centrella, M.; Canalis, E. Insulin-Like Growth Factor I Has Independent Effects on Bone Matrix Formation and Cell Replication. Endocrinology 1988, 122, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Alejandro-Osorio, A.L.; Grorud, K.W.; Martinez, D.A.; Vailas, A.C.; Grindeland, R.E.; Vanderby, R. Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: Evaluation of loaded and unloaded ligaments. BMC Physiol. 2007, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Ruhrberg, C. Growing and shaping the vascular tree: Multiple roles for VEGF. BioEssays 2003, 25, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Schwarz, E.M.; Maloney, M.D. Platelet-rich plasma therapy—Future or trend? Arthritis Res. Ther. 2012, 14, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. In VEGF and Cancer; Springer: Boston, MA, USA, 2004; pp. 133–144. [Google Scholar]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H. Dietary Modulation of Inflammation. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 74–78. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Falcon, D.M. The Role of Immune Cells and Cytokines in Intestinal Wound Healing. Int. J. Mol. Sci. 2019, 20, 6097. [Google Scholar] [CrossRef] [Green Version]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine To Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Melissa, A.; Guise, T.A.; David, R.G. Cytokine Regulation of Bone Cell Differentiation. In Vitamins and Hormones; Elsevier Masson SAS: Issy-les-Moulineaux, France, 1996; Volume 52, pp. 63–98. [Google Scholar]
- Rossi, C.; Cusimano, M.; Zambito, M.; Finardi, A.; Capotondo, A.; Garcia-Manteiga, J.M.; Comi, G.; Furlan, R.; Martino, G.; Muzio, L. Interleukin 4 modulates microglia homeostasis and attenuates the early slowly progressive phase of amyotrophic lateral sclerosis. Cell Death Dis. 2018, 9, 250. [Google Scholar] [CrossRef]
- Salmon-Ehr, V.; Ramont, L.; Godeau, G.; Birembaut, P.; Guenounou, M.; Bernard, P.; Maquart, F.-X. Implication of Interleukin-4 in Wound Healing. Lab. Investig. 2000, 80, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.A.; O’Neill, L.A.J.; Gearing, A.J.H.; Callard, R.E. TNFα. In The Cytokine FactsBook and Webfacts; Elsevier: Amsterdam, The Netherlands, 2001; pp. 474–480. [Google Scholar]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, L.; Giannou, A.; Gagliani, N.; Huber, S. Regulation of TH17 Cells and Associated Cytokines in Wound Healing, Tissue Regeneration, and Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. An opportunity in paro-implantology: PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, M.; Marshal, V.; Naik, B.; Karunakar, P. Role of Platelet rich fibrin in wound healing: A critical review. J. Conserv. Dent. 2013, 16, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.A.; Campbell, E.J. The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 1999, 65, 137–150. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, T.; Dohan Ehrenfest, D.M. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF): Surgical Adjuvants, Preparations for In Situ Regenerative Medicine and Tools for Tissue Engineering. Curr. Pharm. Biotechnol. 2012, 13, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Bennardo, L.; Del Duca, E.; Patruno, C.; Fortunato, L.; Giudice, A.; Nisticò, S.P. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 2020, 33, e13334. [Google Scholar] [CrossRef] [PubMed]
- Kour, P.; Pudakalkatti, P.S.; Vas, A.M.; Swetalin, D.; Padmanabhan, S. Comparative Evaluation of Antimicrobial Efficacy of Platelet-rich Plasma, Platelet-rich Fibrin, and Injectable Platelet—Rich Fibrin on the Standard Strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Contemp. Clin. Dent. 2018, 9, S325–S330. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261. [Google Scholar] [CrossRef]
- Nurden, A. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105, S13–S33. [Google Scholar] [CrossRef]
- Turitto, V.; Weiss, H. Red blood cells: Their dual role in thrombus formation. Science 1980, 207, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Vinholt, P.J. The role of platelets in bleeding in patients with thrombocytopenia and hematological disease. Clin. Chem. Lab. Med. 2019, 57, 1808–1817. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan Ehrenfest, D.M.; Everts, P.A.; Wiczkowski, A. The Role of Leukocytes from L-PRP/L-PRF in Wound Healing and Immune Defense: New Perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound Healing—Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Wend, S.; Kubesch, A.; Orlowska, A.; Al-Maawi, S.; Zender, N.; Dias, A.; Miron, R.J.; Sader, R.; Booms, P.; Kirkpatrick, C.J.; et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J. Mater. Sci. Mater. Med. 2017, 28, 188. [Google Scholar] [CrossRef] [PubMed]
- Boyce, D.E. The role of lymphocytes in human dermal wound healing. Br. J. Dermatol. 2000, 143, 59. [Google Scholar] [CrossRef] [PubMed]
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Loya, K. Stem Cells. In Handbook of Pharmacogenomics and Stratified Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 207–231. [Google Scholar]
- Lin, S.C.; Talbot, P. Stem Cells. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 390–394. [Google Scholar]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced Platelet-Rich Fibrin: A New Concept for Cell-Based Tissue Engineering by Means of Inflammatory Cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 25, 3747–3755. [Google Scholar] [CrossRef]
- Ghanaati, S.; Mourão, C.; Adam, E.; Sader, R.; Zadeh, H.; Al-Maawi, S. The role of centrifugation process in the preparation of therapeutic blood concentrates: Standardization of the protocols to improve reproducibility. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 41. [Google Scholar] [CrossRef]
- Caruana, A.; Savina, D.; Macedo, J.P.; Soares, S.C. From Platelet-Rich Plasma to Advanced Platelet-Rich Fibrin: Biological Achievements and Clinical Advances in Modern Surgery. Eur. J. Dent. 2019, 13, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Cabaro, S.; D’Esposito, V.; Gasparro, R.; Borriello, F.; Granata, F.; Mosca, G.; Passaretti, F.; Sammartino, J.C.; Beguinot, F.; Sammartino, G.; et al. White cell and platelet content affects the release of bioactive factors in different blood-derived scaffolds. Platelets 2018, 29, 463–467. [Google Scholar] [CrossRef]
- Heal, C.; Buettner, P.; Browning, S. Risk factors for wound infection after minor surgery in general practice. Med. J. Aust. 2006, 185, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, Q.; Huang, C.; Mo, A.; Li, J.; Zuo, Y. Biological properties of an anti-bacterial membrane for guided bone regeneration: An experimental study in rats. Clin. Oral Implants Res. 2010, 21, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.; Hiss, D.; Stephen, L.; Maboza, E. Antibiotic release from leukocyte-and platelet-rich fibrin (L-PRF)—An observational study. S. Afr. Dent. J. 2018, 73, 268–270. [Google Scholar]
- Feng, M.; Wang, Y.; Zhang, P.; Zhao, Q.; Yu, S.; Shen, K.; Miron, R.J.; Zhang, Y. Antibacterial effects of platelet-rich fibrin produced by horizontal centrifugation. Int. J. Oral Sci. 2020, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Jasmine, S.; Thangavelu, A.; Janarthanan, K.; Krishnamoorthy, R.; Alshatwi, A.A. Antimicrobial and antibiofilm potential of injectable platelet rich fibrin—A second-generation platelet concentrate—Against biofilm producing oral staphylococcus isolates. Saudi J. Biol. Sci. 2020, 27, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Moojen, D.J.F.; Everts, P.A.M.; Schure, R.-M.; Overdevest, E.P.; van Zundert, A.; Knape, J.T.A.; Castelein, R.M.; Creemers, L.B.; Dhert, W.J.A. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J. Orthop. Res. 2008, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, T.M.; Gazdzik, T.S.; Arendt, J.; Szczepanski, T.; Kròl, W.; Wielkoszynski, T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances. J. Bone Joint Surg. Br. 2007, 89-B, 417–420. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Greenstein, G.; Polson, A. The Role of Local Drug Delivery in the Management of Periodontal Diseases: A Comprehensive Review. J. Periodontol. 1998, 69, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Clemer-Shamai, N.; Shapira, L. Incorporating antibiotics into platelet-rich fibrin: A novel antibiotics slow-release biological device. J. Clin. Periodontol. 2019, 46, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Knafl, D.; Thalhammer, F.; Vossen, M.G. In-vitro release pharmacokinetics of amikacin, teicoplanin and polyhexanide in a platelet rich fibrin—layer (PRF)—A laboratory evaluation of a modern, autologous wound treatment. PLoS ONE 2017, 12, e0181090. [Google Scholar] [CrossRef] [Green Version]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Cranio-Maxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef]
- Maestre-Vera, J.R. Treatment options in odontogenic infection. Med. Oral Patol. Oral Cir. Bucal. 2004, 9, 19–24. [Google Scholar]
- Bahaa, M.H.D.; Alhaffar, A. New Protocol to Improve the Antibacterial Activity of Platelet Rich Fibrin—In Vitro Study. Res. Sq. 2021, 1–17. [Google Scholar] [CrossRef]
- Bahaa, M.H.D.; Alhaffar, A. Modi ed Protocol to Use Platelet Rich Fibrin (PRF) as a local antibiotic delivery system—In Vitro study. Res. Sq. 2021, 1–16. [Google Scholar] [CrossRef]
- Kanoriya, D.; Pradeep, A.R.; Garg, V.; Singhal, S. Mandibular Degree II Furcation Defects Treatment With Platelet-Rich Fibrin and 1% Alendronate Gel Combination: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 250–258. [Google Scholar] [CrossRef]
- Kanoriya, D.; Pradeep, A.R.; Singhal, S.; Garg, V.; Guruprasad, C.N. Synergistic Approach Using Platelet-Rich Fibrin and 1% Alendronate for Intrabony Defect Treatment in Chronic Periodontitis: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Martande, S.S.; Kumari, M.; Pradeep, A.R.; Singh, S.P.; Suke, D.K.; Guruprasad, C.N. Platelet-Rich Fibrin Combined with 1.2% Atorvastatin for Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2016, 87, 1039–1046. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Garg, V.; Kanoriya, D.; Singhal, S. Platelet-Rich Fibrin with 1.2% Rosuvastatin for Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2016, 87, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Grover, H.; Masamatti, S.; Saksena, N. A clinicoradiographic assessment of 1% metformin gel with platelet-rich fibrin in the treatment of mandibular grade II furcation defects. J. Indian Soc. Periodontol. 2018. [Google Scholar] [CrossRef]
- Shah, M.; Patel, J.; Dave, D.; Shah, S. Comparative evaluation of platelet-rich fibrin with demineralized freeze-dried bone allograft in periodontal infrabony defects: A randomized controlled clinical study. J. Indian Soc. Periodontol. 2015, 19, 56. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Nagpal, K.; Karvekar, S.; Patnaik, K.; Naik, S.B.; Guruprasad, C.N. Platelet-Rich Fibrin With 1% Metformin for the Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2015, 86, 729–737. [Google Scholar] [CrossRef]
- Pillai, A.K.; Thomas, S.; Seth, S.; Jain, N.; Chobey, A. Platelet Rich Fibrin ( PRF ) Gel as Efficient Vehicle for Local Drug Delivery in Minor Oral Surgical Defects. SVOA Dent. 2021, 2, 185–191. [Google Scholar]
- Rafiee, A.; Memarpour, M.; Taghvamanesh, S.; Karami, F.; Karami, S.; Morowvat, M.H. Drug Delivery Assessment of a Novel Triple Antibiotic-Eluting Injectable Platelet-Rich Fibrin Scaffold: An In Vitro Study. Curr. Pharm. Biotechnol. 2021, 22, 380–388. [Google Scholar] [CrossRef]
- Simonpieri, A.; Del Corso, M.; Sammartino, G.; Dohan Ehrenfest, D.M. The Relevance of Choukroun’s Platelet-Rich Fibrin and Metronidazole During Complex Maxillary Rehabilitations Using Bone Allograft. Part I: A New Grafting Protocol. Implant Dent. 2009, 18, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Riaz, L.; Anjum, M.; Yang, Q.; Safeer, R.; Sikandar, A.; Ullah, H.; Shahab, A.; Yuan, W.; Wang, Q. Treatment technologies and management options of antibiotics and AMR/ARGs. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 369–393. ISBN 9780128188828. [Google Scholar]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Sauberan, J.B.; Bradley, J.S. Antimicrobial Agents. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1499.e3–1531.e3. ISBN 9780323401814. [Google Scholar]
- Morar, M.; Bhullar, K.; Hughes, D.W.; Junop, M.; Wright, G.D. Structure and Mechanism of the Lincosamide Antibiotic Adenylyltransferase LinB. Structure 2009, 17, 1649–1659. [Google Scholar] [CrossRef] [Green Version]
- Deane, J.; Rea, M.C.; Fouhy, F.; Stanton, C.; Ross, R.P.; Plant, B.J. Long-Term Implications of Antibiotic Use on Gut Health and Microbiota in Populations Including Patients With Cystic Fibrosis. In The Gut-Brain Axis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–259. ISBN 9780128025444. [Google Scholar]
- Spížek, J.; Řezanka, T. Lincomycin, clindamycin and their applications. Appl. Microbiol. Biotechnol. 2004, 64, 455–464. [Google Scholar] [CrossRef]
- Sancho-Puchades, M.; Herráez-Vilas, J.M.; Berini-Aytés, L.; Gay-Escoda, C. Antibiotic prophylaxis to prevent local infection in Oral Surgery: Use or abuse? Med. Oral Patol. Oral Cir. Bucal. 2009, 14, E28–E33. [Google Scholar]
- Blanchard, R.; Thomas, C.D.L.; Hardiman, R.; Clement, J.G.; Cooper, D.C.; Pivonka, P. Structural and Material Changes of Human Cortical Bone With Age: Lessons from the Melbourne Femur Research Collection. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1–3, pp. 246–264. ISBN 9780128051443. [Google Scholar]
- Chang, C.; Greenspan, A.; Gershwin, M.E. Osteonecrosis. In Kelley’s Textbook of Rheumatology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1692–1711.e5. [Google Scholar]
- Saini, R.; Marawar, P.; Shete, S.; Saini, S. Periodontitis, a true infection. J. Glob. Infect. Dis. 2009, 1, 149. [Google Scholar] [CrossRef]
- Shukla, S.; Chug, A.; Mahesh, L.; Singh, S.; Singh, K. Optimal management of intrabony defects: Current insights. Clin. Cosmet. Investig. Dent. 2019, 11, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Xiao, Z.-J.; Zhao, S.-P. The Benefits and Risks of Statin Therapy in Ischemic Stroke: A Review of the Literature. Neurol. India 2019, 67, 983. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Karvekar, S.; Nagpal, K.; Patnaik, K.; Raju, A.; Singh, P. Rosuvastatin 1.2 mg In Situ Gel Combined With 1:1 Mixture of Autologous Platelet-Rich Fibrin and Porous Hydroxyapatite Bone Graft in Surgical Treatment of Mandibular Class II Furcation Defects: A Randomized Clinical Control Trial. J. Periodontol. 2016, 87, 5–13. [Google Scholar] [CrossRef]
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926. [Google Scholar] [CrossRef]
- Tupas, G.D.; Otero, M.C.B.; Ebhohimen, I.E.; Egbuna, C.; Aslam, M. Antidiabetic lead compounds and targets for drug development. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 127–141. ISBN 9780128178911. [Google Scholar]
- Radi, Z.A.; Khan, K.N. Cardio-renal safety of non-steroidal anti-inflammatory drugs. J. Toxicol. Sci. 2019, 44, 373–391. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zou, D.; Dai, T.; Xu, H.; An, R.; Liu, Y.; Liu, B. Effects of incorporation of granule-lyophilised platelet-rich fibrin into polyvinyl alcohol hydrogel on wound healing. Sci. Rep. 2018, 8, 14042. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zhang, M.; Liu, N.-X.; Lv, X.; Zhang, J.; Chen, F.-M.; Chen, Y.-J. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 2013, 34, 5506–5520. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef]
- Yang, K.-C.; Wang, C.-H.; Chang, H.-H.; Chan, W.P.; Chi, C.-H.; Kuo, T.-F. Fibrin glue mixed with platelet-rich fibrin as a scaffold seeded with dental bud cells for tooth regeneration. J. Tissue Eng. Regen. Med. 2012, 6, 777–785. [Google Scholar] [CrossRef]
- Mu, Z.; Chen, K.; Yuan, S.; Li, Y.; Huang, Y.; Wang, C.; Zhang, Y.; Liu, W.; Luo, W.; Liang, P.; et al. Gelatin Nanoparticle-Injectable Platelet-Rich Fibrin Double Network Hydrogels with Local Adaptability and Bioactivity for Enhanced Osteogenesis. Adv. Healthc. Mater. 2020, 9, 1901469. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Herrera-Vizcaíno, C.; Orlowska, A.; Willershausen, I.; Sader, R.; Miron, R.J.; Choukroun, J.; Ghanaati, S. Biologization of Collagen-Based Biomaterials Using Liquid-Platelet-Rich Fibrin: New Insights into Clinically Applicable Tissue Engineering. Materials 2019, 12, 3993. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.-S.; Park, J.-W.; Kweon, H.; Lee, K.-G.; Kang, S.-W.; Baek, D.-H.; Choi, J.-Y.; Kim, S.-G. Restoration of peri-implant defects in immediate implant installations by Choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Sambhav, J.; Rohit, R.; Ranjana, M.; Shalabh, M. Platelet rich fibrin (PRF) and β -Tricalcium phosphate with coronally advanced flap for the management of grade-II furcation defect. Ethiop. J. Health Sci. 2014, 24, 269. [Google Scholar] [CrossRef] [Green Version]
- Sezgin, Y.; Uraz, A.; Taner, I.L.; Çulhaoğlu, R. Effects of platelet-rich fibrin on healing of intra-bony defects treated with anorganic bovine bone mineral. Braz. Oral Res. 2017, 31, e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef]
- Stone, W.L.; Leavitt, L.; Varacallo, M. Physiology, Growth Factor; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chia-Lai, P.; Orlowska, A.; Al-Maawi, S.; Dias, A.; Zhang, Y.; Wang, X.; Zender, N.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin. Oral Investig. 2018, 22, 1851–1863. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Vorakulpipat, C.; Orlowska, A.; Zrnc, T.A.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. In vivo Implantation of a Bovine-Derived Collagen Membrane Leads to Changes in the Physiological Cellular Pattern of Wound Healing by the Induction of Multinucleated Giant Cells: An Adverse Reaction? Front. Bioeng. Biotechnol. 2018, 6, 104. [Google Scholar] [CrossRef]
- Gonshor, A.; Tye, C.L. Evaluation of an anorganic bovine bone mineral in post-extraction alveolar sockets: A case series. J. Osseointegration 2010, 2, 25–30. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/results?cond=platelet-rich+fibrin+surgery&term=&cntry=&state=&city=&dist= (accessed on 3 October 2021).


| Abbreviation | Platelet Concentrate | Explanation |
|---|---|---|
| PRP | Platelet-rich plasma | First-generation platelet concentrate with high platelet concentrations [4] |
| PRF | Platelet-rich fibrin | Second-generation platelet concentrate [5] |
| i-PRF | Injectable platelet-rich fibrin | Advanced version of PRF in liquid form which can be injected and contains stem cells with high regenerative potential [6] |
| A-PRF | Advanced platelet-rich fibrin | An autogenous blood product with applications in dento-alveolar surgery [7] |
| Abbreviation | Growth Factor/Cytokine | Properties |
|---|---|---|
| PDGF | Platelet-derived growth factor | Provides fibroblast chemotaxis [11], extracellular matrix modification [12], and increases TGF-β release from macrophages [13]. Its addition ensures the growth of cultured cells [14] and improves bone cell proliferation [15] |
| TGF-β | Transforming growth factor β | A multifunctional cytokine [16] and one of 30 members of the superfamily [5] that has been shown to promote extracellular matrix formation [15]. The most common of the three isoforms [13] of TGF-β is TGF-β1, which has the ability to stimulate the production of collagen and fibronectin in cells [17] |
| IGF-I | Insulin-like growth factor I | A growth hormone-dependent polypeptide that stimulates skeletal growth in vivo [18], has an effect on the behavior of cells, thus providing tissue regeneration [19] |
| VEGF | Vascular endothelial growth factor | Promotes the proliferation [20] of endothelial cells and stimulates their migration [21]. It plays an important role in the cardiovascular system, increasing blood flow and enriching the injury site with nutrients [22]. In addition, it plays a role in bone formation and wound healing [23] |
| IL-1β | Interleukin-1β | Plays an important role in protection against infections and injuries [24], it is also involved in the activation of monocytes [25] |
| IL-6 | Interleukin-6 | Able to respond to infections and tissue injuries by stimulating hematopoiesis [26]. The main signal enhancement pathway [20] upon exposure to epithelium and immune cells [27] |
| IL-4 | Interleukin-4 | Acts as a powerful immune regulator [28] that inhibits the proliferation of osteoblast-like cells in vitro [29] and modulates the regeneration of macrophage cells [30]. It is also able to stimulate the accumulation of extracellular matrix macromolecules [31] |
| TNF-α | Tumor necrosis factor-α | Provides growth and differentiation of different cell types [32]. Stimulates the ability of fibroblasts to transform [20], and regulates the activity of vascular endothelial cells and keratinocytes. Determines the synthesis of extracellular matrix proteins [33]; it plays a key role in healing inflammation and wounds [34] |
| Cell Type | Functions |
|---|---|
| Platelets | Involved in primary wound closure and able to release several growth factors to attract inflammatory cells to the site of injury [46,47] |
| Leukocytes | Essential for tissue regeneration as they direct and attract different types of cells in the wound healing process [44] |
| Red blood cells | Physical and chemical interactions between platelets and the blood surface may be provided [48]. Induces an increase in platelet concentrations at the site of action and in vitro coordination [49] |
| Neutrophils | Play an important role in healing processes [50]. Serves as the first signals for the activation of local fibroblasts and keratinocytes [51] |
| Lymphocytes | It affects the osteogenic differentiation of mesenchymal stromal cells [52] and releases a wide range of cytokines [53] |
| Monocytes | A key role in supporting tissue homeostasis by disseminating immune responses to convenience [54] |
| Stem cells | Play an important role in regenerative medicines [55], also have the opportunity to regenerate and differentiate in different types of cells [56]. PRF is a unique source of hematopoietic stem cells (HSCs) [57] |
| Drug | Incorporation Method | Time of the Study | Reference |
|---|---|---|---|
| Clindamycin | Drug mixing in a blood sample, use of PRF clot | 4 days | [71] |
| Lincomycin | Drug mixing in a blood sample, use of PRF clot | 10 days antibacterial activity | [75,76] |
| Amikacin, teicoplanin or polyhexanide | PRF mixing with drug, using co-delivery applicator | 168 h for amikacin, 120 h for teicoplanin and 24 h for polyhexanide antimicrobial effect | [72] |
| 1% Alendronate gel | PRF combinated with drugs | 9 months | [77,78] |
| 1.2% Atorvastatin | Drug combination with PRF and open flap debridement (OFD) | 9 months | [79] |
| 1.2% Rosuvastatin gel | Drug gel adding into PRF membrane | 9 months | [80] |
| 1% Metformin | Drug combination with PRF and OFD | 9 months | [81,82,83] |
| Diclofenac sodium | Drugs injected in PRF using needle | 7 days | [84] |
| Triple antibiotic mixture (MET + CIP + MINO) | Antibiotic mixture mixing with i-PRF, i-PRF scaffold prepare | 28 days | [85] |
| 0.5% Metronidazole | Metronidazole added to the PRF membrane combinated with freeze-dried bone allograft | 10 weeks | [86] |
| Amoxicillin | Drugs used orally 1 h before blood collection | 48 h | [64] |
| Carrier System | Target | Incorporation Method | Time of the Study | Reference |
|---|---|---|---|---|
| G-L-PRF | Accelerate wound healing | Fresh lyophilized PRF added to PVA hydrogels (simple physical method) | 9 days | [103] |
| PRF granules | Improve periodontal healing | PDLSC cultivated with PRF membrane | 7 days | [104] |
| PRF membrane | Improve wound healing | TGFβ-1, PDGF-AB, VEGF and TSP-1 included in PRF | 7 days | [105] |
| Fibrin glue | Enrich the microenvironment with growth factors | Adding PRF into DBC/fibrin glue | 36 weeks | [106] |
| Gelatin nanoparticles | Get mechanically tough and bioactive hydrogel | Mixing i-PRF with GNPs by repetitive extrusion | 3 weeks | [107] |
| Collagen membrane | Enhance the bioactivity of collagen-based biomaterials | Liquid-PRF is applied to collagen membrane | 24 h | [108] |
| PRF | Prevent peri-implant defect | Silk fibroin mixing with PRF in vivo | 8 weeks | [109] |
| PRF membrane | Treatment of furcation defect | β-TCP granules insertion at the defect site and sealing with a PRF membrane | 9 months | [110] |
| PRF membrane | Treatment of intrabony defects | ABBM mixed with PRF | 6 months | [111] |
| PRF membrane | Treatment for periodontal intrabony defects | BPBM mixed with PRF | 6 months | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egle, K.; Salma, I.; Dubnika, A. From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release. Int. J. Mol. Sci. 2021, 22, 11553. https://doi.org/10.3390/ijms222111553
Egle K, Salma I, Dubnika A. From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release. International Journal of Molecular Sciences. 2021; 22(21):11553. https://doi.org/10.3390/ijms222111553
Chicago/Turabian StyleEgle, Karina, Ilze Salma, and Arita Dubnika. 2021. "From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release" International Journal of Molecular Sciences 22, no. 21: 11553. https://doi.org/10.3390/ijms222111553
APA StyleEgle, K., Salma, I., & Dubnika, A. (2021). From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release. International Journal of Molecular Sciences, 22(21), 11553. https://doi.org/10.3390/ijms222111553






