Serous Membrane Detachment with Ultrasonic Homogenizer Improves Engraftment of Fetal Liver to Liver Surface in a Rat Model of Cirrhosis
Abstract
:1. Introduction
2. Results
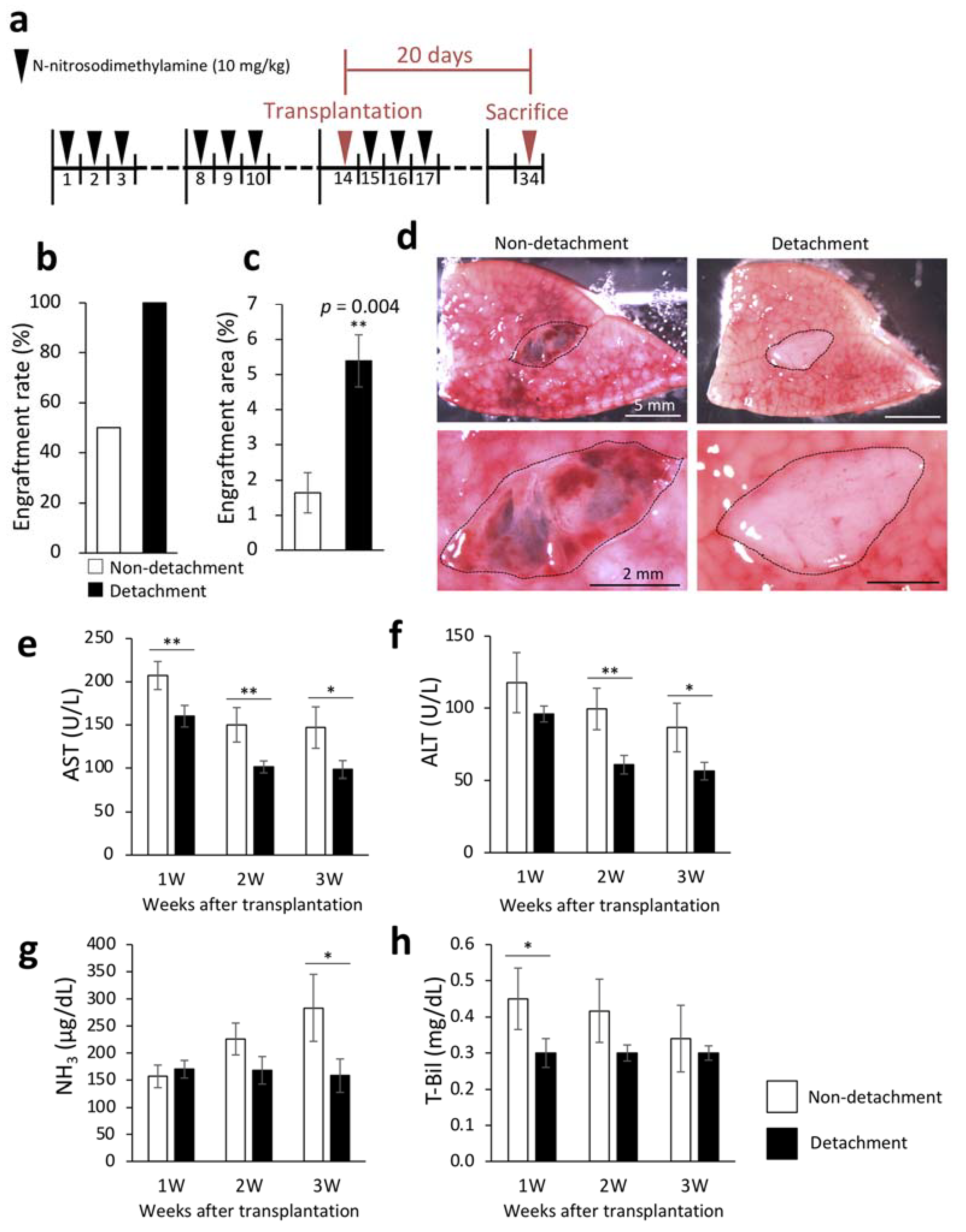
2.1. Effectiveness of Serous Membrane Detachment
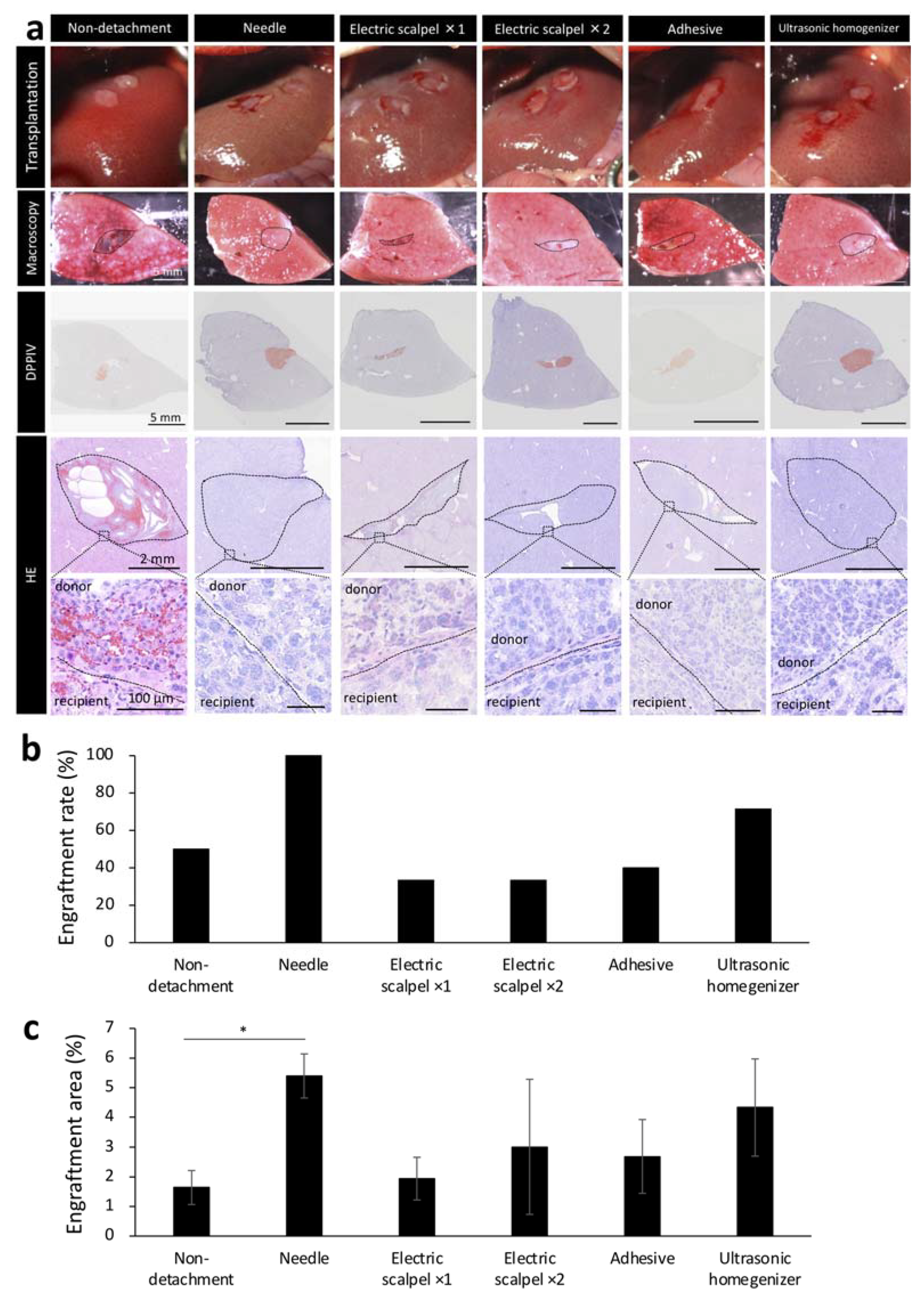
2.2. Amount of Bleeding during Surgery and Tissue Damage after Fetal Liver Transplantation Using Different Detachment Methods
2.3. Engraftment Efficiency and Histological Assessment of the Fetal Liver Transplanted Using Different Detachment Methods
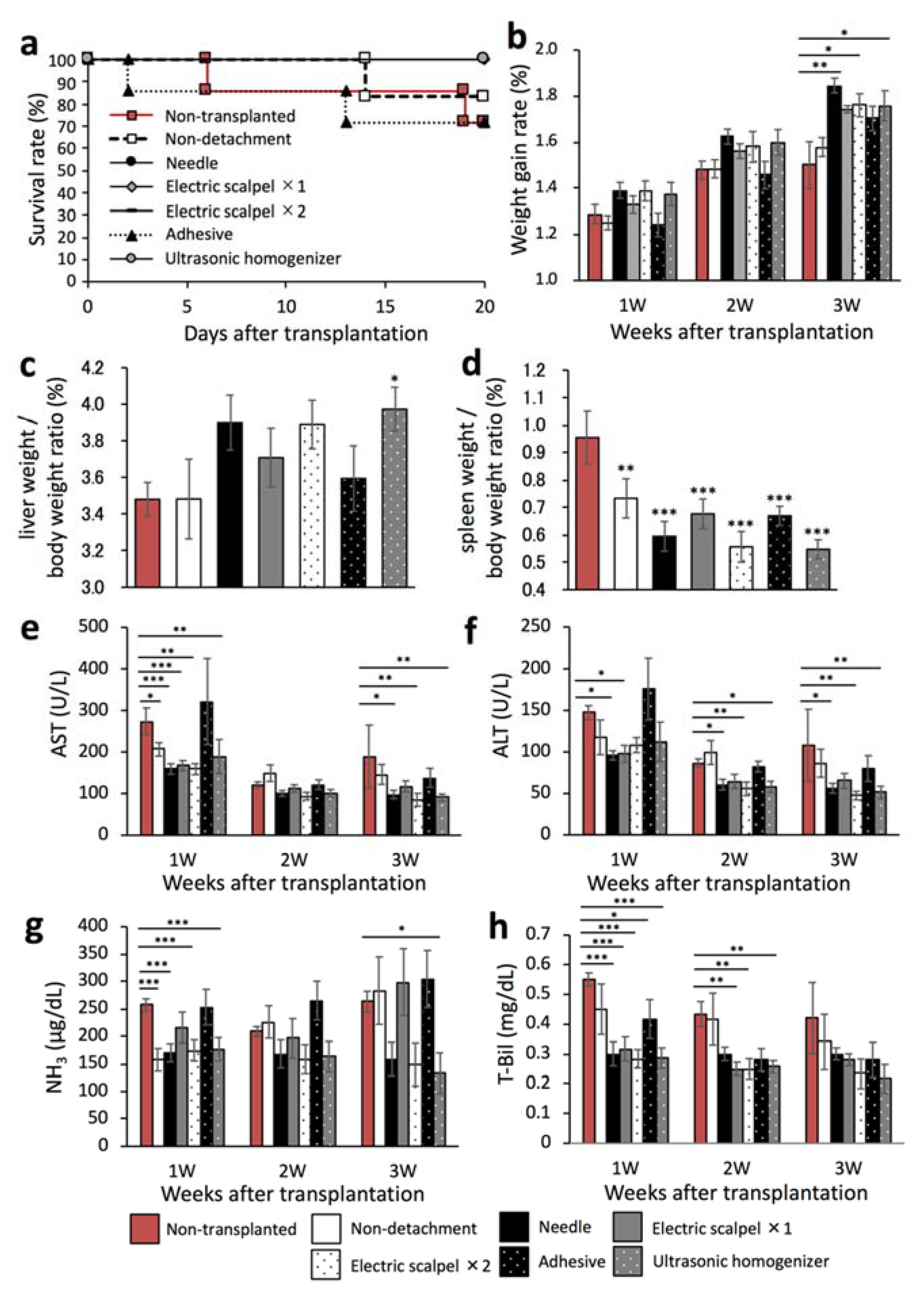
2.4. Therapeutic Effect of the Transplanted Fetal Liver Using Different Detachment Methods
2.5. Evaluation for Maturation of the Transplanted Fetal Liver Using Different Detachment Methods
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Induction of Liver Cirrhosis in Rats
4.3. Methods for Detachment of the Serous Membrane
4.4. Transplantation of Rat Fetal Livers
4.5. Measurement of Bleeding
4.6. Assessment of Liver Function
4.7. Enzyme Histochemistry for DPPIV
4.8. Immunohistochemical Staining
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, P.S.; Runyon, B.A. Treatment of patients with cirrhosis. N. Engl. J. Med. 2016, 375, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, D.; Ditah, I.C.; Saeian, K.; Lalehzari, M.; Aronsohn, A.; Gorospe, E.C.; Charlton, M. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver Disease Among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017, 152, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Viganò, M.; Lampertico, P. Antiviral drugs for HBV liver disease. Expert Opin. Biol. Ther. 2011, 11, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human hepatocyte transplantation for liver disease: Current status and future perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pose, E.; Napoleone, L.; Amin, A.; Campion, D.; Jimenez, C.; Piano, S.; Roux, O.; Uschner, F.E.; de Wit, K.; Zaccherini, G.; et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): A randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol. Hepatol. 2020, 5, 31–41. [Google Scholar] [CrossRef]
- Wang, M.-F.; Li, Y.-B.; Gao, X.-J.; Zhang, H.-Y.; Lin, S.; Zhu, Y.-Y. Efficacy and safety of autologous stem cell transplantation for decompensated liver cirrhosis: A retrospective cohort study. World J. Stem Cells 2018, 10, 138–145. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; Alimoghaddam, K.; Mohyeddin-Bonab, M.; Bagheri, M.; Bashtar, M.; Ghanaati, H.; Baharvand, H.; Ghavamzadeh, A.; Malekzadeh, R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch. Iran. Med. 2007, 10, 459–466. [Google Scholar]
- Patrick, M. Hepatocytes methods and protocols. Hepatocytes Methods Mol. Biol. 2010, 640, 525–534. [Google Scholar]
- Volarevic, V.; Nurković, J.S.; Arsenijevic, N.; Stojkovic, M. Concise Review: Therapeutic Potential of Mesenchymal Stem Cells for the Treatment of Acute Liver Failure and Cirrhosis. Stem Cells 2014, 32, 2818–2823. [Google Scholar] [CrossRef]
- Gupta, S.; Aragona, E.; Vemuru, R.P.; Bhargava, K.K.; Burk, R.D.; Chowdhury, J.R. Permanent engraftment and function of hepatocytes delivered to the liver: Implications for gene therapy and Liver Repopulation. Hepatology 1991, 14, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Chowdhury, J.R. Hepatocyte transplantation. Am. J. Transplant. 2004, 4 (Suppl. 6), 7–13. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Enomura, M.; Yoshizawa, E.; Kimura, M.; Koike, H.; Ueno, Y.; Matsuzaki, T.; Yamazaki, T.; Toyohara, T.; Osafune, K.; et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell 2015, 16, 556–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabol, F.; Vasilenko, T.; Novotný, M.; Tomori, Z.; Bobrov, N.; Zivcak, J.; Hudák, R.; Gal, P. Intradermal Running Suture versus 3M™ Vetbond™ Tissue Adhesive for Wound Closure in Rodents: A Biomechanical and Histological Study. Eur. Surg. Res. 2010, 45, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Fasulo, F.; Giori, A.; Fissi, S.; Bozzetti, F.; Doci, R.; Gennari, L. Cavitron Ultrasonic Surgical Aspirator (CUSA) in liver resection. Int. Surg. 1992, 77, 64–66. [Google Scholar] [PubMed]
- Qiu, R.; Murata, S.; Oshiro, K.; Hatada, Y.; Taniguchi, H. Transplantation of fetal liver tissue coated by ultra-purified alginate gel over liver improves hepatic function in the cirrhosis rat model. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Haggerty, H.G.; Holsapple, M.P. Role of metabolism in dimethylnitrosamine-induced immunosuppression: A review. Toxicology 1990, 63, 1–23. [Google Scholar] [CrossRef]
- Lee, E.-S.; Shin, M.-O.; Yoon, S.; Moon, J.-O. Resveratrol inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharmacal. Res. 2010, 33, 925–932. [Google Scholar] [CrossRef]
- Thompson, N.L.; Hixson, D.C.; Callanan, H.; Panzica, M.; Flanagan, D.; Faris, R.A.; Hong, W.J.; Hartel-Schenk, S.; Doyle, D. A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein. Use as a liver-cell transplantation model. Biochem. J. 1991, 273, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Walborg, E.F., Jr.; Tsuchida, S.; Weeden, D.S.; Thomas, M.W.; Barrick, A.; McEntire, K.D.; Allison, J.P.; Hixson, D.C. Identification of dipeptidylpeptidase IV as a protein shared by the plasma membrane of hepatocytes and liver Biomatrix. Exp. Cell Res. 1985, 158, 509–518. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Petkov, P.; Dabeva, M.D.; Shafritz, D.A. Stem Cell Properties and Repopulation of the Rat Liver by Fetal Liver Epithelial Progenitor Cells. Am. J. Pathol. 2001, 159, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Dabeva, M.D.; Hwang, S.-G.; Vasa, S.R.G.; Hurston, E.; Novikoff, P.M.; Hixson, D.C.; Gupta, S.; Shafritz, D.A. Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc. Natl. Acad. Sci. USA 1997, 94, 7356–7361. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.F.; Carithers, R.L.; AASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005, 41, 1407–1432. [Google Scholar] [CrossRef]
- Kwak, K.-A.; Cho, H.-J.; Yang, J.-Y.; Park, Y.-S. Current perspectives regarding stem cell-based therapy for liver cirrhosis. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–19. [Google Scholar] [CrossRef]
- Jang, Y.O.; Kim, Y.J.; Baik, S.K.; Kim, M.Y.; Eom, Y.W.; Cho, M.Y.; Park, H.J.; Park, S.Y.; Kim, B.R.; Kim, J.W.; et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: A pilot study. Liver Int. 2014, 34, 33–41. [Google Scholar] [CrossRef]
- Peng, L.; Xie, D.-Y.; Lin, B.-L.; Liu, J.; Zhu, H.-P.; Xie, C.; Zheng, Y.-B.; Gao, Z.-L. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology 2011, 54, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Sabry, D.; Rashed, L.A.; Aref, W.M.; El-Ghobary, M.A.; Farhan, M.S.; Fouad, H.A.; Youssef, Y.A.-A. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin. Transplant. 2013, 27, 607–612. [Google Scholar] [CrossRef]
- Nishida, K.; Sato, N.; Sasaki, H.; Nakamura, J. Absorption characteristics of dextrans with different molecular weights from the liver surface membrane in rats: Implications for targeting to the liver. J. Drug Target. 1996, 4, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.-W.; Wu, Y.-Z.; Wang, X.; Pan, H.-X.; Li, P.; Du, W.-Y.; Qi, Z.; Huang, A.; Zhang, L.-W.; Chen, W.; et al. Experimental and clinical study of influence of high-frequency electric surgical knives on healing of abdominal incision. World J. Gastroenterol. 2006, 12, 4082–4085. [Google Scholar] [CrossRef]
- Romano, F.; Garancini, M.; Uggeri, F.; Degrate, L.; Nespoli, L.; Gianotti, L.; Nespoli, A.; Uggeri, F. Bleeding in hepatic surgery: Sorting through methods to prevent it. HPB Surg. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawakatsu-Hatada, Y.; Murata, S.; Mori, A.; Kimura, K.; Taniguchi, H. Serous Membrane Detachment with Ultrasonic Homogenizer Improves Engraftment of Fetal Liver to Liver Surface in a Rat Model of Cirrhosis. Int. J. Mol. Sci. 2021, 22, 11589. https://doi.org/10.3390/ijms222111589
Kawakatsu-Hatada Y, Murata S, Mori A, Kimura K, Taniguchi H. Serous Membrane Detachment with Ultrasonic Homogenizer Improves Engraftment of Fetal Liver to Liver Surface in a Rat Model of Cirrhosis. International Journal of Molecular Sciences. 2021; 22(21):11589. https://doi.org/10.3390/ijms222111589
Chicago/Turabian StyleKawakatsu-Hatada, Yumi, Soichiro Murata, Akihiro Mori, Kodai Kimura, and Hideki Taniguchi. 2021. "Serous Membrane Detachment with Ultrasonic Homogenizer Improves Engraftment of Fetal Liver to Liver Surface in a Rat Model of Cirrhosis" International Journal of Molecular Sciences 22, no. 21: 11589. https://doi.org/10.3390/ijms222111589





