Transcriptome Profiling of Cu Stressed Petunia Petals Reveals Candidate Genes Involved in Fe and Cu Crosstalk
Abstract
1. Introduction
2. Results
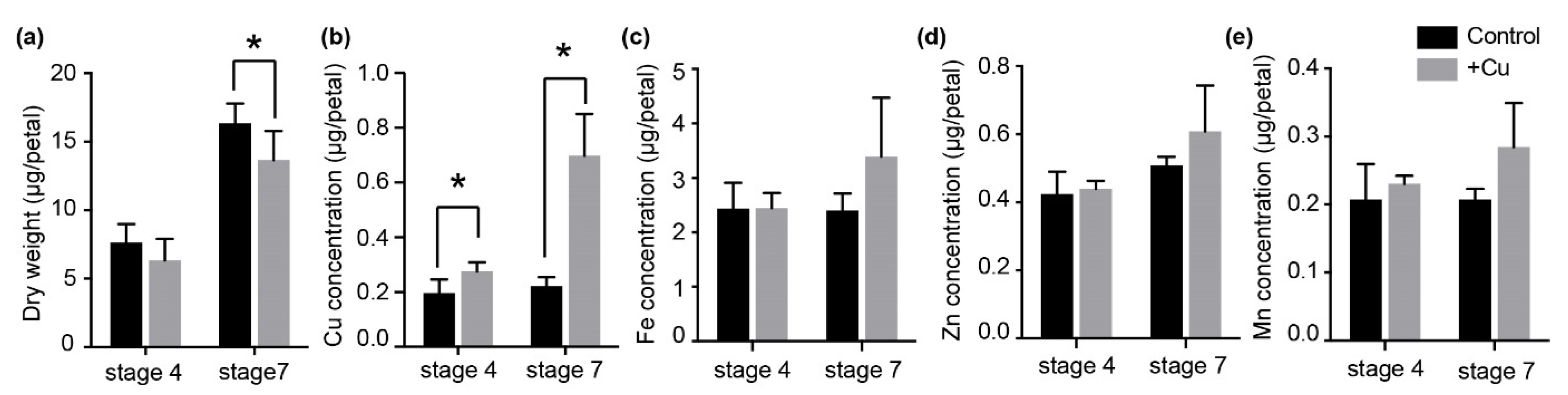
2.1. Dry Weight and Element Content in Petunia Petals under Excess Cu Treatment
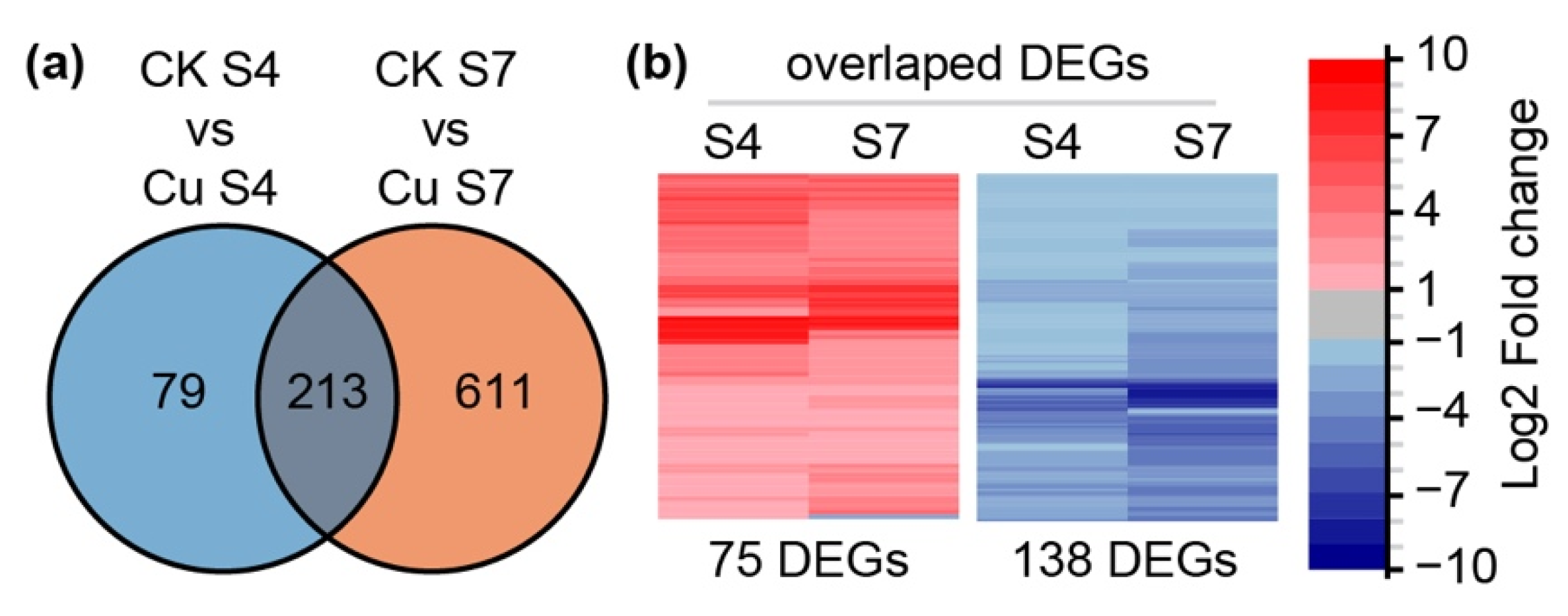
2.2. Transcriptome Profiling Analysis
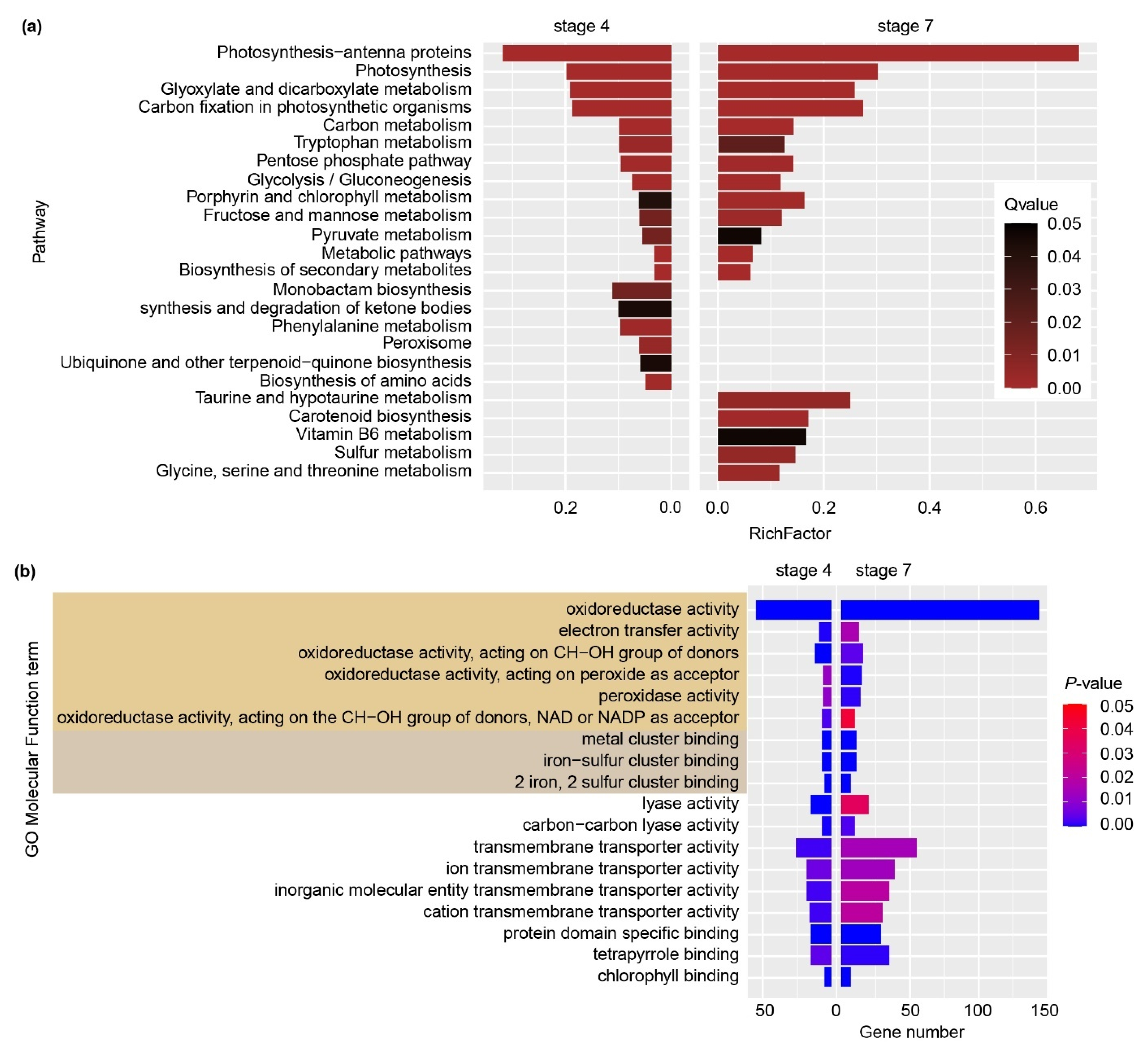
2.3. KEGG and GO Enrichment Analysis
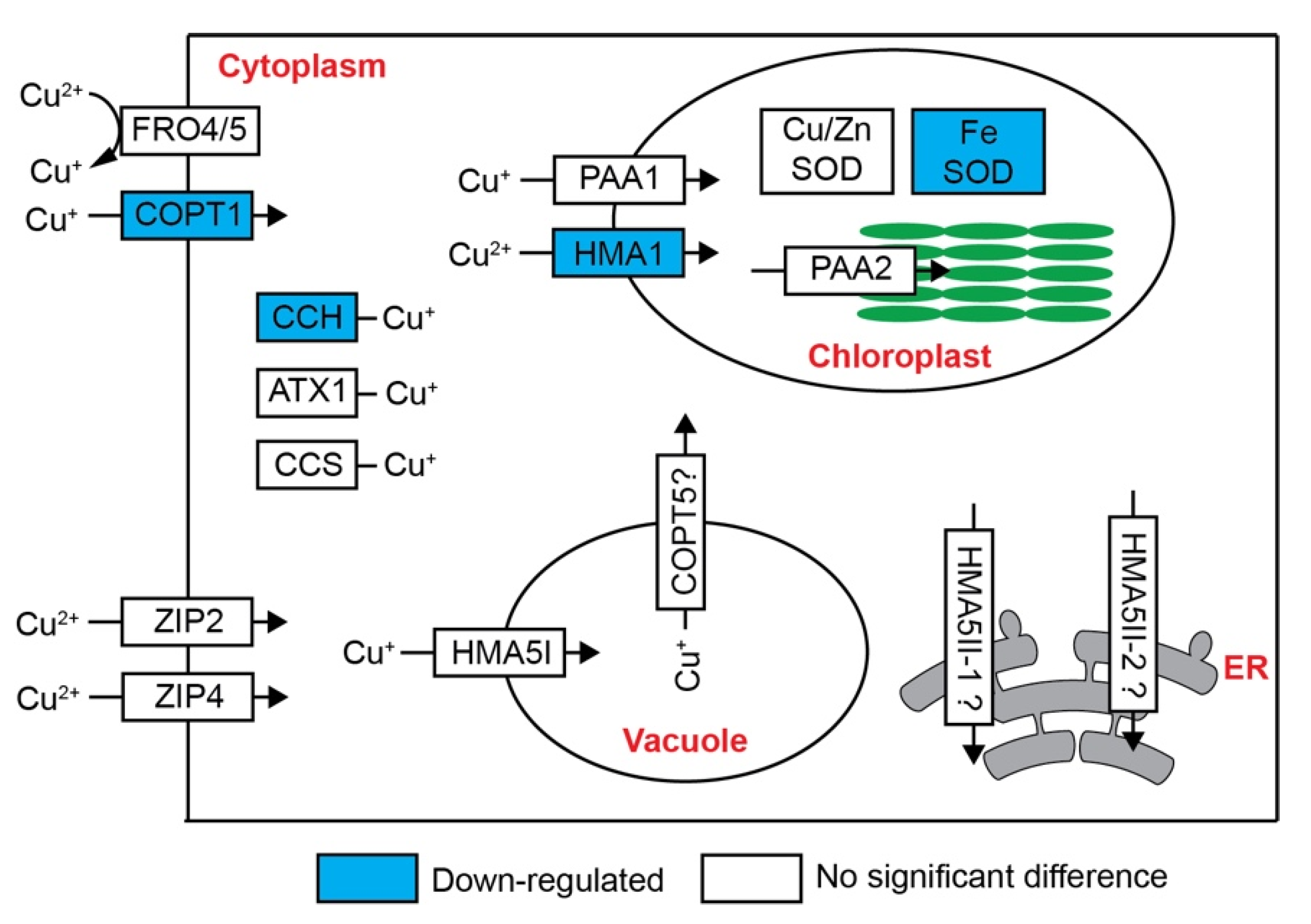
2.4. Expression Pattern of Cu Homeostasis Genes
2.5. Expression Profile of Fe Homeostasis Genes
2.6. Excess Cu Affects Photosynthesis and Fe-Dependent Proteins in Petal
2.7. Expression Levels of DEGs in Petunia Petals under Fe-Deficiency Treatment
3. Discussion
3.1. Fe Deficiency Response in Petunia Petals under Excess Cu
3.2. Cu Detoxification and Crosstalk with Fe in Petunia Petal under Excess Cu
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. RNA-Seq
4.3. GO and KEGG Enrichment
4.4. Real-Time RT-PCR
4.5. Element Analysis
4.6. Chlorophyll Content and Photochemical Activityp Measurement
4.7. Malondialdehyde (MDA) and Catalase (CAT) Measurement
4.8. Phylogenetic Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirayama, T.; Kieber, J.J.; Hirayama, N.; Kogan, M.; Guzman, P.; Nourizadeh, S.; Alonso, J.M.; Dailey, W.P.; Dancis, A.; Ecker, J.R. RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 1999, 97, 383–393. [Google Scholar] [CrossRef]
- Terry, N.; Low, G. Leaf Chlorophyll Content and Its Relation to the Intracellular Localization of Iron. J. Plant Nutr. 1982, 5, 301–310. [Google Scholar] [CrossRef]
- Shikanai, T. PAA1, a P-Type ATPase of Arabidopsis, Functions in Copper Transport in Chloroplasts. Plant Cell 2003, 15, 1333–1346. [Google Scholar] [CrossRef]
- Katoh, S. A new copper protein from Chlorella ellisoidea. Nature 1960, 186, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Castruita, M.; Casero, D.; Karpowicz, S.J.; Kropat, J.; Vieler, A.; Hsieh, S.I.; Yan, W.; Cokus, S.; Loo, J.A.; Benning, C.; et al. Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell 2011, 23, 1273–1292. [Google Scholar] [CrossRef]
- Huang, X.Y.; Deng, F.; Yamaji, N.; Pinson, S.R.M.; Fujii-Kashino, M.; Danku, J.; Douglas, A.; Guerinot, M.L.; Salt, D.E.; Ma, J.F. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun. 2016, 7, 12138. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Colás, N.; Sancenón, V.; Rodríguez-Navarro, S.; Mayo, S.; Thiele, D.J.; Ecker, J.R.; Puig, S.; Peñarrubia, L. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006, 45, 225–236. [Google Scholar] [CrossRef]
- Li, Y.; Iqbal, M.; Zhang, Q.; Spelt, C.; Bliek, M.; Hakvoort, H.W.J.; Quattrocchio, F.M.; Koes, R.; Schat, H. Two Silene vulgaris copper transporters residing in different cellular compartments confer copper hypertolerance by distinct mechanisms when expressed in Arabidopsis thaliana. New Phytol. 2017, 215, 1102–1114. [Google Scholar] [CrossRef]
- Yamasaki, H.; Hayashi, M.; Fukazawa, M.; Kobayashi, Y.; Shikanai, T. SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 2009, 21, 347–361. [Google Scholar] [CrossRef]
- Bernal, M.; Casero, D.; Singh, V.; Wilson, G.T.; Grande, A.; Yang, H.; Dodani, S.C.; Pellegrini, M.; Huijser, P.; Connolly, E.L.; et al. Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 2012, 24, 738–761. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Eisenhut, M.; Schneider, A. Chloroplast Transition Metal Regulation for Efficient Photosynthesis. Trends Plant Sci. 2020, 25, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, H.; Wang, N.; Li, J.; Zhao, W.; Du, J.; Wang, D.; Ling, H.Q. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008, 18, 385–397. [Google Scholar] [CrossRef]
- Wang, N.; Cui, Y.; Liu, Y.; Fan, H.; Du, J.; Huang, Z.; Yuan, Y.; Wu, H.; Ling, H.Q. Requirement and functional redundancy of Ib subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana. Mol. Plant 2013, 6, 503–513. [Google Scholar] [CrossRef]
- Schwarz, B.; Bauer, P. FIT, a regulatory hub for iron deficiency and stress signaling in roots, and FIT-dependent and -independent gene signatures. J. Exp. Bot. 2020, 71, 1694–1705. [Google Scholar] [CrossRef]
- Kim, S.A.; LaCroix, I.S.; Gerber, S.A.; Guerinot, M. Lou The iron deficiency response in Arabidopsis thaliana requires the phosphorylated transcription factor URI. Proc. Natl. Acad. Sci. USA 2019, 116, 24933–24942. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Y.; Liang, G. FIT and bHLH Ib transcription factors modulate iron and copper crosstalk in Arabidopsis. Plant Cell Environ. 2021, 44, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; McInturf, S.A.; Amundsen, K. Transcriptomic and physiological characterization of the fefe mutant of melon (Cucumis melo) reveals new aspects of iron-copper crosstalk. New Phytol. 2014, 203, 1128–1145. [Google Scholar] [CrossRef]
- Garcia-Molina, A.; Marino, G.; Lehmann, M.; Leister, D. Systems biology of responses to simultaneous copper and iron deficiency in Arabidopsis. Plant J. 2020, 103, 2119–2138. [Google Scholar] [CrossRef]
- Kastoori Ramamurthy, R.; Xiang, Q.; Hsieh, E.J.; Liu, K.; Zhang, C.; Waters, B.M. New aspects of iron-copper crosstalk uncovered by transcriptomic characterization of Col-0 and the copper uptake mutant: Spl7 in Arabidopsis thaliana. Metallomics 2018, 10, 1824–1840. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Pak, C.H.; Choi, J.M.; Self, J.R. Induced micronutrient toxicity in Petunia hybrida. J. Plant Nutr. 1992, 15, 327–339. [Google Scholar] [CrossRef]
- Jones, M.L. Mineral nutrient remobilization during corolla senescence in ethylene-sensitive and -insensitive flowers. AoB Plants 2013, 5, plt023. [Google Scholar] [CrossRef] [PubMed]
- Khaodee, W.; Aeungmaitrepirom, W.; Tuntulani, T. Effectively simultaneous naked-eye detection of Cu(II), Pb(II), Al(III) and Fe(III) using cyanidin extracted from red cabbage as chelating agent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 126, 98–104. [Google Scholar] [CrossRef]
- Brun, L.A.; Le Corff, J.; Maillet, J. Effects of elevated soil copper on phenology, growth and reproduction of five ruderal plant species. Environ. Pollut. 2003, 122, 361–368. [Google Scholar] [CrossRef]
- Puig, S.; Andrés-Colás, N.; García-Molina, A.; Peñarrubia, L. Copper and iron homeostasis in Arabidopsis: Responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ. 2007, 30, 271–290. [Google Scholar] [CrossRef]
- Andrés-Colás, N.; Perea-García, A.; Puig, S.; Peñarrubia, L. Deregulated copper transport affects Arabidopsis development especially in the absence of environmental cycles. Plant Physiol. 2010, 153, 170–184. [Google Scholar] [CrossRef]
- Deng, F.; Yamaji, N.; Xia, J.; Ma, J.F. A Member of the Heavy Metal P-Type ATPase OsHMA5 Is Involved in Xylem Loading of Copper in Rice. Plant Physiol. 2013, 163, 1353–1362. [Google Scholar] [CrossRef]
- Rodríguez-Celma, J.; Connorton, J.M.; Kruse, I.; Green, R.T.; Franceschetti, M.; Chen, Y.T.; Cui, Y.; Ling, H.Q.; Yeh, K.C.; Balk, J. Arabidopsis BRUTUS-LIKE E3 ligases negatively regulate iron uptake by targeting transcription factor FIT for recycling. Proc. Natl. Acad. Sci. USA 2019, 116, 17584–17591. [Google Scholar] [CrossRef]
- Selote, D.; Samira, R.; Matthiadis, A.; Gillikin, J.W.; Long, T.A. Iron-binding e3 ligase mediates iron response in plants by targeting basic helix-loop-helix transcription factors. Plant Physiol. 2015, 167, 273–286. [Google Scholar] [CrossRef]
- Palmer, C.M.; Hindt, M.N.; Schmidt, H.; Clemens, S.; Guerinot, M. Lou MYB10 and MYB72 Are Required for Growth under Iron-Limiting Conditions. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.J.; Procter, C.M.; Connolly, E.L.; Guerinot, M. Lou A ferric-chelate reductase for iron uptake from soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Fett, J.P.; Guerinot, M. Lou Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002, 14, 1347–1357. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Gayomba, S.R.; Jung, H.I.; Vimalakumari, N.K.; Piñeros, M.; Craft, E.; Rutzke, M.A.; Danku, J.; Lahner, B.; Punshon, T.; et al. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell 2014, 26, 2249–2264. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Chu, H.H.; DiDonato, R.J.; Roberts, L.A.; Eisley, R.B.; Lahner, B.; Salt, D.E.; Walker, E.L. Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 2006, 141, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shinwari, K.I.; Luo, L.; Zheng, L. OsYSL13 is involved in iron distribution in rice. Int. J. Mol. Sci. 2018, 19, 3537. [Google Scholar] [CrossRef]
- Jean, M.L.; Schikora, A.; Mari, S.; Briat, J.F.; Curie, C. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. Plant J. 2005, 44, 769–782. [Google Scholar] [CrossRef]
- DiDonato, R.J.; Roberts, L.A.; Sanderson, T.; Eisley, R.B.; Walker, E.L. Arabidopsis Yellow Stripe-Like2 (YSL2): A metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J. 2004, 39, 403–414. [Google Scholar] [CrossRef]
- Balk, J.; Schaedler, T.A. Iron Cofactor Assembly in Plants. Annu. Rev. Plant Biol. 2014, 65, 125–153. [Google Scholar] [CrossRef]
- Halimaa, P.; Blande, D.; Baltzi, E.; Aarts, M.G.M.; Granlund, L.; Keinänen, M.; Kärenlampi, S.O.; Kozhevnikova, A.D.; Peräniemi, S.; Schat, H.; et al. Transcriptional effects of cadmium on iron homeostasis differ in calamine accessions of Noccaea caerulescens. Plant J. 2019, 97, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Roosens, N.; Verbruggen, N.; Meerts, P.; Ximénez-Embún, P.; Smith, J.A.C. Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from western Europe. Plant Cell Environ. 2003, 26, 1657–1672. [Google Scholar] [CrossRef]
- Schuler, M.; Keller, A.; Backes, C.; Philippar, K.; Lenhof, H.P.; Bauer, P. Transcriptome analysis by GeneTrail revealed regulation of functional categories in response to alterations of iron homeostasis in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 87. [Google Scholar] [CrossRef][Green Version]
- Colangelo, E.P.; Guerinot, M. Lou The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 2004, 16, 3400–3412. [Google Scholar] [CrossRef]
- Ravet, K.; Touraine, B.; Boucherez, J. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J. 2009, 57, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Ramos, M.S.; Lelièvre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Krämer, U.; Thomine, S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Song, L.; Sengupta, S.; McInturf, S.A.; Grant, D.A.G.; Marjault, H.B.; Castro-Guerrero, N.A.; Burks, D.; Azad, R.K.; Mendoza-Cozatl, D.G.; et al. Expression of a dominant-negative AtNEET-H89C protein disrupts iron–sulfur metabolism and iron homeostasis in Arabidopsis. Plant J. 2020, 101, 1152–1169. [Google Scholar] [CrossRef]
- Nechushtai, R.; Conlan, A.R.; Harir, Y.; Song, L.; Yogev, O.; Eisenberg-Domovich, Y.; Livnah, O.; Michaeli, D.; Rosen, R.; Ma, V.; et al. Characterization of Arabidopsis NEET reveals an ancient role for NEET proteins in iron metabolism. Plant Cell 2012, 24, 2139–2154. [Google Scholar] [CrossRef] [PubMed]
- Rellán-Alvarez, R.; Giner-Martínez-Sierra, J.; Orduna, J.; Orera, I.; Rodríguez-Castrillón, J.A.; García-Alonso, J.I.; Abadía, J.; Alvarez-Fernández, A. Identification of a tri-iron(III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: New insights into plant iron long-distance transport. Plant Cell Physiol. 2010, 51, 91–102. [Google Scholar] [CrossRef]
- Perea-Garcia, A.; Garcia-Molina, A.; Andres-Colas, N.; Vera-Sirera, F.; Perez-Amador, M.A.; Puig, S.; Penarrubia, L. Arabidopsis Copper Transport Protein COPT2 Participates in the Cross Talk between Iron Deficiency Responses and Low-Phosphate Signaling. Plant Physiol. 2013, 162, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Sancenón, V.; Puig, S.; Mateu-Andrés, I.; Dorcey, E.; Thiele, D.J.; Peñarrubia, L. The Arabidopsis Copper Transporter COPT1 Functions in Root Elongation and Pollen Development. J. Biol. Chem. 2004, 279, 15348–15355. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, Y.Y.; Tang, I.C.; Liang, H.M.; Lai, C.C.; Chiou, J.M.; Yeh, K.C. Arabidopsis SUMO E3 ligase SIZ1 is involved in excess copper tolerance. Plant Physiol. 2011, 156, 2225–2234. [Google Scholar] [CrossRef]
- Wintz, H.; Fox, T.; Wu, Y.Y.; Feng, V.; Chen, W.; Chang, H.S.; Zhu, T.; Vulpe, C. Expression Profiles of Arabidopsis thaliana in Mineral Deficiencies Reveal Novel Transporters Involved in Metal Homeostasis. J. Biol. Chem. 2003, 278, 47644–47653. [Google Scholar] [CrossRef] [PubMed]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.L.; Eide, D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Le Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yamaji, N.; Yokosho, K.; Ma, J.F. YSL16 Is a Phloem-Localized Transporter of the Copper-Nicotianamine Complex That Is Responsible for Copper Distribution in Rice. Plant Cell 2012, 24, 3767–3782. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Jiang, Y.; Rahmati, M.; Chia, J.C.; Dokuchayeva, T.; Kavulych, Y.; Zavodna, T.O.; Mendoza, P.N.; Huang, R.; Smieshka, L.M.; et al. YSL3-mediated copper distribution is required for fertility, seed size and protein accumulation in Brachypodium. Plant Physiol. 2021, 186, 655–676. [Google Scholar] [CrossRef] [PubMed]
- Yordem, B.K.; Conte, S.S.; Ma, J.F.; Yokosho, K.; Vasques, K.A.; Gopalsamy, S.N.; Walker, E.L. Brachypodium distachyon as a new model system for understanding iron homeostasis in grasses: Phylogenetic and expression analysis of Yellow Stripe-Like (YSL) transporters. Ann. Bot. 2011, 108, 821–833. [Google Scholar] [CrossRef]
- Mira, H.; Martínez-García, F.; Peñarrubia, L. Evidence for the plant-specific intercellular transport of the Arabidopsis copper chaperone CCH. Plant J. 2001, 25, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Noshi, M.; Mori, D.; Nozawa, K.; Tamoi, M.; Shigeoka, S. The basic helix-loop-helix transcription factor, bHLH11 functions in the iron-uptake system in Arabidopsis thaliana. J. Plant Res. 2019, 132, 93–105. [Google Scholar] [CrossRef]
- Gao, F.; Robe, K.; Dubos, C. Further insights into the role of bHLH121 in the regulation of iron homeostasis in Arabidopsis thaliana. Plant Signal. Behav. 2020. [Google Scholar] [CrossRef]
- Schat, H.; Vooijs, R.; Kuiper, E. Identical major gene loci for heavy metal tolerances that have independently evolved in different local populations and subspecies of Silene vulgaris. Evolution (N. Y.) 1996, 50, 1888–1895. [Google Scholar] [CrossRef]
- Richert-Pöggeler, K.R.; Davies, K.; Franken, P.; Bao, M.; Fernandez-Pozo, N.; Bombarely, A.; Tang, H.; Sims, T.L.; Kuhlemeier, C.; Moser, M.; et al. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants 2016, 2, 16074. [Google Scholar] [CrossRef]
- Van Bel, M.; Diels, T.; Vancaester, E.; Kreft, L.; Botzki, A.; Van de Peer, Y.; Coppens, F.; Vandepoele, K. PLAZA 4.0: An integrative resource for functional, evolutionary and comparative plant genomics. Nucleic Acids Res. 2018, 46, D1190–D1196. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lischewski, S.; Mallona, I.; Hause, B.; Egea-Cortines, M.; Weiss, J. Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida. BMC Plant Biol. 2010, 10, 4. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, S.; Fei, L.; Liu, L.; Wang, Z. Interaction between cd and zn on metal accumulation, translocation and mineral nutrition in tall fescue (Festuca arundinacea). Int. J. Mol. Sci. 2019, 20, 3332. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Lv, A.; Wen, W.; Zhou, P.; An, Y. Auxin Is Involved in Magnesium-Mediated Photoprotection in Photosystems of Alfalfa Seedlings Under Aluminum Stress. Front. Plant Sci. 2020, 11, 746. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Morel, P.; Chambrier, P.; Boltz, V.; Chamot, S.; Rozier, F.; Rodrigues Bento, S.; Trehin, C.; Monniaux, M.; Zethof, J.; Vandenbussche, M. Divergent Functional Diversification Patterns in the SEP/AGL6/AP1 MADS-box Transcription Factor Superclade. Plant Cell 2019, 31, 3033–3056. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]




| Gene Description | PaxiN Number | FPKM (Stage 4 CK) | FPKM (Stage 4 + Cu) | Log2 FC (Stage 4 CK versus + Cu) | FPKM (Stage 7, CK) | FPKM (Stage 7, + Cu) | Log2 FC (S7 CK versus S7 + Cu) |
|---|---|---|---|---|---|---|---|
| COPT1 | Peaxi162Scf00179g00097 | 109.49 | 114.58 | ND | 32.25 | 10.52 | −1.65 |
| COPT protein | Peaxi162Scf00664g00032 | 0 | 0 | ND | 0.05 | 0 | ND |
| COPT protein | Peaxi162Scf00765g00012 | 0 | 0 | ND | 0 | 0 | ND |
| COPT protein | Peaxi162Scf00009g00337 | 0.22 | 0.09 | ND | 0 | 0 | ND |
| COPT protein | Peaxi162Scf00132g00819 | 0.75 | 0.24 | ND | 6.54 | 2.57 | ND |
| COPT5 | Peaxi162Scf00486g00012 | 157.85 | 160.02 | ND | 369.44 | 351.72 | ND |
| ZIP2 | Peaxi162Scf00299g00532 | 0 | 0 | ND | 0.02 | 0 | ND |
| ZIP4 | Peaxi162Scf00158g00077 | 101.6 | 33.84 | ND | 93.45 | 56.48 | ND |
| FRO4/5 | Peaxi162Scf00444g00018 | 0 | 0 | ND | 0 | 0 | ND |
| FRO4/5 | Peaxi162Scf00444g00130 | 0 | 0 | ND | 0 | 0 | ND |
| CCH | Peaxi162Scf00173g01010 | 759.64 | 406.96 | ND | 826.08 | 262.30 | −1.71 |
| ATX1 | Peaxi162Scf01633g00011 | 445.43 | 368.77 | ND | 477.01 | 357.93 | ND |
| CCS | Peaxi162Scf00620g00241 | 10.05 | 14.77 | ND | 22.80 | 34.69 | ND |
| COX17-1 | Peaxi162Scf00069g01430 | 6.68 | 6.39 | ND | 17.11 | 14.96 | ND |
| COX17-2 | Peaxi162Scf00027g00225 | 6.75 | 6.44 | ND | 9.42 | 6.30 | ND |
| MT | Peaxi162Scf00362g00133 | 0 | 0 | ND | 6.33 | 13.93 | ND |
| MT2b-1 | Peaxi162Scf00774g00011 | 160.82 | 200.47 | ND | 1180.83 | 793.71 | ND |
| MT2b-2 | Peaxi162Scf00946g00028 | 13.05 | 10.60 | ND | 8.47 | 4.63 | ND |
| HMA5I | Peaxi162Scf00029g02812 | 47.22 | 37.56 | ND | 31.25 | 30.65 | ND |
| HMA5II-1 | Peaxi162Scf00119g00510 | 0.11 | 0.12 | ND | 0.01 | 0 | ND |
| HMA5II-2 | Peaxi162Scf00119g00519 | 0.62 | 0.62 | ND | 0.56 | 0.62 | ND |
| HMA1 | Peaxi162Scf00945g00216 | 63.80 | 20.52 | −1.64 | 30.56 | 18.21 | ND |
| PAA1 | Peaxi162Scf00001g01039 | 3.17 | 2.90 | ND | 0.82 | 0.67 | ND |
| PAA2 | Peaxi162Scf00486g00096 | 23.15 | 17.08 | ND | 19.62 | 17.38 | ND |
| RAN1 | Peaxi162Scf00199g00810 | 9.60 | 9.46 | ND | 21.06 | 20.17 | ND |
| SPL7 | Peaxi162Scf00095g00001 | 29.70 | 26.56 | ND | 28.10 | 23.88 | ND |
| FIT-like | Peaxi162Scf00490g00018 | 0 | 0 | ND | 0 | 0 | ND |
| FIT-like | Peaxi162Scf00530g00031 | 0 | 0 | ND | 0 | 0.09 | ND |
| FIT-like | Peaxi162Scf00530g00063 | 0 | 0.06 | ND | 0.06 | 0 | ND |
| FIT | Peaxi162Scf00530g00066 | 0.07 | 0 | ND | 0 | 0.09 | ND |
| bHLH121-like | Peaxi162Scf00684g00019 | 6.89 | 0.75 | −3.16 | 13.63 | 0.38 | −5.19 |
| bHLH11-like | Peaxi162Scf00362g01043 | 8.41 | 8.81 | ND | 7.58 | 8.57 | ND |
| PYE1 | Peaxi162Scf00274g00834 | 5.68 | 19.07 | 1.75 | 17.08 | 41.43 | 1.2 |
| PYE2 | Peaxi162Scf00712g00029 | 1.77 | 67.70 | 5.24 | 5.92 | 90.73 | 3.87 |
| bHLH38 | Peaxi162Scf00772g00076 | 0 | 4.03 | 9.05 | 0.12 | 64.04 | 9.0 |
| bHLH39 | Peaxi162Scf00772g00048 | 0.08 | 24.88 | 8.20 | 0.36 | 92.36 | 7.91 |
| BTS1 | Peaxi162Scf00753g00119 | 8.39 | 31.41 | 1.92 | 9.94 | 59.19 | 2.50 |
| BTS2 | Peaxi162Scf00021g00527 | 0.02 | 7.34 | 8.10 | 0.31 | 5.87 | 4.17 |
| BTSL | Peaxi162Scf00009g00529 | 6.47 | 21.58 | 1.75 | 7.00 | 75.48 | 3.37 |
| MYB10-like | Peaxi162Scf00452g00412 | 0.04 | 0.4 | ND | 1.06 | 5.95 | 2.44 |
| FRO2 | Peaxi162Scf00585g00115 | 0.00 | 1.35 | 8.63 | 0.02 | 0.37 | 3.74 |
| FRO1 | Peaxi162Scf00585g00014 | 1.14 | 543.08 | 8.88 | 1.68 | 736.18 | 8.71 |
| FRO8 | Peaxi162Scf00089g01433 | 6.75 | 10.38 | ND | 10.79 | 23.35 | 1.04 |
| AHA2 | Peaxi162Scf01054g00032 | 212.50 | 189.98 | ND | 249.42 | 205.23 | ND |
| IRT-like | Peaxi162Scf00623g00213 | 0 | 0 | ND | 0 | 0 | ND |
| IRT-like | Peaxi162Scf00623g00114 | 0 | 0 | ND | 0 | 0 | ND |
| IRT-like | Peaxi162Scf00149g00128 | 0 | 0 | ND | 0 | 0.12 | ND |
| OPT3 | Peaxi162Scf00442g00710 | 75.43 | 947.23 | 3.65 | 38.56 | 667.36 | 4.03 |
| YSL1 | Peaxi162Scf00074g00232 | 6.29 | 0.27 | −4.50 | 6.88 | 2.04 | −1.85 |
| YSL2 | Peaxi162Scf00542g00622 | 15.25 | 3.34 | −2.17 | 16.23 | 2.62 | −2.72 |
| NRAMP3 | Peaxi162Scf00264g00512 | 68.84 | 293.86 | 2.09 | 151.88 | 460.16 | 1.55 |
| NRAMP2 | Peaxi162Scf00081g00618 | 39.30 | 21.30 | ND | 28.10 | 8.25 | −1.83 |
| NEET | Peaxi162Scf00192g00828 | 39.98 | 0.15 | −7.94 | 110.74 | 0.27 | −8.62 |
| MFL1 | Peaxi162Scf00263g00927 | 35.03 | 38.27 | ND | 15.67 | 15.52 | ND |
| PIC1 | Peaxi162Scf00016g02324 | 52.48 | 49.68 | ND | 44.74 | 47.49 | ND |
| MIT1/2 | Peaxi162Scf00390g00722 | 27.04 | 25.67 | ND | 26.42 | 28.44 | ND |
| Ferritin3 | Peaxi162Scf00284g00521 | 37.81 | 0.33 | −6.79 | 44.99 | 7.04 | −2.76 |
| Ferritin2 | Peaxi162Scf01149g00318 | 53.49 | 11.83 | −2.16 | 104.08 | 16.55 | −2.74 |
| Ferritin1 | Peaxi162Scf01132g00315 | 0.69 | 0.05 | −3.55 | 0.34 | 0.00 | −5.55 |
| NAS1 | Peaxi162Scf00673g00042 | 89.20 | 12.80 | −2.82 | 38.62 | 7.3 | −2.45 |
| NAS2 | Peaxi162Scf00750g00013 | 0 | 0 | ND | 0 | 0 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Li, K.; Li, J.; Schat, H.; Li, Y. Transcriptome Profiling of Cu Stressed Petunia Petals Reveals Candidate Genes Involved in Fe and Cu Crosstalk. Int. J. Mol. Sci. 2021, 22, 11604. https://doi.org/10.3390/ijms222111604
Wu J, Li K, Li J, Schat H, Li Y. Transcriptome Profiling of Cu Stressed Petunia Petals Reveals Candidate Genes Involved in Fe and Cu Crosstalk. International Journal of Molecular Sciences. 2021; 22(21):11604. https://doi.org/10.3390/ijms222111604
Chicago/Turabian StyleWu, Jinglei, Kai Li, Jian Li, Henk Schat, and Yanbang Li. 2021. "Transcriptome Profiling of Cu Stressed Petunia Petals Reveals Candidate Genes Involved in Fe and Cu Crosstalk" International Journal of Molecular Sciences 22, no. 21: 11604. https://doi.org/10.3390/ijms222111604
APA StyleWu, J., Li, K., Li, J., Schat, H., & Li, Y. (2021). Transcriptome Profiling of Cu Stressed Petunia Petals Reveals Candidate Genes Involved in Fe and Cu Crosstalk. International Journal of Molecular Sciences, 22(21), 11604. https://doi.org/10.3390/ijms222111604





