Abstract
Cadmium (Cd2+) pollution occurring in salt-affected soils has become an increasing environmental concern in the world. Fast-growing poplars have been widely utilized for phytoremediation of soil contaminating heavy metals (HMs). However, the woody Cd2+-hyperaccumulator, Populus × canescens, is relatively salt-sensitive and therefore cannot be directly used to remediate HMs from salt-affected soils. The aim of the present study was to testify whether colonization of P. × canescens with ectomycorrhizal (EM) fungi, a strategy known to enhance salt tolerance, provides an opportunity for affordable remediation of Cd2+-polluted saline soils. Ectomycorrhization with Paxillus involutus strains facilitated Cd2+ enrichment in P. × canescens upon CdCl2 exposures (50 μM, 30 min to 24 h). The fungus-stimulated Cd2+ in roots was significantly restricted by inhibitors of plasmalemma H+-ATPases and Ca2+-permeable channels (CaPCs), but stimulated by an activator of plasmalemma H+-ATPases. NaCl (100 mM) lowered the transient and steady-state Cd2+ influx in roots and fungal mycelia. Noteworthy, P. involutus colonization partly reverted the salt suppression of Cd2+ uptake in poplar roots. EM fungus colonization upregulated transcription of plasmalemma H+-ATPases (PcHA4, 8, 11) and annexins (PcANN1, 2, 4), which might mediate Cd2+ conductance through CaPCs. EM roots retained relatively highly expressed PcHAs and PcANNs, thus facilitating Cd2+ enrichment under co-occurring stress of cadmium and salinity. We conclude that ectomycorrhization of woody hyperaccumulator species such as poplar could improve phytoremediation of Cd2+ in salt-affected areas.
Keywords:
annexins; calcium-permeable channels; Cd flux; MAJ; NaCl; NAU; Paxillus involutus; Populus × canescens; PM H+-ATPase 1. Introduction
Cadmium (Cd2+) pollution presents a critical threat to ecological environment and human life [1,2,3,4,5]. The Cd2+ contamination occurring in salt-affected soils has become an increasing environmental concern in recent years [6,7,8,9,10,11,12,13,14,15,16,17]. Coastal areas are polluted by Cd2+ due to rapid urbanization and industrialization. Cadmium is mainly derived from wastewater discharged by electroplating, mining, smelting, fuel, battery and chemical industry [18]. In some coastal saline zones, soil heavy metal pollution also comes from sludge and sewage irrigation [19]. Mining activities cause the release and spread of both hazardous heavy metals (HMs) and soluble salts in inland regions [11]. The Cd2+ contamination in salt-affected soils complicates remediation processes [6,7]. Naturally occurring halophytes may be potentially useful for remediation and phytomanagement [6,20,21,22,23]. However, halophytic species are commonly characterized by slow growth and therefore low biomass production [24]. Poplar trees have been widely utilized for phytoremediation of soils and water resources contaminated with HMs, because of their fast-growth, large biomass and remarkable Cd2+ accumulation in shoots and below-ground [25,26,27,28,29,30,31]. Moreover, several poplars, e.g., Populus tremula, P. × canescens, are known Cd2+ hyperaccumulators [32,33] in terms of the buildup of heavy metals in aerial parts (i.e., 100 times higher than non-accumulators) [34,35,36,37]. However, despite its high ability to tolerate Cd2+ stress [29,33,38], P. × canescens is relatively salt-sensitive [39] and therefore cannot be directly utilized to remediate HMs from salt-affected soils. The use of salt-resistant poplar, P. euphratica, is also hindered because this species is relatively susceptible to Cd2+ stress [40,41,42,43]. Therefore, efficient phytomanagement of heavy metal-contaminated salt soils with fast-growing poplars requires increased abilities of the plants to deal with the ionic stress situations produced by heavy metals and salts [6].
Ectomycorrhization offers great potential and feasibility for remediation of cadmium-contaminated soils [44,45,46,47,48,49,50]. Ectomycorrhization is the formation of symbiosis of a soil fungus with plant roots, whereby the root tip is completely ensheathed by the fungal hyphae. The plant benefits from this interaction by improved mineral nutrition and health [51]. Colonization of roots of P. × canescens with Paxillus involutus, an ectomycorrhizal (EM) fungus, has been repeatedly shown to improve Cd2+ uptake and tolerance [48,52]. The association of Populus canadensis with P. involutus leads to a highly significant increase of Cd2+ uptake and root-to-shoot transport, thus enhancing the total Cd2+ extraction by P. canadensis [44]. P. involutus ameliorates the negative effects of Cd2+ on shoot and root growth and chlorophyll content of old needles in Norway spruce seedlings (Picea abies) [53]. A protective effect against Cd2+ toxicity in the host was observed in Pinus sylvestris colonized with P. involutus [54,55]. P. involutus strains have also been used for phytoremediation of other heavy metals. Inoculation with a lead (Pb2+)-tolerant strain of P. involutus improves growth and Pb2+ tolerance of P. × canescens [56,57]. P. involutus decreases Pb2+ in roots and the translocation from the roots to the stems in Norway spruce (Picea abies) [58,59]. Similarly, P. involutus fungi act as a safety net that can immobilize large amounts of zinc, thus preventing transport to the host plant, Pinus sylvestris [60]. Moreover, ectomycorrhization of P. × canescens with P. involutus increases salt tolerance by maintaining nutrient uptake of K+, Ca2+ and NO3−, and improves Na+ homeostasis in the symbiotic associations [61,62,63,64,65,66]. Thus, it can be hypothesized that P. involutus could increase plant ability for Cd2+ enrichment in salt-affected soils. Arbuscular mycorrhizal fungi are able to enhance growth of pigeonpea (Cajanus cajan) by lowering Cd2+ content and strengthening antioxidant defense under NaCl and Cd stress [67]. Whether the ectomycorrhizal fungus P. involutus can mediate Cd2+ uptake under co-existing stress of NaCl and cadmium needs to be clarified by further experimental investigations.
Under cadmium stress, the P. involutus-facilitated Cd2+ influx is stimulated by plasma membrane (PM) H+-ATPases in EM roots [48]. Upregulated transcription of the PM H+-ATPase genes (HA2.1 and AHA10.1) results in accelerated Cd2+ transport into roots of transgenic [38] and EM poplars [52]. Increased proton pumping activity and transcription of H+-ATPases have also been observed in EM P. × canescens under salt stress [66]. H+-ATPases maintain a proton gradient across PM to drive the entry of Cd2+ [38,48] and nutrient elements, such as K+, Ca2+, and NO3−, in addition to promotion of Na+/H+ antiport [64,65,66]. Moreover, the P. involutus-activated H+-pumps hyperpolarize the membrane potential, facilitating Cd2+ influx via hyperpolarization-activated Ca2+-permeable channels (CaPCs) [48]. Although the P. involutus-stimulated H+-ATPase enhances Cd2+ uptake under single stress of cadmium [48,52], little is known whether the fungi-activated H+-ATPase could improve Cd2+ enrichment in combined stress of CdCl2 and NaCl.
Cellular uptake of Cd2+ also involves the PM CaPCs, as demonstrated for various species [38,41,48,68]. Plant annexins (ANNs) might serve as channels to allow the entry of Ca2+ [69,70,71,72,73,74,75,76] or indirectly mediate Ca2+ conductance [77,78]. Chen et al. suggested that OsANN4 mediates the transmembrane Cd2+ influx along rice roots [73]. The P. euphratica annexin ANN1 facilitates Cd2+ enrichment through CaPCs in roots of transgenic Arabidopsis [79]. P. × canescens colonization with P. involutus leads to Cd2+ enrichment [52] due to stimulation of Cd2+ influx via CaPCs [48]. Cadmium treatment results in increased transcript levels of annexins in maize (ZmAnx9, [80]), peanut (ANNAh3, [81]), and rice (ANN4, [73]). Whether P. × canescens annexins are affected by cadmium and contribute to Cd2+ enrichment in P. involutus ectomycorrhizal associations needs to be investigated. Under sodium chloride salinity, competition between Na+ and Cd2+ for Ca2+ ion channels reduced Cd2+ uptake in Amaranthus mangostanus [82]. The salt effects on annexin-mediated Ca2+ channels remain unclear in ectomycorrhizal roots under co-existing stress conditions of Cd2+ and NaCl.
In this study, we examined the impact of ectomycorrhizal fungi on root Cd2+ uptake under combined stress of salt and cadmium, aiming to elucidate the underlying mechanisms. We used two different P. involutus isolates, MAJ and NAU, for this study. Strain MAJ forms a complete ectomycorrhiza composed of a thick hyphal mantle ensheathing root tip and a typical Hartig net structure inside the roots for nutrient exchange, while strain NAU forms only the outer mantle [83]. We studied Cd2+ uptake in the presence and absence of NaCl and analyzed gene expression of annexins because previous studies show that PeANN1 facilitates Cd2+ enrichment through CaPCs [79]. P. involutus activates H+-pumps and hyperpolarizes membrane potential in EM roots [48,64,65]. Therefore, the PM H+-ATPases-promoted Cd2+ flux was also verified in EM roots under salt stress. Our data reveal that P. involutus inoculation stimulates Cd2+ influx under salt stress, resulting from the upregulated H+-ATPases and annexins in the ectomycorrhizal roots. Both MAJ and NAU conserved the Cd2+ uptake capacities under co-occurring stresses of cadmium and salinity, regardless of the formation of Hartig net in the ectomycorrhizal symbioses.
2. Results
2.1. Cd2+ Concentrations in Roots and Shoots of Ectomycorrhizal Poplars under NaCl Stress
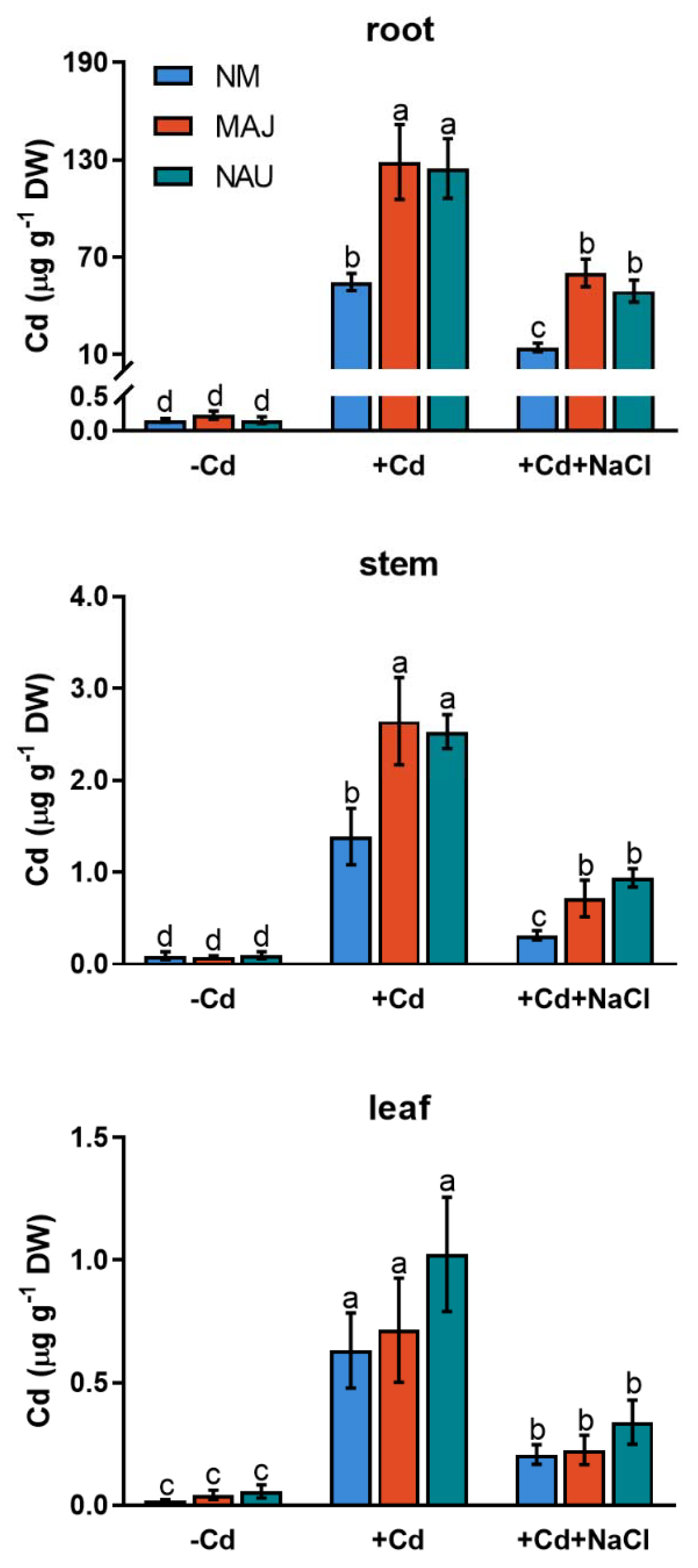
Cd2+ concentrations were analyzed in roots, stems and leaves of NM and EM P. × canescens after 24 h exposure to CdCl2 (50 µM) or combined stress of CdCl2 and NaCl (100 mM). Under CdCl2 stress, non-mycorrhizal (NM) roots displayed remarkably higher Cd2+ concentrations than stem and leaves (Figure 1). Compared to NM plants, Cd2+ concentrations were 0.8- to 1.4-fold higher in roots and stems of poplars colonized with P. involutus isolates, MAJ and NAU (Figure 1). However, the addition of NaCl (100 mM) significantly decreased Cd2+ accumulation in roots and shoots of both NM- and EM-plants (Figure 1). Of note, EM-plants retained significantly higher Cd2+ concentrations in roots and stems than NM poplars under salt stress (Figure 1). Therefore, EM fungi enhanced Cd2+ enrichment in both root and aerial parts of P. × canescens under co-occurring stresses of cadmium and salinity.
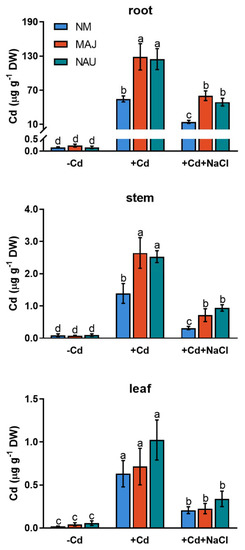
Figure 1.
Cd2+ concentrations in roots, stems and leaves of non-mycorrhizal (NM) and ectomycorrhizal (EM) Populus × canescens under cadmium and salt stress. Poplar plantlets inoculated with or without Paxillus involutus isolates (MAJ or NAU, 30 d), were hydroponically acclimated and subjected to 24 h of CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM). Mean values of Cd2+ concentrations in control (−Cd), CdCl2 stress (+Cd), and combined stress of CdCl2 and NaCl (+Cd + NaCl) are shown. Each column is mean ± SD obtained from 3 individual plants. Statistically significant differences (p < 0.05) among treatments are indicated with different letters (a–d).
2.2. Steady-State Cd2+ Influx in Ectomycorrhizal Poplar Roots and Fungal Mycelia under NaCl Stress
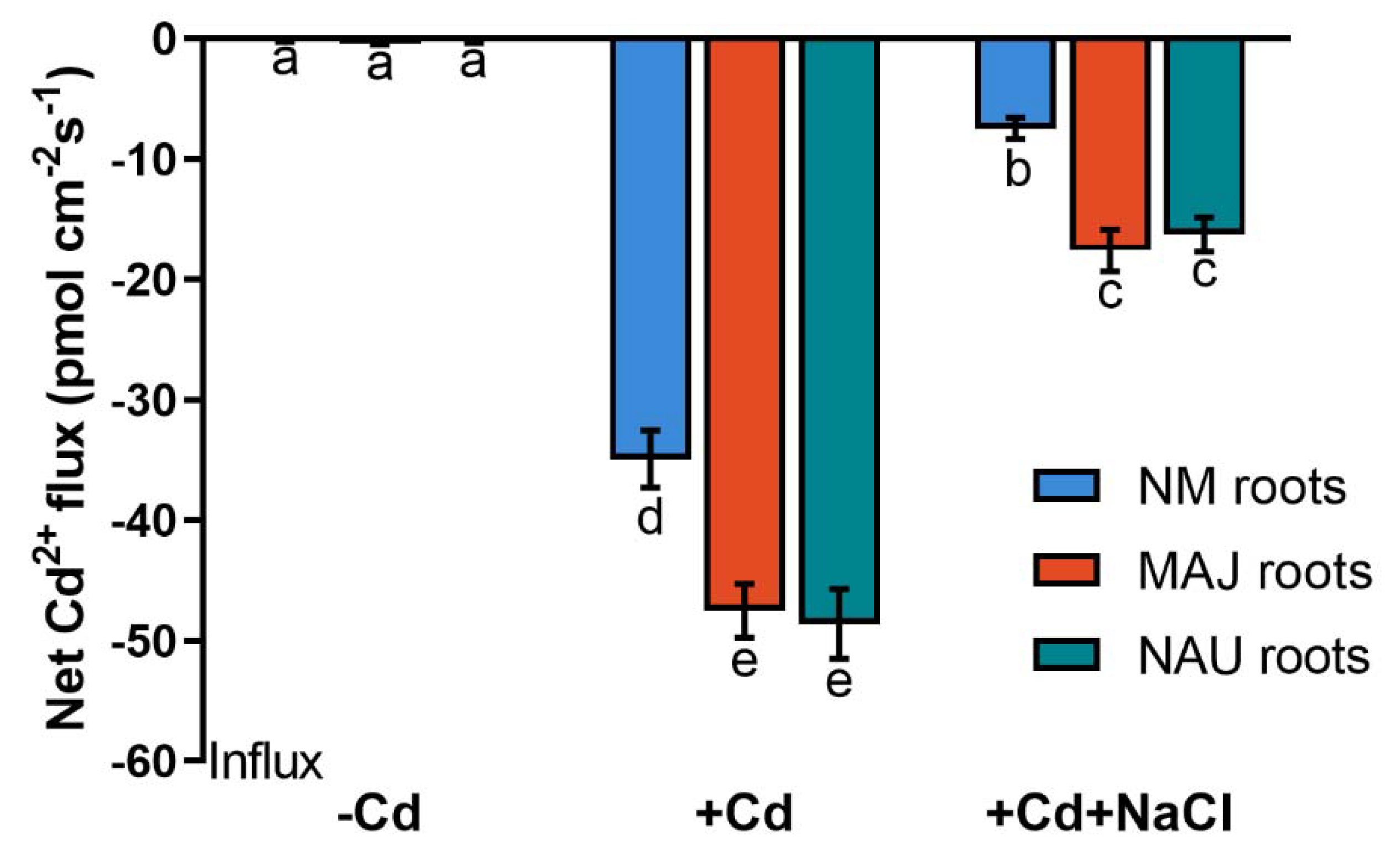
To determine whether the Cd2+ enrichment in EM P. × canescens resulted from the P. involutus-stimulated uptake, Cd2+ fluxes were examined in NM-, EM-roots and fungal mycelia under CdCl2 and NaCl stress. CdCl2 exposure (50 µM, 24 h) resulted in an apparent Cd2+ uptake, 34.9 pmol cm−2 s−1, along NM-roots of the hyperaccumulator, Populus × canescens (Figure 2 and Figure S1). EM-roots exhibited 36% to 39% higher Cd2+ fluxes than the NM-roots (Figure 2). The presence of NaCl (100 mM) significantly decreased the flux rates in both NM- and EM-roots but the EM-roots still exhibited 1.2–1.4-fold greater Cd2+ uptake than the NM-roots (Figure 2 and Figure S1). The effect of NaCl on root Cd2+ fluxes resembles the trend of Cd2+ accumulation in salinized NM- and EM-roots (Figure 1 and Figure 2).
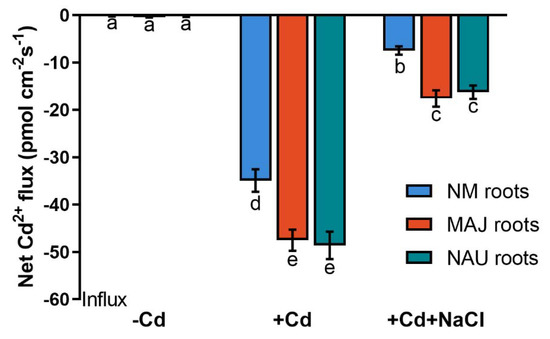
Figure 2.
Steady-state Cd2+ fluxes in non-mycorrhizal (NM) Populus × canescens and ectomycorrhizal (EM) roots under cadmium and salt stress. Poplar plantlets inoculated with or without Paxillus involutus isolates (MAJ or NAU, 30 d), were hydroponically acclimated and subjected to 24 h of CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM). Root tips were excised from EM- and NM-poplars and equilibrated for 30 min in measuring solution. Net fluxes of Cd2+ along root axis (100 to 2300 μm) were monitored at an interval of 200–300 μm (Figure S1). Mean values of Cd2+ fluxes in control (−Cd), CdCl2 stress (+Cd), and combined stress of CdCl2 and NaCl (+Cd + NaCl) are shown. Cd2+ flux was not detectable in salt controls that were treated without CdCl2. Each column is mean ± SD obtained from 5 individual plants. Statistically significant differences (p < 0.05) among treatments are indicated with different letters (a–e).
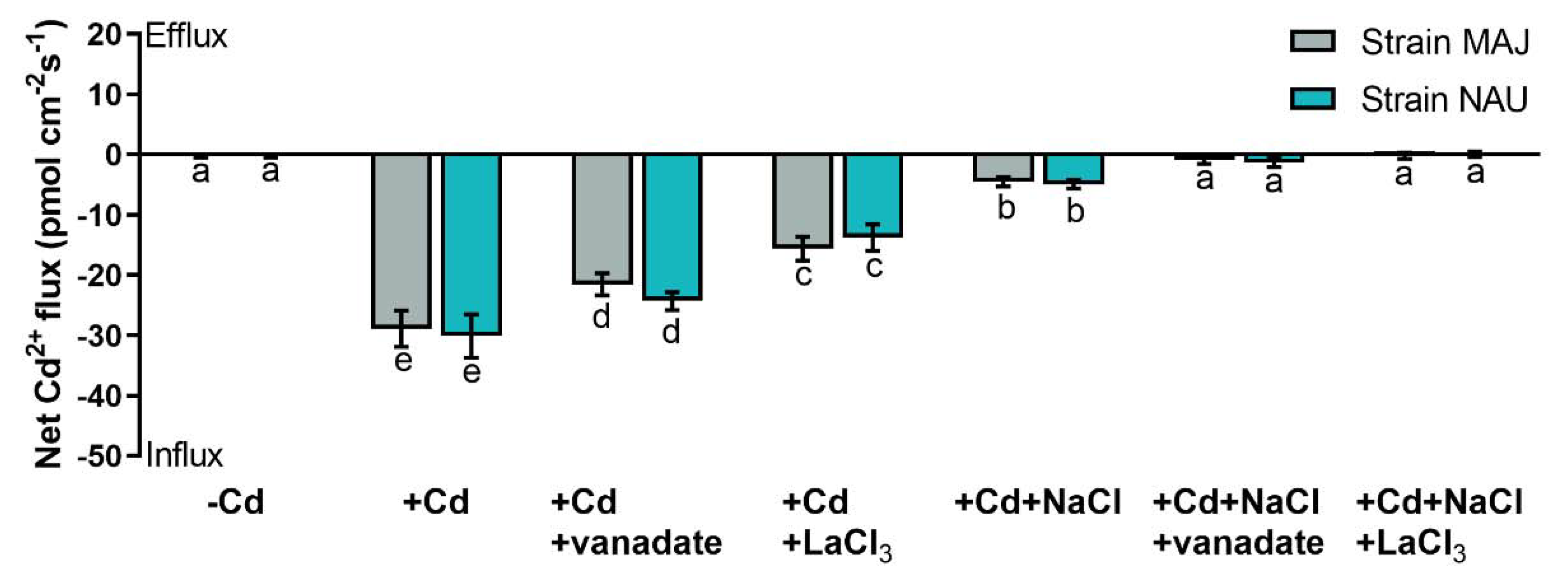
Fungal hyphae of the two tested P. involutus isolates, MAJ and NAU, showed a drastic Cd2+ influx, 28.9–30.1 pmol cm−2 s−1, under CdCl2 treatment (50 µM, 24 h, Figure 3). NaCl reduced the Cd2+ influx by 84–85% in the mycelia (Figure 3), which is similar to the reduction in EM-roots upon salinity stress (Figure 2).
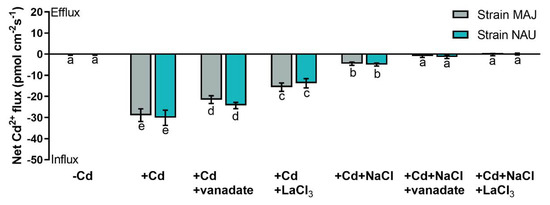
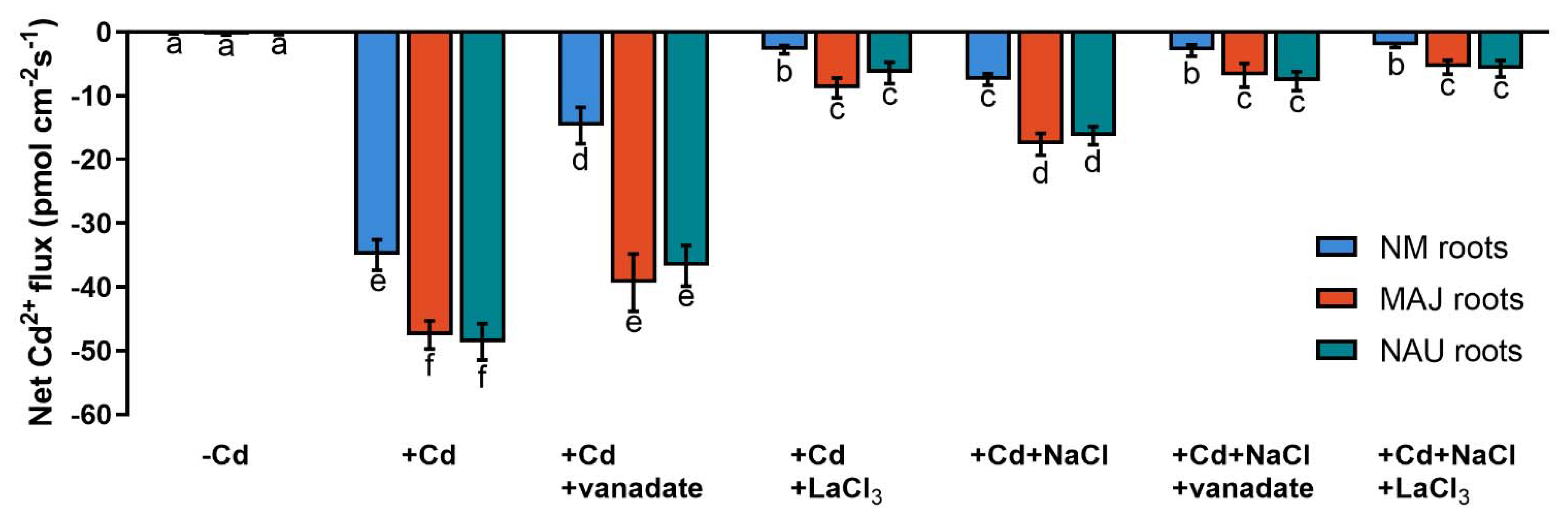
Figure 3.
Net Cd2+ fluxes in fungal hyphae of Paxillus involutus isolates (MAJ and NAU) under cadmium, salt, and inhibitor treatments. MAJ and NAU mycelia (the youngest and active hyphae) were hydroponically acclimated and subjected to 24 h of CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM). The short-term Cd- and Cd + NaCl-stressed fungal mycelia were treated with an inhibitor of plasmalemma H+-ATPase (sodium orthovanadate, 0 or 500 μM) or an inhibitor of Ca2+-permeable channels (LaCl3, 0 or 5 mM) for 30 min. Following 30 min equilibration in measuring solutions, Cd2+ flux recordings were continued for 15 min on the surface of pelleted hyphae. Mean values of Cd2+ fluxes in control (−Cd), CdCl2 stress (+Cd), and combined stress of CdCl2 and NaCl (+Cd + NaCl) in the presence and absence of inhibitors are shown. Cd2+ flux was not detectable in salt controls that were treated without CdCl2. Each column is mean ± SD obtained from 5 fungal cultures. Statistically significant differences (p < 0.05) among treatments are indicated with different letters (a–e).
2.3. Transient Cd2+ Kinetics and Membrane Potential upon Salt Shock
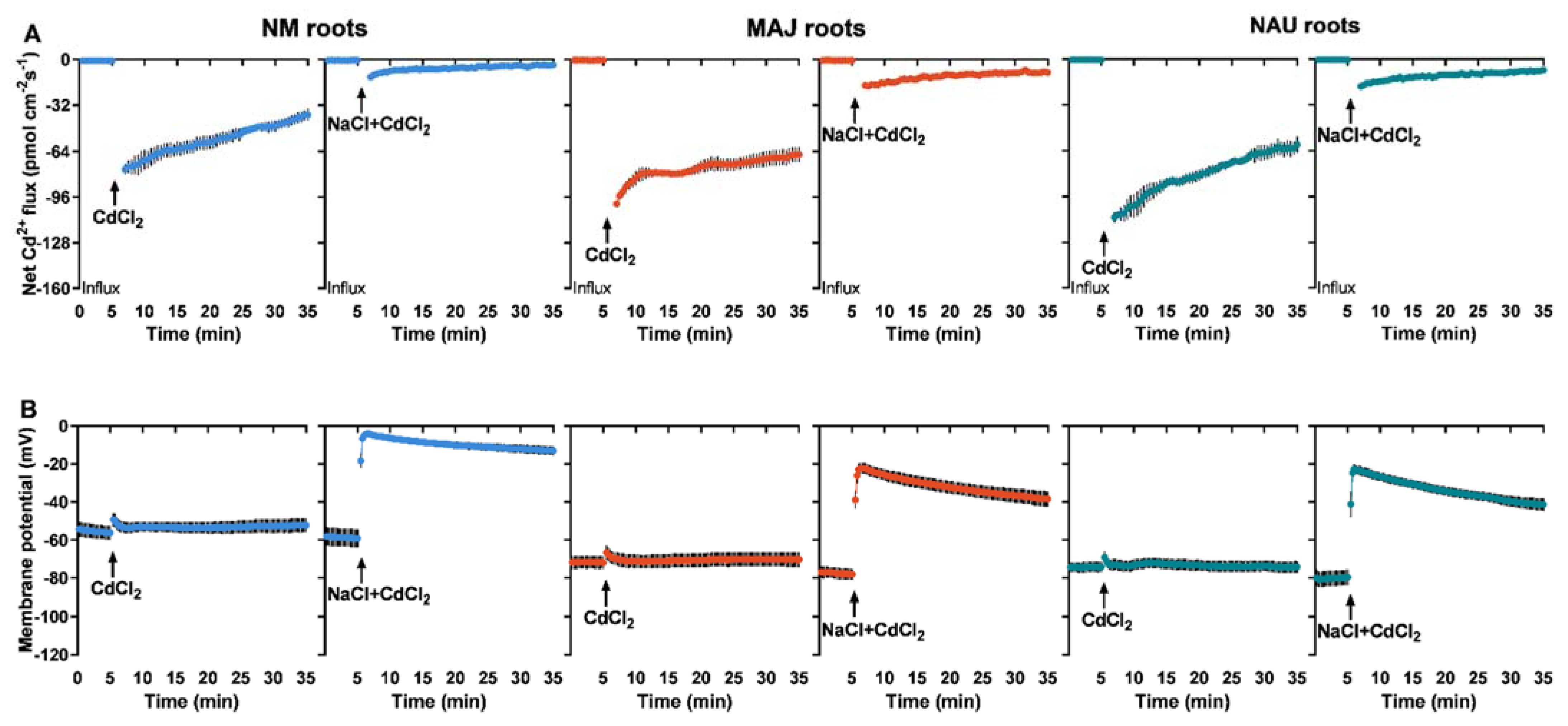
CdCl2 shock (50 µM) created a transient Cd2+ influx in roots of NM P. × canescens, although the flux gradually decreased with prolonged exposure time (Figure 4A). EM-roots exhibited a pattern similar to NM-roots but with typically higher influx rates (Figure 4A). The Cd2+ influxes in both NM- and EM-roots were markedly reduced upon the NaCl addition (Figure 4A), similar to reduction found for the steady-state Cd2+ influx in salinized roots (Figure 2). Compared with the EM-roots, the restriction effect of NaCl was more pronounced in NM-roots (Figure 4A).
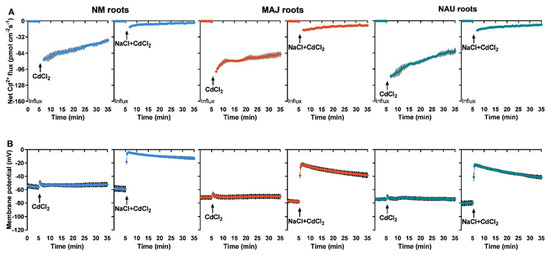
Figure 4.
CdCl2 and NaCl shock-altered Cd2+ kinetics and membrane potential in non-mycorrhizal (NM) Populus × canescens and ectomycorrhizal (EM) roots. (A) Cd2+ flux kinetics. (B) Membrane potential. Poplar plantlets were inoculated with or without Paxillus involutus isolates (MAJ or NAU) for 30 d. Root tips were excised from EM- and NM-poplars and equilibrated for 30 min in Cd2+ or H+ measuring solution. At the apical zones Cd2+ fluxes and membrane potential were recorded before and after the addition of CdCl2 (100 μM) or a combined solution of CdCl2 (50 μM) and NaCl (100 mM). The recordings continued respectively for 5 and 30 min before and after the cadmium and salt shock. Each data point is mean ± SD obtained from 5 individual plants.
Transient kinetics of membrane potential upon CdCl2 (50 µM) and NaCl (100 mM) shocks were compared between roots of NM- and EM-poplars because the membrane potential indicates activity of PM H+-ATPase [66]. NMT recordings showed that the resting membrane potential ranged from −54.4 to −59.2 mV in NM-roots under control conditions (Figure 4B). EM-roots had a more strongly hyperpolarized PM, with a membrane potential ranging from −71.7 to −80.8 mV (Figure 4B). CdCl2 shock exerted no significant effects on the membrane potential in NM- and EM-roots, although a marginal rise (5.0–6.1 mV) was observed after the onset of CdCl2 addition, which returned to the pretreatment level 1–2 min after Cd2+ addition (Figure 4B). However, the addition of NaCl together with CdCl2 caused an immediate and substantial depolarization of the membrane potential in NM- and EM-roots, although the PM tended to be rehyperpolarized during prolonged exposure to NaCl + CdCl2 (Figure 4B). In comparison, the membrane potential in EM-roots was less depolarized (−22.2 to −41.4 mV) after the onset of CdCl2 + NaCl shock as compared to NM-roots (−4.1 to −13.0 mV, Figure 4B).
2.4. Effects of PM H+-ATPase Inhibitor and Activator on Cd2+ Uptake
Cd2+ transport in poplar trees is accelerated by the PM H+-ATPase [38,48,52]. An H+-pump inhibitor, orthovanadate, was used to testify the crucial role of H+-pumps for Cd2+ uptake in NM-, EM-roots, and fungal hyphae under CdCl2 and salt stress. In NM-roots, orthovanadate decreased the Cd2+ influx approximately two-fold, while in EM-roots only 17–25% decreases were found (Figure 5 and Figure S2). In mycelia, vanadate also caused moderately reduced Cd2+ influx (Figure 3). In the presence of NaCl, the inhibition of orthovanadate was evident in the fungus and roots, although the Cd2+ influx had been significantly lowered by the salt treatment (Figure 3, Figure 5, and Figure S2).
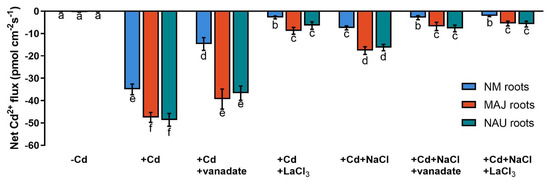
Figure 5.
Net Cd2+ fluxes in non-mycorrhizal (NM) Populus × canescens and ectomycorrhizal (EM) roots under cadmium, salt, and inhibitor treatments. Poplar plantlets inoculated with or without Paxillus involutus isolates (MAJ or NAU, 30 d), were hydroponically acclimated and subjected to 24 h of CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM). Root tips were excised from EM- and NM-poplars and subjected to an inhibitor of plasmalemma H+-ATPase (sodium orthovanadate, 0 or 500 μM) or an inhibitor of Ca2+-permeable channels (LaCl3, 0 or 5 mM) for 30 min. Following 30 min equilibration in measuring solutions, net fluxes of Cd along root axis (100 to 2300 μm) were monitored at an interval of 200–300 μm (Figures S2 and S3). Mean values of Cd2+ fluxes in control (−Cd), CdCl2 stress (+Cd), and combined stress of CdCl2 and NaCl (+Cd + NaCl) in the presence and absence of inhibitors are shown. Cd2+ flux was not detectable in salt controls that were treated without CdCl2. Each column is mean ± SD obtained from 5 individual plants. Statistically significant differences (p < 0.05) among treatments are indicated with different letters (a–f).
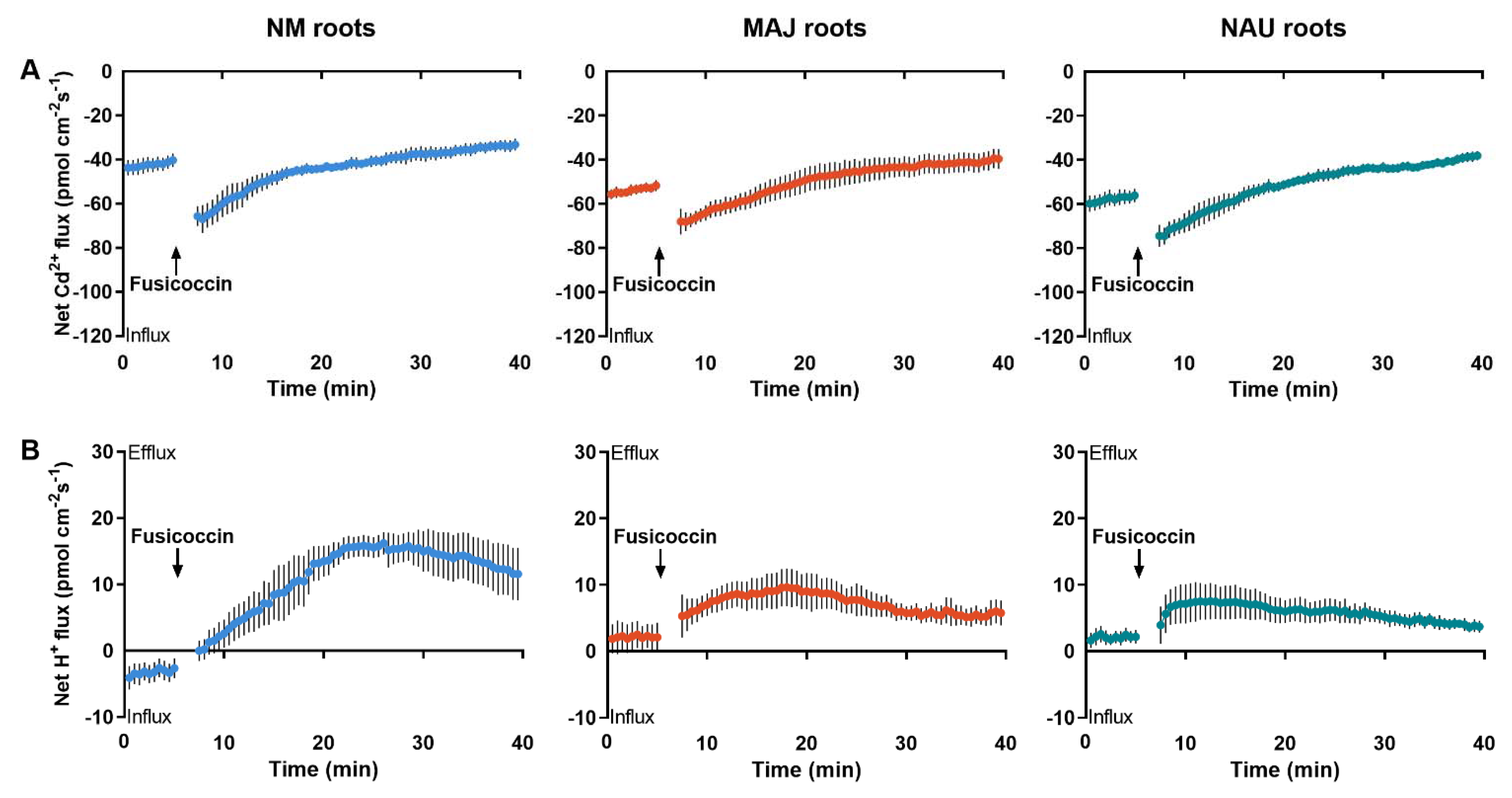
Furthermore, the activator of PM H+-ATPase, fusicoccin (FC), was used to test the effect of H+ pumping on Cd2+ uptake in short-term stressed roots. Following the CdCl2 treatment (50 µM, 24 h), roots of NM- and EM-poplars were subjected to FC activation. Immediately after the onset of FC addition, a stimulation of Cd2+ influxes was observed at the surface of NM- and EM-roots (Figure 6A). H+ efflux was correspondingly increased in FC-treated NM- and EM-roots (Figure 6B), indicating that H+ pumps were transiently activated [84,85,86,87]. The observation that the increase in H+ efflux corresponded to the Cd2+ influx in P. × canescens roots suggests that the uptake of Cd2+ was promoted by the H+-ATPases in the PM.
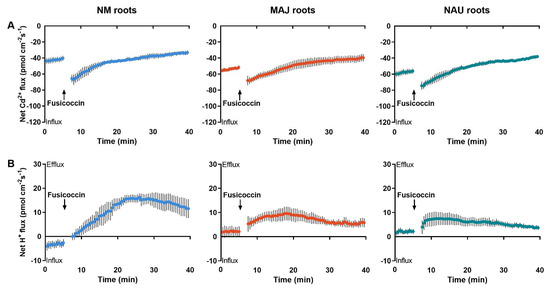
Figure 6.
Fusicoccin shock-altered Cd2+ and H+ kinetics in non-mycorrhizal (NM) Populus × canescens and ectomycorrhizal (EM) roots. (A) Cd2+ flux kinetics. (B) H+ flux kinetics. Poplar plantlets inoculated with or without Paxillus involutus isolates (MAJ or NAU, 30 d) were hydroponically acclimated and subjected to 24 h of CdCl2 (50 μM). Root tips were excised from EM- and NM-poplars and equilibrated for 30 min in Cd2+ or H+ measuring solution. At the apical zones, Cd2+ and H+ fluxes were recorded before and after the addition of fusicoccin (10 μM). The recordings continued, respectively, for 5 and 35 min before and after fusicoccin shock. Each data point is mean ± SD obtained from 5 individual plants.
2.5. Transcriptional Activation of H+-ATPase in Ectomycorrhizal P. × canescens
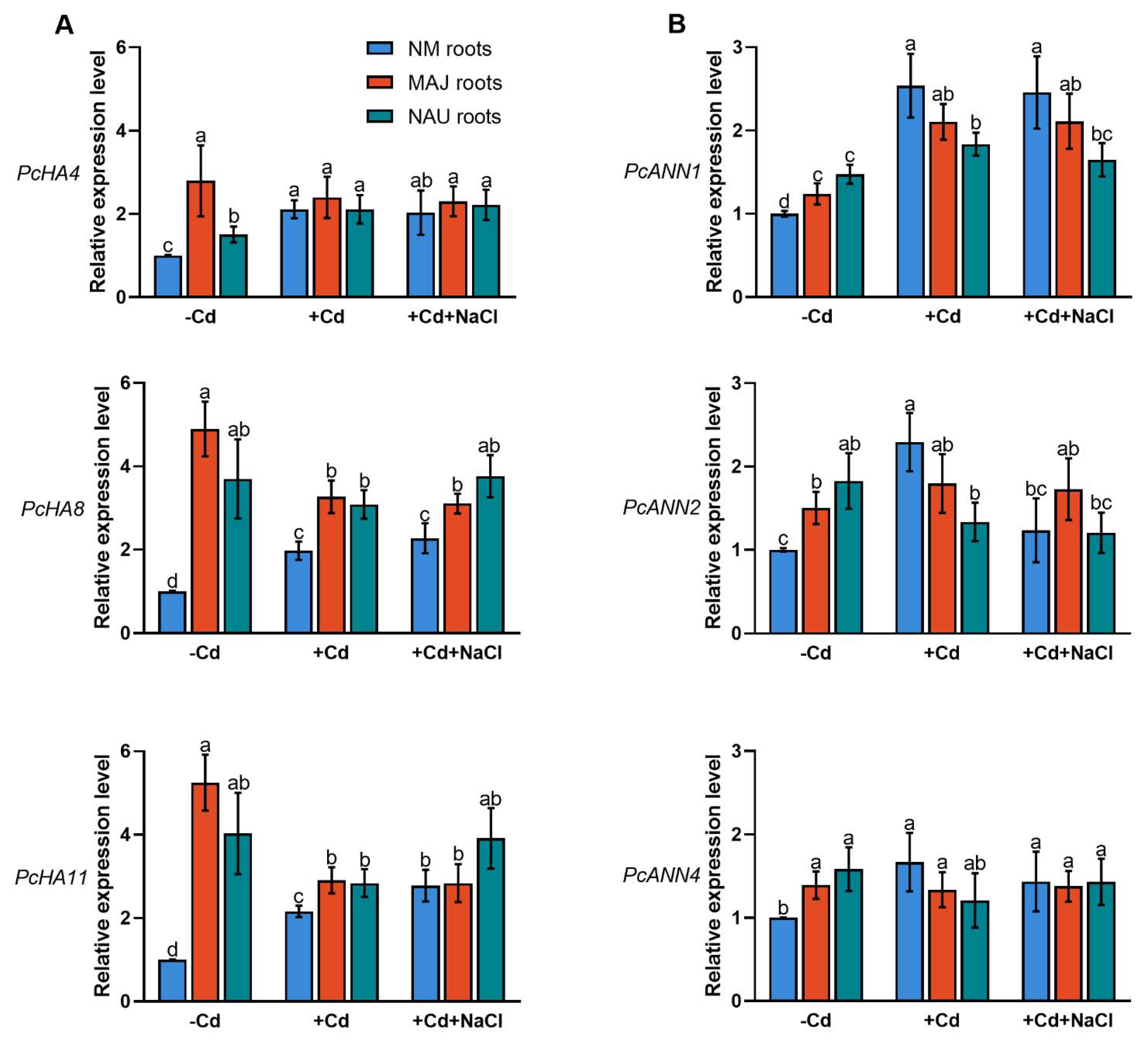
Transcript levels of the PM H+-ATPase-encoding genes, PcHA4, PcHA8 and PcHA11, were examined in NM and EM roots since these three PcHAs were previously shown to be differently expressed under control and Na+ stress conditions [66]. EM-roots showed significantly higher (0.5–4.2 fold) transcript levels of PcHA4, PcHA8 and PcHA11 than NM-roots (Figure 7A). This observation agrees with Sa et al. (2019) [66]. Cadmium treatment (50 µM CdCl2, 24 h) resulted in upregulation of PcHA4, PcHA8, and PcHA11 in NM-roots (Figure 7A). In contrast, Cd2+ caused a 14–45% decline of PcHAs in EM-roots, with the exception of PcHA4 in NAU roots (Figure 7A). It is notable that the transcript levels, in particular those of PcHA8, and PcHA11, still remained higher in the EM- than in NM-roots, despite the decline caused by Cd2+ stress (Figure 7A).
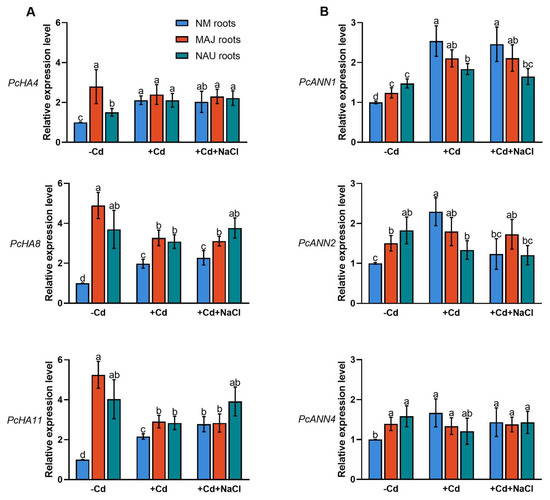
Figure 7.
Effects of CdCl2 as single stress factor or in combination with NaCl on transcriptional profiles of plasmalemma H+-ATPase (PcHAs) and annexins (PcANNs) in roots of non-mycorrhizal or ectomycorrhizal (EM) Populus × canescens. (A) PcHA4, 8, 11. (B) PcANN1, 2, 4. Poplar plantlets inoculated with or without Paxillus involutus isolates (MAJ or NAU, 30 d), were hydroponically acclimated and subjected to 24 h of CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM). Roots were harvested from EM- and NM-poplars and used for total RNA isolation and RT-qPCR. 18S rRNA was used as a reference gene. Specific primers designed to target PcHA4, 8, 11, PcANN1, 2, 4 and 18S rRNA are shown in Table S1. Mean values of PcHAs and PcANNs relative transcript levels in control (−Cd), CdCl2 stress (+Cd), and combined CdCl2 and NaCl stress (+Cd + NaCl) are shown. Each column is mean ± SD obtained from 3 independent experiments. Statistically significant differences (p < 0.05) among treatments are indicated with different letters (a–d).
NaCl treatment (100 mM, 24 h) lowers the transcript levels of PcHAs (4, 8, 11) in NM-roots [66]. Here, exposure to NaCl of the Cd2+-treated roots did not result in decreased PcHA4 and PcHA8 transcript levels and an increase of PcHA11 was observed (Figure 7A). Similarly, NaCl did not significantly change PcHAs transcription in EM-roots in the presence of Cd2+ (Figure 7A). We noticed that EM-roots retained overall higher transcript levels of PcHAs than NM-roots under co-occurring stresses of cadmium and salinity.
2.6. Calcium Channel Inhibitor Blocks Cd2+ Fluxes
Cadmium ions enter the plasma membrane through CaPCs in plant cells [48,79,88]. To determine whether CaPCs contributed to the mediation of Cd2+ influx under combined CdCl2 and NaCl stress, LaCl3 was used to block Ca2+-channels in the roots of NM- and EM-poplars. The inhibitor significantly decreased root Cd2+ uptake in the presence and absence of NaCl, although NaCl treatment reduced the apparent Cd2+ influx under coexisting stress (Figure 5 and Figure S3). Similarly, the LaCl3 significantly reduced Cd2+ uptake in fungal hyphae regardless of the NaCl addition (Figure 3).
2.7. Transcript Levels of Annexin Genes in Ectomycorrhizal P. × canescens
Plant annexins (ANNs), such as ANN1, ANN2, ANN4, function as Ca2+-permeable channels in higher plants [70,71,72,73,74,75,76,79,89]. We have shown that P. euphratica PeANN1 facilitates cadmium enrichment by regulation of calcium-permeable channels [79]. Here, we examined the P. × canescens orthologs PcANN1, PcANN2 and PcANN4 in NM- and EM-roots. In the absence of Cd and salt, PcANN1, PcANN2 and PcANN4 showed significantly higher transcripts in EM-roots than in the NM (Figure 7B). This observation is in accord with previous findings that EM-roots retain typically higher influx of Ca2+ than NM-roots [64,65]. Short-term cadmium exposure (50 µM, 24 h) caused significant increases of PcANN transcript levels in NM roots (Figure 7B), supporting Cd2+ enrichment in the woody hyperaccumulator [29,33,38,52]. The Cd2+ stimulation of annexin transcript levels was less pronounced in EM-roots (Figure 7B). For example, PcANN1 levels which increased by 25–70% in MAJ and NAU roots under Cd2+ treatment were still lower than those in CdCl2-treated NM-roots (Figure 7B). The PcANN2 responded differently to short-term cadmium exposure in the EM-roots colonized with the strain MAJ (increase) and the strain NAU (decrease) (Figure 7B). Cadmium exposure also slightly decreased PcANN4 in EM-roots (4–24%, Figure 7B). In CdCl2-stressed NM roots, NaCl lowered the transcripts of PcANNs by 3–46% (Figure 7B). As a result, the cadmium stimulation of annexin genes (with the exception of PcANN1) was lost by the addition of NaCl (Figure 7B). Compared to NM-roots, PcANNs was either less (PcANN1, PcANN2) or not reduced (PcANN4) by NaCl in EM-roots under cadmium treatment (Figure 7B).
3. Discussion
3.1. The P. Involutus-Activated PM H+-ATPase Contributes to Cd2+ Enrichment in EM Roots
Our data show that the woody hyperaccumulator, P. × canescens, exhibited strong Cd2+ uptake and accumulation in root and shoots, which is further enhanced by colonizing with EM-fungus P. involutus (Figure 1). These findings are similar to previous reports in long-term studies [29,33,48,52]. The root flux recordings confirmed that the enhanced Cd2+ entry in P. × canescens roots was due to the colonization with MAJ and NAU isolates, which were characterized by a remarkable Cd2+ enrichment in the hyphae (Figure 2, Figure 3, and Figure S1) [48,90]. However, we observed that salt stress caused by NaCl reduced the Cd2+ influx in roots and fungus (Figure 2, Figure 3, and Figure S1). Similarly, NaCl reduced root cadmium uptake and translocation in the halophyte Carpobrotus rossii [7,8] and Atriplex halimus [91]. An important novel result was that the P. involutus could alleviate the salt suppression of Cd2+ uptake in P. × canescens roots (Figure 2, Figure 4, and Figure S1). To obtain a mechanistic understanding of the underlying processes, we inhibited and stimulated the Cd2+ fluxes with pharmacological agents. The entry of Cd2+ in the roots and fungal hyphae declined when the plasmalemma H+-ATPase was inhibited by vanadate (Figure 3, Figure 5, and Figure S2) [48] and increased when the plasmalemma H+-ATPase was stimulated by FC (Figure 6). These data suggest that Cd2+ uptake required a proton gradient [48,52]. Moreover, P. involutus colonization resulted in a higher H+ efflux and correspondingly a more negative membrane potential (Figure 6), indicating that the PM H+-ATPases were activated by the ectomycorrhiza [48,64,66]. This is similar to the enhanced proton-ATPase in arbuscular-mycorrhizal symbiosis [92,93]. The highly activated H+-pumps hyperpolarize the PM, thereby facilitating Cd2+ influx via hyperpolarization-activated CaPCs [48,73]. In accordance with our flux analyses, transcript levels of the PM H+-ATPase-encoding genes, PcHA4, PcHA8, PcHA11, generally remained at higher levels in ectomycorrhizal roots under control and CdCl2 stress compared to NM P. × canescens roots, although two or three of the tested PcHAs were down-regulated by CdCl2 in MAJ and NAU roots (Figure 7). Of note, EM-roots maintained higher transcripts of PcHA4, 8, 11 than non-colonized roots under combined stress of CdCl2 and NaCl (Figure 7). Similarly, Sa et al. showed that both MAJ and NAU roots retain higher transcript levels of PcHA4 and/or PcHA8 than NM-roots under control and NaCl stress conditions [66]. Increased abundances of PM H+-ATPase transcripts are expected to contribute to the activated H+-pumps because the plasmalemma H+-ATPases are transcriptionally regulated in poplars [85,86,94]. Thus, the retained H+-pumping activity resulted in less depolarization of membrane potential under NaCl stress (Figure 4) [66], thereby upkeeping Cd2+ influx into the EM-roots. This result concurs with those of Ma et al. (2014), who found that upregulation of HA2.1 and AHA10.1 leads to Cd2+ uptake in EM poplar roots [52].
3.2. The Fungus-Elicited Annexins Mediated Cd2+ Uptake in EM Roots
Since LaCl3 inhibited Cd2+ uptake into roots and fungal hyphae, our results support that Cd2+ uptake involves CaPCs in the PM (Figure 3, Figure 5, and Figure S3) [41,48,68,73,88]. Plant annexins, in particular ANN1, ANN2 and ANN4, have been shown to function as CaPCs in Arabidopsis, maize and rice [70,71,72,73,75,76]. Zhang et al. suggested that PeANN1 facilitates the flow of cadmium ions through CaPCs [79]. CdCl2 treatment upregulated transcripts of PcANN1, PcANN2 and PcANN4 in roots of NM P. × canescens (Figure 7), similar to the findings in crop species, such as maize, peanut and rice [73,80,81]. Accordingly, the cadmium-elicited annexins might mediate root Cd2+ inflow through CaPCs in the poplar, contributing to its hyperaccumulator character [33]. Noteworthy, PcANN1, PcANN2 and PcANN4 showed remarkably higher transcripts in EM-roots than in the non-colonized under control conditions; CdCl2 treatment caused a further increase in PcANN1 in EM-roots and PcANN2 was specifically increased in MAJ-colonized roots (Figure 7). The arbuscular mycorrhiza-stimulated transcription of GmAnn1a was observed in soybean roots [95]. In addition, annexin proteins also showed enhanced accumulation in arbuscular mycorrhizal roots of Medicago sativa and M. truncatula following cadmium application [96,97]. Therefore, the fungus-induced annexins might have collectively contributed to the CaPCs-mediated Cd2+ enrichment in root cells of the poplar [73,79]. In accordance with this notion, we have previously shown that Paxillus-colonized roots showed higher Ca2+ and Cd2+ influxes than NM-roots [48,64,65]. We noticed that the transcripts of PcANN1, 2, 4 in EM-roots exhibited lower levels than non-colonized roots under CdCl2 stress (Figure 7). However, root Cd2+ influx remained higher in EM than in NM (Figure 2 and Figure 4). Thus, it can be inferred that the annexin-mediated uptake of Cd2+ was mainly promoted by the electrochemical gradient across the PM that was established by H+-ATPases. NaCl decreased PcANN2 and PcANN4 in NM-roots, but the transcript levels of PcANN1, 2, 4 were less reduced by NaCl in EM-roots (Figure 7). It is worth noting that in these ectomycorrhizal roots, transcription of PcHAs was retained at high levels under cadmium and salinity stress (Figure 7). Taken together, this suggests that the fungus-stimulated transcription of annexins contributed to Cd2+ enrichment in EM-roots under combined stresses of cadmium and salt.
4. Materials and Methods
4.1. Fungal Inoculation with Populus × canescens
The two isolates of EM fungus P. involutus (MAJ and NAU) from Büsgen-Institute: Forest Botany and Tree Physiology (Göttingen University, Büsgenweg 2, Göttingen, Germany) were cultured on modified Melin Norkrans medium [83]. P. × canescens plantlets were micropropagated and rooted in modified Murashige and Skoog (MMS) medium [98]. Uniform and healthy plantlets were inoculated with MAJ or NAU for 30 d using a Petri-dish culture system [99].
4.2. Cadmium and NaCl Treatment
The agar plugs with hyphae, and plants colonized with or without EM fungus, were hydroponically acclimated in MMS nutrient solution for 2–3 d [66]. Then fungal mycelia, NM- and EM-plants were treated with CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM) in MMS solution. Following 24 h of CdCl2 treatment and combined stress of CdCl2 and NaCl, steady-state Cd2+ fluxes were recorded in fungal mycelia, NM- and EM-roots. Transcript levels of genes encoding annexins (PcANN1, 2, 4) and PM H+-ATPases (PcHA4, 8, 11) were examined in control and stressed roots.
4.3. Inhibitor and Activator Treatment
The fungal mycelia, NM-, and EM-roots pretreated with short-term CdCl2 or CdCl2 + NaCl were exposed to inhibitors of Ca2+ channels (LaCl3, 0 or 5 mM) [48,100] or PM H+-ATPases (sodium orthovanadate, 0 or 500 μM) [100,101] for 30 min. Steady-state Cd2+ fluxes were recorded on the surface of roots and pelleted hyphae, respectively [48].
After 24 h exposure to 50 μM CdCl2, roots from NM- and EM-poplars were subjected to an activator of PM H+-ATPase, Fusicoccin (FC). FC produced by Fusicoccum amygdali, has the function of activating H+-ATPase in the PM [102,103]. Cd2+ and H+ transient kinetics were continuously recorded for 35 min after FC (10 μM) were added to measuring solutions.
4.4. Assessed of Cd2+ Concentrations
After 24 h exposure to CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM), roots, stems and leaves of NM- and EM-poplars were sampled and oven dried at 70–80 °C for 5 d. Dried samples was weighed 0.1 g and digested in 5 mL of concentrated HNO3 and 2 mL 30% H2O2 in a microwave accelerated reaction system (Titan MPS Microwave Sample Preparation System, Perkin-Elmer, Waltham, MA, USA). Concentrations of Cd2+ were assessed by a PerkinElmer Optima 8000 ICP-OES Spectrometer (Perkin-Elmer, Waltham, MA, USA).
4.5. Flux Recordings of Cd2+ and H+
4.5.1. Microelectrodes Preparation and Calibration
Cd2+ and H+ flux profiles were recorded using an NMT system (NMT-YG-100, Younger USA LLC, Amherst, MA, USA). The glass microelectrodes were prepared as previously described [42,43,48,84,104]. Prior to flux recordings, the calibration of Cd2+- and H+-selective microelectrodes were carried out in the following standards (concentrations in mM):
- (a)
- H+ microelectrodes: 0.1 NaCl, 0.1 CaCl2, 0.1 MgCl2, and 0.5 KCl, pH 4.5, 5.5, and 6.5 (pH was adjusted to 5.3 during H+ flux recordings); and
- (b)
- Cd2+ microelectrodes: 0.05 CaCl2, 0.1 MgCl2, 0.5 KCl, 0 or 100 NaCl, and CdCl2 series (0.01, 0.05, and 0.1), pH 5.3 (Cd2+ concentration was 0.05 mM during Cd2+ flux recordings).
After calibration, the microelectrodes that showed Nernstian slopes of 58 ± 6 mV/decade (H+) and 29 ± 4 mV/decade (Cd2+) were used in our NMT recordings.
4.5.2. Steady-State Cd2+ Flux Recordings
After 24 h exposure to CdCl2 (0 or 50 μM) in combination with NaCl (0 or 100 mM), sodium orthovanadate (0 or 500 μM), and LaCl3 (0 or 5 mM), fungal mycelia and root tips excised from NM- and EM-poplars were subjected to 30 min equilibration in the following measuring solutions (concentrations in mM), respectively:
- (i)
- Control (−Cd): 0.05 CaCl2, 0.1 MgCl2, 0.5 KCl, pH 5.3;
- (ii)
- +Cd: 0.05 CaCl2, 0.05 CdCl2, 0.1 MgCl2, 0.5 KCl, pH 5.3; and
- (iii)
- Cd+NaCl: 0.05 CaCl2, 0.05 CdCl2, 0.1 MgCl2, 0.5 KCl, 100 NaCl, pH 5.3.
Following equilibration, net fluxes of Cd2+ along root axis (100 to 2300 μm) were monitored at an interval of 200–300 μm. The flux recording at each point was continued for 6–8 min [41,64,101,105]. For the fungal mycelia, Cd2+ flux recording of pelleted hyphae was continued 15 min [48]. Cd2+ fluxes were recorded from at least five individual plants or fungal cultures for each treatment. The flux oscillations in EM fungus and poplars are not so pronounced as that observed in crop seedlings [48,106].
4.5.3. Transient Recordings of Cd2+, H+ Flux and Membrane Potential
Transient Cd2+ Kinetics and Membrane Potential.
NM- and EM-roots were incubated in basic solutions of Cd2+ (concentration in mM: 0.05 CaCl2, 0.1 MgCl2, 0.5 KCl, pH 5.3) and H+ (0.1 CaCl2, 0.1 MgCl2, 0.1 NaCl, 0.5 KCl, pH 5.3) for 30 min. Cd2+ fluxes and membrane potentials at apical regions were recorded for 5 min prior to CdCl2 and NaCl shocks. Membrane potential was measured using Ag/AgCl microelectrodes (XY-CGQ03; Xuyue (Beijing) Sci and Tech Co. Ltd., Suzhou street 49, Haidian District, Beijing, China) as previously described [66]. Then, CdCl2 (100 μM) stock, or a combined stock solution of CdCl2 (100 μM) and NaCl (200 mM) was added slowly to reach final concentrations of 50 μM (CdCl2) and 100 mM (NaCl). Kinetics of membrane potential and Cd2+ uptake were recorded up to 30 min in NM- and EM-roots. Cd2+ fluxes and membrane potentials were recorded from at least five individual plants for each treatment.
Transient Kinetics of Cd2+ and H+ upon FC.
The NM- and EM-roots pretreated with CdCl2 (50 μM, 24 h) were excised and equilibrated in measuring solutions of Cd2+ or H+ for 30 min. Fluxes of Cd2+ and H+ at apical regions were recorded for 5 min before the addition of FC (Sigma-Aldrich, St. Louis, MO, USA). Then, FC stock solution (dissolved in DMSO) was added to Cd2+ and H+ measuring solutions, reaching a final concentration of 10 μM [103]. Cd2+ and H+ transient kinetics in FC-treated roots were further recorded for 35 min. Fluxes of Cd2+ and H+ were recorded from at least five individual plants for NM-, MAJ- and NAU-roots.
4.6. Determination of Gene Expression of Annexins and PM H+-ATPases
After 24 h exposure to CdCl2 (0 or 50 μM), or to CdCl2 (50 μM) in combination with NaCl (100 mM), total RNA was isolated from NM and fungus-colonized roots and used for real-time quantitative PCR (RT-qPCR) [66]. The primer sequences for annexins (PcANN1, 2, 4) [79], plasmalemma H+ ATPase (PcHAs, PcHA4, 8, 11) [66], and reference genes (18S rRNA) [107], are shown in Table S1. The RT-qPCR amplification was performed as previously described [66,79,86]. Expression profiles for PcANNs and PcHAs were normalized to the transcripts of 18S rRNA [108]. The RT-qPCR experiment was repeated three times.
4.7. Data Analysis
The calculations of flux rate and membrane potential were processed using JCal V3.2.1 program (Xuyue (Beijing) Sci and Tech Co. Ltd., Suzhou street 49, Haidian District, Beijing, China, Available online: http://www.xuyue.net/, accessed on 12 March 2021). All experimental data were subjected to SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Differences between means were considered significant at p < 0.05.
5. Conclusions
Our data provide further evidence that cadmium can be enriched in ectomycorrhizal poplars under co-existing stress conditions of Cd2+ and NaCl. P. involutus stimulated Cd2+ influx through CaPCs in ectomycorrhizal P. × canescens roots, depending on the plasmalemma H+-ATPase. NaCl lowered the uptake of Cd2+ in poplar roots, which was alleviated by ectomycorrhization with P. involutus. Ectomycorrhizal fungus colonization upregulated transcription of PM H+-ATPases (PcHA4, 8, 11) and increased transcripts of annexins (PcANN1, 2, 4), which might mediate Cd2+ conductance through PM CaPCs. NaCl-treated EM-roots retained relatively highly expressed PcHAs and PcANNs. We hypothesize that the sustained transcription of PcHAs resulted in H+ pumping activity and PM hyperpolarization in the ectomycorrhiza, thus promoting Cd2+ enrichment through the PcANNs-mediated Ca2+ channels in EM-roots under co-occurring stresses of cadmium and salinity. Although the colonization of MAJ and NAU varies with regard to the formation of intraradical hyphae, i.e., the Hartig net, both strains conserved higher Cd2+ uptake under salt stress than NM-roots. We propose that P. involutus strains, which have been repeatedly shown to improve salt tolerance, may be applied as beneficial microbes to improve plant phytoremediation for cadmium in salt-affected areas.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222111651/s1.
Author Contributions
C.D.: Investigation, Data curation, Visualization, Writing-original draft preparation. Z.Z., Investigation, Data curation, Visualization, Writing-original draft preparation. J.L.: Investigation, Data curation, Visualization. Y.Z. (Ying Zhang): Investigation, Visualization, Data curation. Y.Z. (Yinan Zhang): Investigation, Visualization. D.Y.: Visualization, Validation. S.H.: Investigation, Data curation. Y.Z. (Yanli Zhang): Software, Validation. J.Y.: Methodology, Software. H.Z.: Methodology, Software. N.Z.: Methodology, Visualization. G.S.: Visualization, Validation. Y.Z. (Yuhong Zhang): Visualization, Validation. X.M.: Visualization. R.Z.: Methodology, Software. A.P.: Conceptualization, Resources, Writing-reviewing & editing, Project administration. S.C.: Conceptualization, Supervision, Writing-reviewing & editing, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers 31770643, 32071730), Beijing Advanced Innovation Center for Tree Breeding by Molecular Design, Beijing Municipal Education Commission (China), the Alexander von Humboldt-Stiftung (Germany), the Guest Lecturer Scheme of Göttingen University (Germany), and travel grants by the Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz (BMELV) (Germany).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
We thank Jinke Li (The Plat form of Large Instruments and Equipment, Beijing Forestry University) for allowing and assisting the use of ICP-OES.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Nawrot, T.; Plusquin, M.; Hogervorst, J.; Roels, H.A.; Celis, H.; Thijs, L.; Vangronsveld, J.; Van Hecke, E.; Staessen, J.A. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol. 2006, 7, 119–126. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, O.; Ince, M.; Yaman, M. Sequential extraction of cadmium in different soil phases and plant parts from a former industrialized area. Environ. Chem. Lett. 2011, 9, 397–404. [Google Scholar] [CrossRef]
- Luo, Z.B.; He, J.L.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef]
- Shi, W.G.; Zhang, Y.H.; Chen, S.L.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef]
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef]
- Cheng, M.M.; Kopittke, P.M.; Wang, A.A.; Tang, C.X. Salinity decreases Cd translocation by altering Cd speciation in the halophytic Cd-accumulator Carpobrotus rossii. Ann. Bot. 2018, 123, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.M.; Wang, A.A.; Liu, Z.Q.; Gendall, A.R.; Rochfort, S.; Tang, C.X. Sodium chloride decreases cadmium accumulation and changes the response of metabolites to cadmium stress in the halophyte Carpobrotus rossii. Ann. Bot. 2018, 122, 373–385. [Google Scholar] [CrossRef]
- Guo, S.H.; Jiang, L.Y.; Xu, Z.M.; Li, Q.S.; Wang, J.F.; Ye, H.J.; Wang, L.L.; He, B.Y.; Zhou, C.; Zeng, E.Y. Biological mechanisms of cadmium accumulation in edible Amaranth (Amaranthus mangostanus L.) cultivars promoted by salinity: A transcriptome analysis. Environ. Pollut. 2020, 262, 114304. [Google Scholar] [CrossRef]
- Hao, L.T.; Chen, L.H.; Zhu, P.; Zhang, J.; Zhang, D.J.; Xiao, J.J.; Xu, Z.F.; Zhang, L.; Liu, Y.; Li, H.; et al. Sex-specific responses of Populus deltoides to interaction of cadmium and salinity in root systems. Ecotoxicol. Environ. Saf. 2020, 195, 110437. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef]
- Nosek, M.; Kaczmarczyk, A.; Jędrzejczyk, R.J.; Supel, P.; Kaszycki, P.; Miszalski, Z. Expression of genes involved in heavy metal trafficking in plants exposed to salinity stress and elevated Cd concentrations. Plants 2020, 9, 475. [Google Scholar] [CrossRef]
- Pastuszak, J.; Kopeć, P.; Płażek, A.; Gondek, K.; Szczerba, A.; Hornyák, M.; Dubert, F. Cadmium accumulation in the grain of durum wheat is associated with salinity resistance degree. Plant Soil Environ. 2020, 66, 257–263. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Kamińska, I.; Hanus-Fajerska, E.; Sliwinska, E.; Koźmińska, A. Distinct co-tolerance responses to combined salinity and cadmium exposure in metallicolous and non-metallicolous ecotypes of Silene vulgaris. Ecotoxicol. Environ. Saf. 2020, 201, 110823. [Google Scholar] [CrossRef]
- Zhang, S.L.; Ni, X.L.; Arif, M.; Yuan, Z.X.; Li, L.J.; Li, C.X. Salinity influences Cd accumulation and distribution characteristics in two contrasting halophytes, Suaeda glauca and Limonium aureum. Ecotoxicol. Environ. Saf. 2020, 191, 110230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Ni, X.L.; Arif, M.; Zheng, J.; Stubbs, A.; Li, C.X. NaCl improved Cd tolerance of the euhalophyte Suaeda glauca but not the recretohalophyte Limonium aureum. Plant Soil 2020, 449, 303–318. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Bao, J.J.; Liu, J.H.; Zheng, J.L. High salinity acclimatization alleviated cadmium toxicity in Dunaliella salina: Transcriptomic and physiological evidence. Aquat. Toxicol. 2020, 223, 105492. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Mao, J.H.; Shen, W.R. Reflection of soil salination pollution research and landuse of Binhai area in Tianjin. Tianjin Agric. Sci. 2005, 11, 15–17. [Google Scholar]
- Amari, T.; Ghnaya, T.; Debez, A.; Taamali, M.; Youssef, N.B.; Lucchini, G.; Sacchi, G.A.; Abdelly, C. Comparative Ni tolerance and accumulation potentials between Mesembryanthemum crystallinum (halophyte) and Brassica juncea: Metal accumulation, nutrient status and photosynthetic activity. J. Plant Physiol. 2014, 171, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Tian, C.Y.; Jiang, L.; Wang, L. Remediation of heavy metals contaminated saline soils: A halophyte choice? Environ. Sci. Technol. 2014, 48, 21–22. [Google Scholar] [CrossRef]
- Liang, L.C.; Liu, W.T.; Sun, Y.B.; Huo, X.H.; Li, S.; Zhou, Q.X. Phytoremediation of heavy metal contaminated saline soils using halophytes: Current progress and future perspectives. Environ. Rev. 2016, 25, 269–281. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity–cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Masters, D.G.; Norman, H.C. 15—Genetic and Environmental Management of Halophytes for Improved Livestock Production. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 243–257. [Google Scholar]
- Schützendübel, A.; Nikolova, P.; Rudolf, C.; Polle, A. Cadmium and H2O2-induced oxidative stress in Populus × canescens roots. Plant Physiol. Bioch. 2002, 40, 577–584. [Google Scholar] [CrossRef]
- Rockwood, D.L.; Naidu, C.V.; Carter, D.R.; Rahmani, M.; Spriggs, T.A.; Lin, C.; Alker, G.R.; Isebrands, J.G.; Segrest, S.A. Short-rotation woody crops and phytoremediation: Opportunities for agroforestry? In New Vistas in Agroforestry; Nair, P.K.R., Rao, M.R., Buck, L.E., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 51–63. [Google Scholar]
- Unterbrunner, R.; Puschenreiter, M.; Sommer, P.; Wieshammer, G.; Tlustoš, P.; Zupan, M.; Wenzel, W.W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ. Pollut. 2007, 148, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Di Lonardo, S.; Capuana, M.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. Exploring the metal phytoremediation potential of three Populus alba L. clones using an in vitro screening. Environ. Sci. Pollut. Res. 2011, 18, 82–90. [Google Scholar] [CrossRef]
- He, J.L.; Li, H.; Luo, J.; Ma, C.F.; Li, S.J.; Qu, L.; Gai, Y.; Jiang, X.N.; Janz, D.; Polle, A.; et al. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus × canescens. Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef]
- He, J.L.; Ma, C.F.; Ma, Y.L.; Li, H.; Kang, J.Q.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Cadmium tolerance in six poplar species. Environ. Sci. Pollut. Res. 2013, 20, 163–174. [Google Scholar] [CrossRef]
- Pajević, S.; Borišev, M.; Nikolić, N.; Arsenov, D.D.; Orlović, S.; Župunski, M. Phytoextraction of heavy metals by fast-growing trees: A review. In Phytoremediation; Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 29–64. [Google Scholar]
- Kieffer, P.; Planchon, S.; Oufir, M.; Ziebel, J.; Dommes, J.; Hoffmann, L.; Hausman, J.F.; Renaut, J. Combining proteomics and metabolite analyses to unravel cadmium stress-response in poplar leaves. J. Proteome Res. 2009, 8, 400–417. [Google Scholar] [CrossRef]
- He, J.L.; Qin, J.J.; Long, L.Y.; Ma, Y.L.; Li, H.; Li, K.; Jiang, X.N.; Liu, T.X.; Polle, A.; Liang, Z.S.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol. Plant. 2011, 143, 50–63. [Google Scholar] [CrossRef]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffré, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar] [CrossRef]
- Brooks, R.R. Phytochemistry of hyperaccumulators. In Plants That Hyperaccumulate Heavy Metals: Their Role in Phytoremediation, Microbiology, Archaeology, Mineral Exploration, and Phytomining; Brooks, R.R., Ed.; CAB International: New York, NY, USA, 1998; pp. 15–53. [Google Scholar]
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Li, H.; Ma, C.F.; Zhang, Y.L.; Polle, A.; Rennenberg, H.; Cheng, X.Q.; Luo, Z.B. Overexpression of bacterial γ-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015, 205, 240–254. [Google Scholar] [CrossRef]
- Janz, D.; Behnke, K.; Schnitzler, J.P.; Kanawati, B.; Schmitt-Kopplin, P.; Polle, A. Pathway analysis of the transcriptome and metabolome of salt sensitive and tolerant poplar species reveals evolutionary adaption of stress tolerance mechanisms. BMC Plant Biol. 2010, 10, 150. [Google Scholar] [CrossRef]
- Polle, A.; Klein, T.; Kettner, C. Impact of cadmium on young plants of Populus euphratica and P. × canescens, two poplar species that differ in stress tolerance. New Forest. 2013, 44, 13–22. [Google Scholar] [CrossRef]
- Sun, J.; Wang, R.G.; Zhang, X.; Yu, Y.C.; Zhao, R.; Li, Z.Y.; Chen, S.L. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Bioch. 2013, 65, 67–74. [Google Scholar] [CrossRef]
- Han, Y.S.; Sa, G.; Sun, J.; Shen, Z.D.; Zhao, R.; Ding, M.Q.; Deng, S.R.; Lu, Y.J.; Zhang, Y.H.; Shen, X.; et al. Overexpression of Populus euphratica xyloglucan endotransglucosylase/hydrolase gene confers enhanced cadmium tolerance by the restriction of root cadmium uptake in transgenic tobacco. Environ. Exp. Bot. 2014, 100, 74–83. [Google Scholar] [CrossRef]
- Han, Y.S.; Wang, S.J.; Zhao, N.; Deng, S.R.; Zhao, C.J.; Li, N.F.; Sun, J.; Zhao, R.; Yi, H.L.; Shen, X.; et al. Exogenous abscisic acid alleviates cadmium toxicity by restricting Cd2+ influx in Populus euphratica cells. J. Plant Growth Regul. 2016, 35, 827–837. [Google Scholar] [CrossRef]
- Sell, J.; Kayser, A.; Schulin, R.; Brunner, I. Contribution of ectomycorrhizal fungi to cadmium uptake of poplars and willows from a heavily polluted soil. Plant Soil 2005, 277, 245–253. [Google Scholar] [CrossRef]
- Krpata, D.; Peintner, U.; Langer, I.; Fitz, W.J.; Schweiger, P. Ectomycorrhizal communities associated with Populus tremula growing on a heavy metal contaminated site. Mycol. Res. 2008, 112, 1069–1079. [Google Scholar] [CrossRef]
- Krpata, D.; Fitz, W.; Peintner, U.; Langer, I.; Schweiger, P. Bioconcentration of zinc and cadmium in ectomycorrhizal fungi and associated aspen trees as affected by level of pollution. Environ. Pollut. 2009, 157, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Wu, C.H.; Zhang, C.; Li, H.; Lipka, U.; Polle, A. The role of ectomycorrhizas in heavy metal stress tolerance of host plants. Environ. Exp. Bot. 2014, 108, 47–62. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Sa, G.; Zhang, Y.N.; Zhu, Z.M.; Deng, S.R.; Sun, J.; Li, N.F.; Li, J.; Yao, J.; Zhao, N.; et al. Paxillus involutus-facilitated Cd2+ influx through plasma membrane Ca2+-permeable channels is stimulated by H2O2 and H+-ATPase in ectomycorrhizal Populus × canescens under cadmium stress. Front. Plant Sci. 2017, 7, 1975. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shi, L.; Zhong, K.; Shen, Z.; Chen, Y. Ectomycorrhizal fungi may not act as a barrier inhibiting host plant absorption of heavy metals. Chemosphere 2019, 215, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hachani, C.; Lamhamedi, M.S.; Cameselle, C.; Gouveia, S.; Zine El Abidine, A.; Khasa, D.P.; Béjaoui, Z. Effects of Ectomycorrhizal Fungi and Heavy Metals (Pb, Zn, and Cd) on Growth and Mineral Nutrition of Pinus halepensis Seedlings in North Africa. Microorganisms 2020, 8, 2033. [Google Scholar] [CrossRef]
- Dreischhoff, S.; Das, I.S.; Jakobi, M.; Kasper, K.; Polle, A. Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Front. Plant Sci. 2020, 11, 1098. [Google Scholar] [CrossRef]
- Ma, Y.L.; He, J.L.; Ma, C.F.; Luo, J.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus × canescens. Plant Cell Environ. 2014, 37, 627–642. [Google Scholar] [CrossRef]
- Jentschke, G.; Winter, S.; Godbold, D.L. Ectomycorrhizas and cadmium toxicity in Norway spruce seedlings. Tree Physiol. 1999, 19, 23–30. [Google Scholar] [CrossRef][Green Version]
- Colpaert, J.V.; Vanassche, J.A. The effects of cadmium on ectomycorrhizal Pinus sylvestris L. New Phytol. 1993, 123, 325–333. [Google Scholar] [CrossRef]
- Hartley-Whitaker, J.; Cairney, J.W.G.; Meharg, A.A. Sensitivity to Cd or Zn of host and symbiont of ectomycorrhizal Pinus sylvestris L. (Scots pine) seedlings. Plant Soil 2000, 218, 31–42. [Google Scholar] [CrossRef]
- Szuba, A.; Karliński, L.; Krzesłowska, M.; Hazubska-Przybył, T. Inoculation with a Pb-tolerant strain of Paxillus involutus improves growth and Pb tolerance of Populus × canescens under in vitro conditions. Plant. Soil 2017, 412, 253–266. [Google Scholar] [CrossRef]
- Szuba, A.; Marczak, Ł.; Kozłowski, R. Role of the proteome in providing phenotypic stability in control and ectomycorrhizal poplar plants exposed to chronic mild Pb stress. Environ. Pollut. 2020, 264, 114585. [Google Scholar] [CrossRef]
- Marschner, P.; Godbold, D.L.; Jentschke, G. Dynamics of lead accumulation in mycorrhizal and non-mycorrhizal Norway spruce (Picea abies (L) Karst). Plant Soil 1996, 178, 239–245. [Google Scholar] [CrossRef]
- Jentschke, G.; Marschner, P.; Vodnik, D.; Marth, C.; Bredemeier, M.; Rapp, C.; Fritz, E.; Gogala, N.; Godbold, D.L. Lead uptake by Picea abies seedlings: Effects of nitrogen source and mycorrhizas. J. Plant Physiol. 1998, 153, 97–104. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Vanassche, J.A. Zinc toxicity in ectomycorrhizal Pinus sylvestris. Plant Soil 1992, 143, 201–211. [Google Scholar] [CrossRef]
- Langenfeld-Heyser, R.; Gao, J.; Ducic, T.; Tachd, P.; Lu, C.F.; Fritz, E.; Gafur, A.; Polle, A. Paxillus involutus mycorrhiza attenuate NaCl-stress responses in the salt-sensitive hybrid poplar Populus × canescens. Mycorrhiza 2007, 17, 121–131. [Google Scholar] [CrossRef]
- Luo, Z.B.; Janz, D.; Jiang, X.N.; Göbel, C.; Wildhagen, H.; Tan, Y.P.; Rennenberg, H.; Feussner, I.; Polle, A. Upgrading root physiology for stress tolerance by ectomycorrhizas: Insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiol. 2009, 151, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Li, K.; Gai, Y.; Göbel, C.; Wildhagen, H.; Jiang, X.N.; Feußner, I.; Rennenberg, H.; Polle, A. The ectomycorrhizal fungus (Paxillus involutus) modulates leaf physiology of poplar towards improved salt tolerance. Environ. Exp. Bot. 2011, 72, 304–311. [Google Scholar] [CrossRef]
- Li, J.; Bao, S.Q.; Zhang, Y.H.; Ma, X.J.; Mishra-Knyrim, M.; Sun, J.; Sa, G.; Shen, X.; Polle, A.; Chen, S.L. Paxillus involutus strains MAJ and NAU mediate K+/Na+ homeostasis in ectomycorrhizal Populus × canescens under sodium chloride stress. Plant Physiol. 2012, 159, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Sun, M.; Sa, G.; Zhang, Y.H.; Li, J.; Sun, J.; Shen, X.; Polle, A.; Chen, S.L. Ion fluxes in Paxillus involutus-inoculated roots of Populus × canescens under saline stress. Environ. Exp. Bot. 2014, 108, 99–108. [Google Scholar] [CrossRef]
- Sa, G.; Yao, J.; Deng, C.; Liu, J.; Zhang, Y.N.; Zhu, Z.M.; Zhang, Y.H.; Ma, X.J.; Zhao, R.; Lin, S.Z.; et al. Amelioration of nitrate uptake under salt stress by ectomycorrhiza with and without a Hartig net. New Phytol. 2019, 222, 1951–1964. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhiza in arresting reactive oxygen species (ROS) and strengthening antioxidant defense in Cajanus cajan (L.) Millsp. nodules under salinity (NaCl) and cadmium (Cd) stress. Plant Growth Regul. 2015, 75, 521–534. [Google Scholar] [CrossRef]
- Li, L.Z.; Liu, X.L.; Peijnenburg, W.J.; Zhao, J.M.; Chen, X.B.; Yu, J.B.; Wu, H.F. Pathways of cadmium fluxes in the root of the halophyte Suaeda salsa. Ecotoxicol. Environ. Saf. 2012, 75, 1–7. [Google Scholar] [CrossRef]
- Hofmann, A.; Proust, J.; Dorowski, A.; Schantz, R.; Huber, R. Annexin 24 from Capsicum annuum: X-ray structure and biochemical characterization. J. Biol. Chem. 2000, 275, 8072–8082. [Google Scholar] [CrossRef]
- Laohavisit, A.; Mortimer, J.C.; Demidchik, V.; Coxon, K.M.; Stancombe, M.A.; Macpherson, N.; Brownlee, C.; Hofmann, A.; Webb, A.A.; Miedema, H.; et al. Zea mays Annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 2009, 21, 479–493. [Google Scholar] [CrossRef]
- Laohavisit, A.; Shang, Z.L.; Rubio, L.; Cuin, T.A.; Véry, A.A.; Wang, A.H.; Mortimer, J.C.; Macpherson, N.; Coxon, K.M.; Battey, N.H.; et al. Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 2012, 24, 1522–1533. [Google Scholar] [CrossRef]
- Liao, C.C.; Zheng, Y.; Guo, Y. MYB30 transcription factor regulates oxidative and heat stress responses through ANNEXIN-mediated cytosolic calcium signaling in Arabidopsis. New Phytol. 2017, 216, 163–177. [Google Scholar] [CrossRef]
- Chen, X.H.; Ouyang, Y.N.; Fan, Y.C.; Qiu, B.Y.; Zhang, G.P.; Zeng, F.R. The pathway of transmembrane cadmium influx via calcium-permeable channels and its spatial characteristics along rice root. J. Exp. Bot. 2018, 69, 5279–5291. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ye, J.M.; Yang, Y.Q.; Lin, H.X.; Yue, L.L.; Luo, J.; Long, Y.; Fu, H.Q.; Liu, X.N.; Zhang, Y.L.; et al. The SOS2-SCaBP8 Complex Generates and Fine-Tunes an AtANN4-Dependent Calcium Signature under Salt Stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef]
- Liu, Q.B.; Ding, Y.L.; Shi, Y.T.; Ma, L.; Wang, Y.; Song, C.P.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef] [PubMed]
- Konopka-Postupolska, D.; Clark, G.; Hofmann, A. Structure, function and membrane interactions of plant annexins: An update. Plant Sci. 2011, 18, 230–241. [Google Scholar] [CrossRef]
- Konopka-Postupolska, D.; Clark, G. Annexins as overlooked regulators of membrane trafficking in plant cells. Int. J. Mol. Sci. 2017, 18, 863. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Sa, G.; Zhang, Y.; Hou, S.Y.; Wu, X.; Zhao, N.; Zhang, Y.H.; Deng, S.R.; Deng, C.; Deng, J.Y.; et al. Populus euphratica annexin1 facilitates cadmium enrichment in transgenic Arabidopsis. J. Hazard. Mater. 2021, 405, 124063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.L.; Yang, X.B.; Zhang, Q.; Zhou, M.; Zhao, E.Z.; Tang, Y.X.; Zhu, X.M.; Shao, J.R.; Wu, Y.M. Induction of annexin by heavy metals and jasmonic acid in Zea mays. Funct. Integr. Genom. 2013, 13, 241–251. [Google Scholar] [CrossRef]
- He, M.J.; Yang, X.L.; Cui, S.L.; Mu, G.J.; Hou, M.Y.; Chen, H.Y.; Liu, L.F. Molecular cloning and characterization of annexin genes in peanut (Arachis hypogaea L.). Gene 2015, 568, 40–49. [Google Scholar] [CrossRef]
- Mei, X.; Li, S.; Li, Q.; Yang, Y.; Luo, X.; He, B.; Li, H.; Xu, Z. Sodium chloride salinity reduces Cd uptake by edible amaranth (Amaranthus mangostanus L.) via competition for Ca channels. Ecotoxicol. Environ. Saf. 2014, 105, 59–64. [Google Scholar] [CrossRef]
- Gafur, A.; Schützendübel, A.; Langenfeld-Heyser, R.; Fritz, E.; Polle, A. Compatible and incompetent Paxillus involutus isolates for ectomycorrhiza formation in vitro with poplar (Populus × canescens) differ in H2O2 production. Plant Biol. 2004, 6, 91–99. [Google Scholar] [PubMed]
- Sun, J.; Chen, S.L.; Dai, S.X.; Wang, R.G.; Li, N.Y.; Shen, X.; Zhou, X.Y.; Lu, C.F.; Zheng, X.J.; Hu, Z.M.; et al. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 2009, 149, 1141–1153. [Google Scholar] [CrossRef]
- Wang, M.J.; Wang, Y.; Sun, J.; Ding, M.Q.; Deng, S.R.; Hou, P.C.; Ma, X.J.; Zhang, Y.H.; Wang, F.F.; Sa, G.; et al. Overexpression of PeHA1 enhances hydrogen peroxide signaling in salt-stressed Arabidopsis. Plant Physiol. Biochem. 2013, 71, 37–48. [Google Scholar] [CrossRef]
- Yao, J.; Shen, Z.D.; Zhang, Y.L.; Wu, X.; Wang, J.H.; Sa, G.; Zhang, Y.H.; Zhang, H.L.; Deng, C.; Liu, J.; et al. Populus euphratica WRKY1 binds the promoter of H+-ATPase gene to enhance gene expression and salt tolerance. J. Exp. Bot. 2020, 71, 1527–1539. [Google Scholar] [CrossRef]
- Zhang, H.L.; Deng, C.; Wu, X.; Yao, J.; Zhang, Y.L.; Zhang, Y.N.; Deng, S.R.; Zhao, N.; Zhao, R.; Zhou, X.Y.; et al. Populus euphratica remorin 6.5 activates plasma membrane H+-ATPases to mediate salt tolerance. Tree Physiol. 2020, 40, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef]
- Laohavisit, A.; Brown, A.T.; Cicuta, P.; Davies, J.M. Annexins: Components of the calcium and reactive oxygen signaling network. Plant Physiol. 2010, 152, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.; Fritz, E.; Polle, A.; Schützendübel, A. Characterisation of antioxidative systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol. Ecol. 2002, 42, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, I.; Marchal, G.; Meerts, P.; Corréal, E.; Lutts, S. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environ. Exp. Bot. 2009, 65, 142–152. [Google Scholar] [CrossRef]
- Ramos, A.C.; Martins, M.A.; Façanha, A.R. ATPase and pyrophosphatase activities in corn root microsomes colonized with arbuscular mycorrhizal fungi. Braz. J. Plant Physiol. 2005, 29, 207–213. [Google Scholar]
- Rosewarne, G.M.; Smith, F.A.; Schachtman, D.P.; Smith, S.E. Localization of proton-ATPase genes expressed in arbuscular mycorrhizal tomato plants. Mycorrhiza 2007, 17, 249–258. [Google Scholar] [CrossRef]
- Ding, M.Q.; Hou, P.C.; Shen, X.; Wang, M.J.; Deng, S.R.; Sun, J.; Xiao, F.; Wang, R.G.; Zhou, X.Y.; Lu, C.F.; et al. Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol. Biol. 2010, 73, 251–269. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Gresshoff, P.M.; Hause, B. Analyzing the soybean transcriptome during autoregulation of mycorrhization identifies the transcription factors GmNF-YA1a/b as positive regulators of arbuscular mycorrhization. Genome Biol. 2013, 14, R62. [Google Scholar] [CrossRef] [PubMed]
- Repetto, O.; Bestel-Corre, G.; Dumas-Gaudot, E.; Berta, G.; Gianinazzi-Pearson, V.; Gianinazzi, S. Targeted proteomics to identify cadmium-induced protein modifications in Glomus mosseae-inoculated pea roots. New Phytol. 2003, 157, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Aloui, A.; Recorbet, G.; Gollotte, A.; Robert, F.; Valot, B.; Gianinazzi-Pearson, V.; Aschi-Smiti, S.; Dumas-Gaudot, E. On the mechanisms of cadmium stress alleviation in Medicago truncatula by arbuscular mycorrhizal symbiosis: A root proteomic study. Proteomics 2009, 9, 420–433. [Google Scholar] [CrossRef]
- Leple, J.C.; Brasileiro, A.C.M.; Michel, M.F.; Delmotte, F.; Jouanin, L. Transgenic poplars: Expression of chimeric genes using four different constructs. Plant Cell Rep. 1992, 11, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Volmer, K.; Mishra-Knyrim, M.; Polle, A. Growing poplars for research with and without mycorrhizas. Front. Plant Sci. 2013, 4, 332. [Google Scholar] [CrossRef]
- Sun, J.; Wang, M.J.; Ding, M.Q.; Deng, S.R.; Liu, M.Q.; Lu, C.F.; Zhou, X.Y.; Shen, X.; Zheng, X.J.; Zhang, Z.K.; et al. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 2010, 33, 943–958. [Google Scholar] [CrossRef]
- Lu, Y.J.; Li, N.Y.; Sun, J.; Hou, P.C.; Jing, X.S.; Zhu, H.P.; Deng, S.R.; Han, Y.S.; Huang, X.X.; Ma, X.J.; et al. Exogenous hydrogen peroxide, nitric oxide and calcium mediate root ion fluxes in two non-secretor mangrove species subjected to NaCl stress. Tree Physiol. 2013, 33, 81–95. [Google Scholar] [CrossRef]
- De Boer, B. Fusicoccin—A key to multiple 14-3-3 locks? Trends Plant Sci. 1997, 2, 60–66. [Google Scholar] [CrossRef]
- Kinoshita, T.; Shimazaki, K.I. Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol. 2001, 42, 424–432. [Google Scholar] [CrossRef]
- Sun, J.; Dai, S.X.; Wang, R.G.; Chen, S.L.; Li, N.Y.; Zhou, X.Y.; Lu, C.F.; Shen, X.; Zheng, X.J.; Hu, Z.M.; et al. Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol. 2009, 29, 1175–1186. [Google Scholar] [CrossRef]
- Sun, J.; Wang, R.G.; Liu, Z.Q.; Ding, Y.Z.; Li, T.Q. Non-invasive microelectrode cadmium flux measurements reveal the spatial characteristics and real-time kinetics of cadmium transport in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii. J. Plant Physiol. 2013, 170, 355–359. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L.; Gradmann, D.; Chen, Z.; Newman, I.; Mancuso, S. Oscillations in plant membrane transport: Model predictions, experimental validation, and physiological implications. J. Exp. Bot. 2006, 57, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Junghans, U.; Polle, A.; Düchting, P.; Weiler, E.; Kuhlman, B.; Gruber, F.; Teichmann, T. Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant Cell Environ. 2006, 29, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).