Nanoparticles for Targeted Brain Drug Delivery: What Do We Know?
Abstract
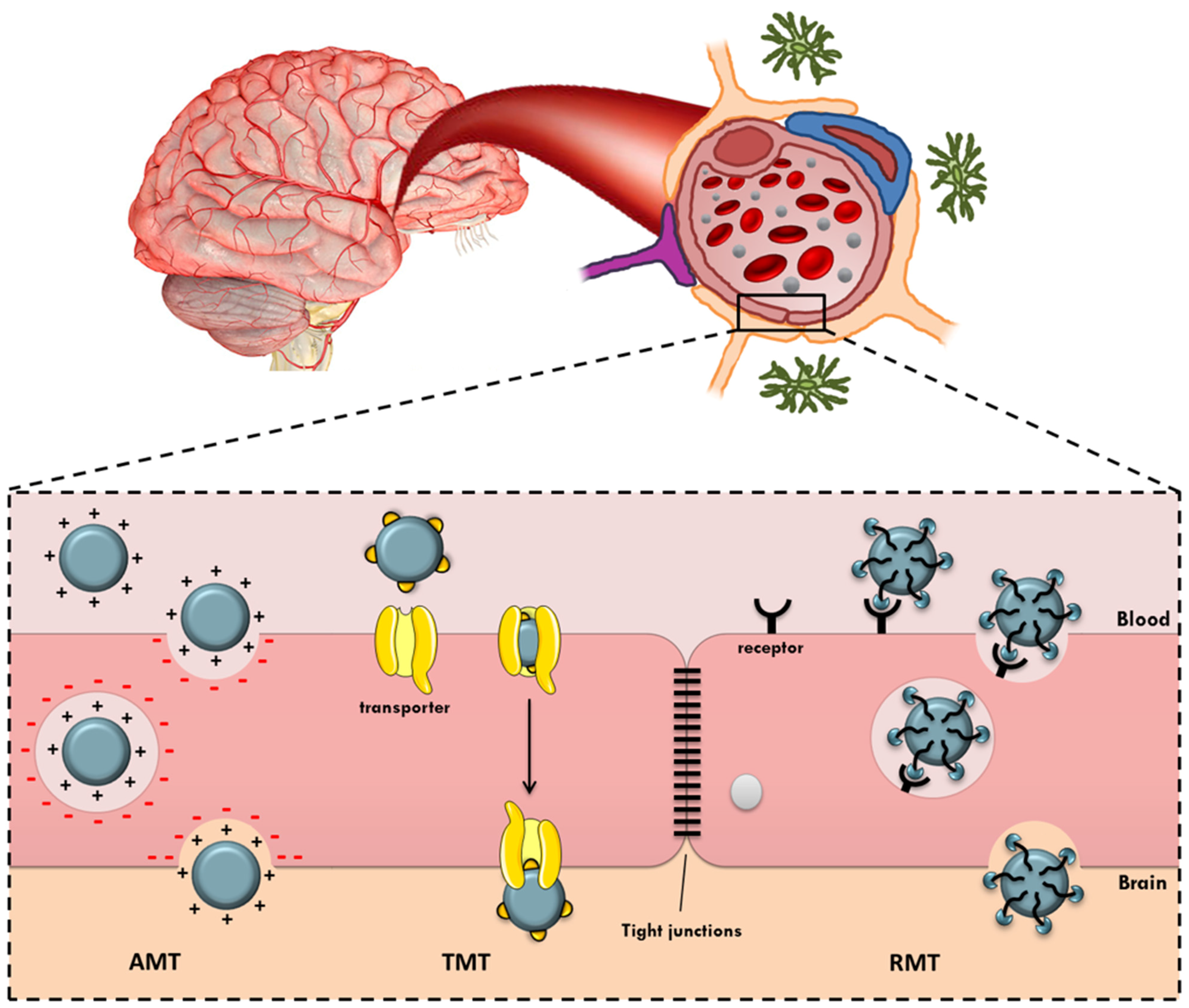
:1. Introduction
2. Active Targeted Brain Delivery
2.1. Adsorptive-Mediated Transcytosis
2.1.1. Lectin
2.1.2. Cardiolipin
2.1.3. Heparin
2.1.4. Cell Penetrating Peptides
2.2. Transporter-Mediated Transcytosis
2.2.1. Glucose Transporter 1
2.2.2. Glutathione Transporter
2.2.3. Amino Acids Transporters
2.3. Receptor-Mediated Transcytosis
2.3.1. Transferrin Receptor
2.3.2. Lactoferrin Receptor
2.3.3. Low-Density Lipoprotein Receptors
2.3.4. Nicotinic Acetylcholine Receptors
2.3.5. αvβ3 Integrin Receptors
2.3.6. CD13/APN Receptor
3. Marketed Formulations and Clinical Trials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockman, P.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle Surface Charges Alter Blood–Brain Barrier Integrity and Permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-Based in Vitro Blood–Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification. Int. J. Mol. Sci. 2014, 15, 1812–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2015, 20, 1–11. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nano-Med. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalin, I.; Alyautdin, R.; Ismail, N.M.; Haron, M.H.; Kuznetsov, D. Nanoscale drug delivery systems and the blood–brain barrier. Int. J. Nanomed. 2014, 9, 795–811. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS Delivery Via Adsorptive Transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Vorbrodt, A.W. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J. Neurocytol. 1989, 18, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.K.; Eisenberg, J.B.; Pardridge, W.M. Absorptive-mediated endocytosis of cationized albumin and a be-ta-endorphin-cationized albumin chimeric peptide by isolated brain capillaries. Model system of blood-brain barrier transport. J. Biol. Chem. 1987, 262, 15214–15219. [Google Scholar] [CrossRef]
- Broadwell, R.D. Transcytosis of macromolecules through the blood-brain barrier: A cell biological perspective and critical appraisal. Acta Neuropathol. 1989, 79, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.E. Caveolae: From basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv. Drug Deliv. Rev. 2001, 49, 265–280. [Google Scholar] [CrossRef]
- Tuma, P.L.; Hubbard, A.L. Transcytosis: Crossing Cellular Barriers. Physiol. Rev. 2003, 83, 871–932. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tao, W.; Lu, W.; Zhang, Q.; Zhang, Y.; Jiang, X.; Fu, S. Lectin-conjugated PEG–PLA nanoparticles: Preparation and brain delivery after intranasal administration. Biomaterials 2006, 27, 3482–3490. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef]
- Piazza, J.; Hoare, T.; Molinaro, L.; Terpstra, K.; Bhandari, J.; Selvaganapathy, P.R.; Gupta, B.; Mishra, R.K. Haloperidol-loaded intranasally administered lectin functionalized poly(ethylene glycol)–block-poly(d,l)-lactic-co-glycolic acid (PEG–PLGA) nanoparticles for the treatment of schizophrenia. Eur. J. Pharm. Biopharm. 2014, 87, 30–39. [Google Scholar] [CrossRef]
- Wen, Z.; Yan, Z.; Hu, K.; Pang, Z.; Cheng, X.; Guo, L.; Zhang, Q.; Jiang, X.; Fang, L.; Lai, R. Odorranalectin-conjugated nanoparticles: Preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J. Control. Release 2011, 151, 131–138. [Google Scholar] [CrossRef]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W.; Rong, Z.; Chen, H.; Jiang, X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Bereczki, E.; Re, F.; Masserini, M.E.; Winblad, B.; Pei, J.J. Liposomes functionalized with acidic lipids rescue Aβ-induced toxicity in murine neuroblastoma cells. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Gutiérrez, L.; Re, F.; Bereczki, E.; Ioja, E.; Gregori, M.; Andersen, A.J.; Antón, M.; Moghimi, S.M.; Pei, J.-J.; Masserini, M.; et al. Repeated intraperitoneal injections of liposomes containing phosphatidic acid and cardiolipin reduce amyloid-β levels in APP/PS1 transgenic mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.; Moore, S.; Mourtas, S.; Niarakis, A.; Re, F.; Zona, C.; La Ferla, B.; Nicotra, F.; Masserini, M.; Antimisiaris, S.; et al. Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer’s Aβ peptide. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Re, F.; Canovi, M.; Beeg, M.; Gregori, M.; Sesana, S.; Sonnino, S.; Brogioli, D.; Musicanti, C.; Gasco, P.; et al. Lipid-based nanoparticles with high binding affinity for amyloid-β1–42 peptide. Biomaterials 2010, 31, 6519–6529. [Google Scholar] [CrossRef]
- Wang, P.; Kouyoumdjian, H.; Zhu, D.C.; Huang, X. Heparin nanoparticles for β amyloid binding and mitigation of β amyloid associated cytotoxicity. Carbohydr. Res. 2014, 405, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, M.; Hallbrink, M.; Prochiantz, A.; Langel, U. Cell-penetrating peptides. Trends. Pharmacol. Sci. 2000, 21, 99–103. [Google Scholar] [CrossRef]
- Drin, G.; Rousselle, C.; Scherrmann, J.-M.; Rees, A.R.; Temsamani, J. Peptide delivery to the brain via adsorptive-mediated endocytosis: Advances with SynB vectors. AAPS Pharm. Sci. 2002, 4, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Rousselle, C.; Smirnova, M.; Clair, P.; Lefauconnier, J.M.; Chavanieu, A.; Calas, B.; Scherrmann, J.M.; Temsamani, J. En-hanced delivery of doxorubicin into the brain via a peptide-vector-mediated strategy: Saturation kinetics and specificity. J. Pharmacol. Exp. Ther. 2001, 296, 124–131. [Google Scholar]
- Vives, E.; Richard, J.; Rispal, C.; Lebleu, B. TAT Peptide Internalization: Seeking the Mechanism of Entry. Curr. Protein Pept. Sci. 2003, 4, 125–132. [Google Scholar] [CrossRef]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorén, P.E.; Persson, D.; Karlsson, M.; Nordén, B. The antennapedia peptide penetratin translocates across lipid bilayers—The first direct observation. FEBS Lett. 2000, 482, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Fittipaldi, A.; Ferrari, A.; Zoppe’, M.; Arcangeli, C.; Pellegrini, V.; Beltram, F.; Giacca, M. Cell Membrane Lipid Rafts Mediate Caveolar Endocytosis of HIV-1 Tat Fusion Proteins. J. Biol. Chem. 2003, 278, 34141–34149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Console, S.; Marty, C.; García-Echeverría, C.; Schwendener, R.; Ballmer-Hofer, K. Antennapedia and HIV Transactivator of Transcription (TAT) “Protein Transduction Domains” Promote Endocytosis of High Molecular Weight Cargo upon Binding to Cell Surface Glycosaminoglycans. J. Biol. Chem. 2003, 278, 35109–35114. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, T.; Morisaki, K.; Suzuki, S.; Takashima, Y. Prolongation of Life in Rats with Malignant Glioma by Intranasal siRNA/Drug Codelivery to the Brain with Cell-Penetrating Peptide-Modified Micelles. Mol. Pharm. 2014, 11, 1471–1478. [Google Scholar] [CrossRef]
- Xia, H.; Gao, X.; Gu, G.; Liu, Z.; Hu, Q.; Tu, Y.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Penetratin-functionalized PEG–PLA nanoparticles for brain drug delivery. Int. J. Pharm. 2012, 436, 840–850. [Google Scholar] [CrossRef]
- Santra, S.; Yang, H.; Stanley, J.T.; Holloway, P.H.; Moudgil, B.M.; Walter, G.; Mericle, R.A. Rapid and effective labeling of brain tissue using TAT-conjugated CdS∶Mn/ZnS quantum dots. Chem. Commun. 2005, 25, 3144–3146. [Google Scholar] [CrossRef]
- Allen, D.D.; Geldenhuys, W.J. Molecular modeling of blood–brain barrier nutrient transporters: In silico basis for evaluation of potential drug delivery to the central nervous system. Life Sci. 2006, 78, 1029–1033. [Google Scholar] [CrossRef]
- Tsuji, A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRX 2005, 2, 54–62. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose transporters in healthy heart and in cardiac disease. Int. J. Cardiol. 2017, 230, 70–75. [Google Scholar] [CrossRef]
- Béduneau, A.; Saulnier, P.; Benoit, J.-P. Active targeting of brain tumors using nanocarriers. Biomaterials 2007, 28, 4947–4967. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Oda, Y.; Seino, Y.; Yamamoto, T.; Inagaki, N.; Yano, H.; Imura, H.; Shigemoto, R.; Kikuchi, H. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992, 52, 3972–3979. [Google Scholar] [PubMed]
- Zeller, K.; Rahner-Welsch, S.; Kuschinsky, W. Distribution of Glut1 Glucose Transporters in Different Brain Structures Compared to Glucose Utilization and Capillary Density of Adult Rat Brains. Br. J. Pharmacol. 1997, 17, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannucci, S.J.; Maher, F.; Simpson, I.A. Glucose transporter proteins in brain: Delivery of glucose to neurons and glia. Glia 1997, 21, 2–21. [Google Scholar] [CrossRef]
- Singh, I.; Swami, R.; Jeengar, M.K.; Khan, W.; Sistla, R. p-Aminophenyl-α-d-mannopyranoside engineered lipidic nanoparticles for effective delivery of docetaxel to brain. Chem. Phys. Lipids 2015, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.J.; Thao, L.Q.; Lee, S.; Min, S.Y.; Lee, E.S.; Shin, B.S.; Choi, H.-G.; Youn, Y.S. Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J. Control. Release 2016, 225, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.; Mbimba, T.; Harrison, T.; Sutariya, V. Brain-targeted delivery of paclitaxel using glutathione-coated nano-particles for brain cancers. J. Drug Target. 2011, 19, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schlachetzki, F.; Pardridge, W.M. Global non-viral gene transfer to the primate brain following intravenous administration. Mol. Ther. 2003, 7, 11–18. [Google Scholar] [CrossRef]
- Acharya, S.R.; Reddy, P.R. Brain targeted delivery of paclitaxel using endogenous ligand. Asian J. Pharm. Sci. 2016, 11, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Englert, C.; Trützschler, A.-K.; Raasch, M.; Bus, T.; Borchers, P.; Mosig, A.S.; Traeger, A.; Schubert, U.S. Crossing the blood-brain barrier: Glutathione-conjugated poly(ethylene imine) for gene delivery. J. Control. Release 2016, 241, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar] [PubMed]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Ylikangas, H.; Peura, L.; Malmioja, K.; Leppänen, J.; Laine, K.; Poso, A.; Lahtela-Kakkonen, M.; Rautio, J. Structure–activity relationship study of compounds binding to large amino acid transporter 1 (LAT1) based on pharmacophore modeling and in situ rat brain perfusion. Eur. J. Pharm. Sci. 2013, 48, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Di, X.; Zhang, S.; Kan, Q.; Liu, H.; Lu, T.; Wang, Y.; Fu, Q.; Sun, J.; He, Z. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf. B Biointerfaces 2016, 141, 260–267. [Google Scholar] [CrossRef]
- Kharya, P.; Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Majumdar, S.; Jain, S.K. Phenylalanine-coupled solid lipid nanoparticles for brain tumor targeting. J. Nanopart. Res. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- Jones, A.R.; Shusta, E.V. Blood–Brain Barrier Transport of Therapeutics via Receptor-Mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin alpha v beta 3 an-tagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Friedlander, M.; Brooks, P.C.; Shaffer, R.W.; Kincaid, C.M.; Varner, J.; Cheresh, D.A. Definition of Two Angiogenic Pathways by Distinct alpha(v) Integrins. Science 1995, 270, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Van Hensbergen, Y.; Broxterman, H.J.; Hanemaaijer, R.; Jorna, A.S.; Van Lent, N.A.; Verheul, H.M.W.; Pinedo, H.M.; Hoekman, K. Soluble aminopeptidase N/CD13 in malignant and nonmalignant effusions and intratumoral fluid. Clin. Cancer Res. 2002, 8, 3747–3754. [Google Scholar] [PubMed]
- Moos, T.; Morgan, E.H. Transferrin and Transferrin Receptor Function in Brain Barrier Systems. Cell. Mol. Neurobiol. 2000, 20, 77–95. [Google Scholar] [CrossRef]
- Rouault, T.A. Iron metabolism in the CNS: Implications for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 551–564. [Google Scholar] [CrossRef]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Wang, L.-J. Transferrin-grafted catanionic solid lipid nanoparticles for targeting delivery of saquinavir to the brain. J. Taiwan Inst. Chem. Eng. 2014, 45, 755–763. [Google Scholar] [CrossRef]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.-B.; Feng, W.; Kang, C.-S.; Pu, P.-Y.; Betbeder, D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood–brain barrier. Int. J. Pharm. 2009, 379, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Z.; Dallman, J.; Baker, J.; Othman, A.M.; Blackwelder, P.; Leblanc, R.M. Crossing the blood–brain–barrier with transferrin conjugated carbon dots: A zebrafish model study. Colloids Surf. B Biointerfaces 2016, 145, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Xia, H.; Cheng, Z.; Cheng, Y.; Xu, Y. Investigating the passage of tetramethylpyrazine-loaded liposomes across blood-brain barrier models in vitro and ex vivo. Mater. Sci. Eng. C 2016, 69, 1010–1017. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Q.; Chow, P.; Wang, D.; Wang, C.-H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef]
- Somani, S.; Blatchford, D.R.; Millington, O.; Stevenson, M.L.; Dufès, C. Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J. Control. Release 2014, 188, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Bickel, U.; Yoshikawa, T.; Pardridge, W. Delivery of peptides and proteins through the blood–brain barrier. Adv. Drug Deliv. Rev. 2001, 46, 247–279. [Google Scholar] [CrossRef]
- Béduneau, A.; Hindré, F.; Clavreul, A.; Leroux, J.-C.; Saulnier, P.; Benoit, J.-P. Brain targeting using novel lipid nanovectors. J. Control. Release 2008, 126, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Gomes, B.; Fricker, G.; Coelho, M.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Calon, F.; Zhu, C.; Boado, R.J.; Pardridge, W.M. Intravenous Nonviral Gene Therapy Causes Normalization of Striatal Tyrosine Hydroxylase and Reversal of Motor Impairment in Experimental Parkinsonism. Hum. Gene Ther. 2003, 14, 1–12. [Google Scholar] [CrossRef]
- Loureiro, J.; Gomes, B.; Fricker, G.; Cardoso, I.; Ribeiro, C.A.; Gaiteiro, C.; Coelho, M.A.; Pereira, M.D.C.; Rocha, S. Dual ligand immunoliposomes for drug delivery to the brain. Colloids Surf. B Biointerfaces 2015, 134, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wally, J.; Buchanan, S.K. A structural comparison of human serum transferrin and human lactoferrin. BioMetals 2007, 20, 249–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuijens, J.H.; van Berkel, P.H.C.; Schanbacher, F.L. Structure and biological actions of lactoferrin. J. Mammary Gland. Biol. Neoplasia 1996, 1, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Maeda, J.; Higuchi, M.; Inoue, K.; Akita, H.; Harashima, H.; Suhara, T. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci. 2006, 78, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, J.; Shen, Y.; Lu, W.; Gao, X.; Zhang, Q.; Jiang, X. Lactoferrin-conjugated PEG–PLA nanoparticles with improved brain delivery: In vitro and in vivo evaluations. J. Control. Release 2009, 134, 55–61. [Google Scholar] [CrossRef]
- Xu, L.; Yeudall, W.A.; Yang, H. Dendrimer-Based RNA Interference Delivery for Cancer Therapy. In Tailored Polymer Ar-chitectures for Pharmaceutical and Biomedical Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1135, pp. 197–213. [Google Scholar]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Shi, Y.; Jiang, W.; Han, J.; Huang, S.; Jiang, X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease. Int. J. Pharm. 2011, 415, 273–283. [Google Scholar] [CrossRef]
- Somani, S.; Robb, G.; Pickard, B.; Dufès, C. Enhanced gene expression in the brain following intravenous administration of lactoferrin-bearing polypropylenimine dendriplex. J. Control. Release 2015, 217, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Cheng, S.-J. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int. J. Pharm. 2015, 499, 10–19. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Chen, Y.-C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015, 479, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Fu, C.; Li, M.; Li, X.; Wang, M.; He, L.; Zhang, L.-M.; Peng, Y. A pH-sensitive hyaluronic acid prodrug modified with lactoferrin for glioma dual-targeted treatment. Mater. Sci. Eng. C 2016, 67, 159–169. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Zhang, Q.; Jiang, W.; Tang, L.; Liu, J.; He, Q. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur. J. Pharm. Sci. 2011, 44, 164–173. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A Receptor-Mediated Pathway for Cholesterol Homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Nykjaer, A.; Willnow, T. The low-density lipoprotein receptor gene family: A cellular Swiss army knife? Trends Cell Biol. 2002, 12, 273–280. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Cerebrovascular Effects of Apolipoprotein E. JAMA Neurol. 2013, 70, 440–444. [Google Scholar] [CrossRef]
- Hatters, D.; Peters-Libeu, C.A.; Weisgraber, K.H. Apolipoprotein E structure: Insights into function. Trends Biochem. Sci. 2006, 31, 445–454. [Google Scholar] [CrossRef]
- Peters-Libeu, C.A.; Newhouse, Y.; Hatters, D.M.; Weisgraber, K.H. Model of Biologically Active Apolipoprotein E Bound to Dipalmitoylphosphatidylcholine. J. Biol. Chem. 2006, 281, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.; Couraud, P.-O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: Two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 495103. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef]
- Magro, R.D.; Ornaghi, F.; Cambianica, I.; Beretta, S.; Re, F.; Musicanti, C.; Rigolio, R.; Donzelli, E.; Canta, A.R.; Ballarini, E.; et al. ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J. Control. Release 2017, 249, 103–110. [Google Scholar] [CrossRef]
- Kreuter, J. Application of nanoparticles for the delivery of drugs to the brain. Int. Congr. Ser. 2005, 1277, 85–94. [Google Scholar] [CrossRef]
- Sun, W.; Xie, C.; Wang, H.; Hu, Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004, 25, 3065–3071. [Google Scholar] [CrossRef]
- Ruan, Y.; Yao, L.; Zhang, B.; Zhang, S.; Guo, J. Antinociceptive properties of nasal delivery of Neurotoxin-loaded nanoparticles coated with polysorbate-80. Peptides 2011, 32, 1526–1529. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2008, 70, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008, 1200, 159–168. [Google Scholar] [CrossRef]
- Jose, S.; Sowmya, S.; Cinu, T.; Aleykutty, N.; Thomas, S.; Souto, E. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef]
- Wang, C.-X.; Huang, L.-S.; Hou, L.-B.; Jiang, L.; Yan, Z.-T.; Wang, Y.-L.; Chen, Z.-L. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009, 1261, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Currie, J.-C.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.-P.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Sha, X.; Jiang, X.; Chen, L.; Law, K.; Gu, J.; Chen, Y.; Wang, X.; Fang, X. The brain targeting mechanism of Angiopep-conjugated poly(ethylene glycol)-co-poly(ɛ-caprolactone) nanoparticles. Biomaterials 2012, 33, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Huile, G.; Shuaiqi, P.; Zhi, Y.; Shijie, C.; Chen, C.; Xinguo, J.; Shun, S.; Zhiqing, P.; Yu, H. A cascade targeting strategy for brain neuroglial cells employing nanoparticles modified with angiopep-2 peptide and EGFP-EGF1 protein. Biomaterials 2011, 32, 8669–8675. [Google Scholar] [CrossRef]
- Shao, K.; Huang, R.; Li, J.; Han, L.; Ye, L.; Lou, J.; Jiang, C. Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain. J. Control. Release 2010, 147, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Wu, J.; Chen, Z.; Huang, S.; Li, J.; Ye, L.; Lou, J.; Zhu, L.; Jiang, C. A brain-vectored angiopep-2 based polymeric micelles for the treatment of intracranial fungal infection. Biomaterials 2012, 33, 6898–6907. [Google Scholar] [CrossRef]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2014, 37, 425–435. [Google Scholar] [CrossRef]
- Ren, J.; Shen, S.; Wang, D.; Xi, Z.; Guo, L.; Pang, Z.; Qian, Y.; Sun, X.; Jiang, X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 2012, 33, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Han, L.; Liu, S.; Ma, H.; Huang, R.; Jiang, C. Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials 2011, 32, 6832–6838. [Google Scholar] [CrossRef] [PubMed]
- McGehee, D.S.; Role, L. Physiological Diversity of Nicotinic Acetylcholine Receptors Expressed by Vertebrate Neurons. Annu. Rev. Physiol. 1995, 57, 521–546. [Google Scholar] [CrossRef]
- Galzi, J.L.; Revah, F.; Bessis, A.; Changeux, J.P. Functional Architecture of the Nicotinic Acetylcholine Receptor: From Electric Organ to Brain. Annu. Rev. Pharmacol. Toxicol. 1991, 31, 37–72. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef]
- Sine, S.M.; Engel, A.G. Recent advances in Cys-loop receptor structure and function. Nature 2006, 440, 448–455. [Google Scholar] [CrossRef]
- Lester, H.A. Cys-loop receptors: New twists and turns. Trends Neurosci. 2004, 27, 329–336. [Google Scholar] [CrossRef]
- Corringer, P.-J.; Poitevin, F.; Prevost, M.S.; Sauguet, L.; Delarue, M.; Changeux, J.-P. Structure and Pharmacology of Pentameric Receptor Channels: From Bacteria to Brain. Structure 2012, 20, 941–956. [Google Scholar] [CrossRef] [Green Version]
- Le Novère, N.; Corringer, P.-J.; Changeux, J.-P. The diversity of subunit composition in nAChRs: Evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 2002, 53, 447–456. [Google Scholar] [CrossRef]
- Millar, N.S.; Gotti, C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Gotti, C.; Clementi, F.; Fornari, A.; Gaimarri, A.; Guiducci, S.; Manfredi, I.; Moretti, M.; Pedrazzi, P.; Pucci, L.; Zoli, M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 2009, 78, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lukas, R.J. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem. Pharmacol. 2011, 82, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, B.T.; Egleton, R.D.; Davis, T.P. Modulation of cerebral microvascular permeability by endothelial nicotinic acetylcholine receptors. Am. J. Physiol. Circ. Physiol. 2005, 289, H212–H219. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.L.; Householder, K.T.; Chung, E.P.; Prakapenka, A.V.; DiPerna, D.M.; Sirianni, R.W. A critical evaluation of drug delivery from ligand modified nanoparticles: Confounding small molecule distribution and efficacy in the central nervous system. J. Control. Release 2015, 220, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-Y.; Choi, W.I.; Kim, Y.H.; Tae, G. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 2013, 34, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, R.; Han, L.; Ke, W.; Shao, K.; Ye, L.; Lou, J.; Jiang, C. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials 2009, 30, 4195–4202. [Google Scholar] [CrossRef]
- Liu, Y.; An, S.; Li, J.; Kuang, Y.; He, X.; Guo, Y.; Ma, H.; Zhang, Y.; Ji, B.; Jiang, C. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials 2016, 80, 33–45. [Google Scholar] [CrossRef]
- Kumar, P.; Wu, H.; McBride, J.L.; Jung, K.-E.; Kim, M.H.; Davidson, B.; Lee, S.K.; Shankar, P.; Manjunath, N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.N.; Charpantier, E.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.-E.; Cheah, L.-S.; Bertrand, D.; Kini, M. Candoxin, a Novel Toxin from Bungarus candidus, Is a Reversible Antagonist of Muscle (αβγδ) but a Poorly Reversible Antagonist of Neuronal α7 Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2002, 277, 17811–17820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, C.; Li, B.; Hu, L.; Wei, X.; Feng, L.; Fu, W.; Lu, W. Micelle-Based Brain-Targeted Drug Delivery Enabled by a Nicotine Acetylcholine Receptor Ligand. Angew. Chem. Int. Ed. 2011, 50, 5482–5485. [Google Scholar] [CrossRef]
- Wei, X.; Gao, J.; Zhan, C.; Xie, C.; Chai, Z.; Ran, D.; Ying, M.; Zheng, P.; Lu, W. Liposome-based glioma targeted drug delivery enabled by stable peptide ligands. J. Control. Release 2015, 218, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Wei, X.; Qian, J.; Feng, L.; Zhu, J.; Lu, W. Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo. J. Control. Release 2012, 160, 630–636. [Google Scholar] [CrossRef]
- Humphries, M.J. Integrin structure. Biochem. Soc. Trans. 2000, 28, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Parise, L.V.; Phillips, D.R. Platelet membrane glycoprotein IIb-IIIa complex incorporated into phospholipid vesicles. Preparation and morphology. J. Biol. Chem. 1985, 260, 1750–1756. [Google Scholar] [CrossRef]
- Nermut, M.V.; Green, N.M.; Eason, P.; Yamada, S.S.; Yamada, K. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988, 7, 4093–4099. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.; Nagaswami, C.; Vilaire, G.; Bennett, J. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J. Biol. Chem. 1992, 267, 16637–16643. [Google Scholar] [CrossRef]
- Erb, E.-M.; Tangemann, K.; Bohrmann, B.; Müller, B.; Engel, J. Integrin αIIbβ3 Reconstituted into Lipid Bilayers Is Nonclustered in Its Activated State but Clusters after Fibrinogen Binding. Biochemistry 1997, 36, 7395–7402. [Google Scholar] [CrossRef]
- Schnell, O.; Krebs, B.; Wagner, E.; Romagna, A.; Beer, A.J.; Grau, S.J.; Thon, N.; Goetz, C.; Kretzschmar, H.A.; Tonn, J.-C.; et al. Expression of Integrin αvβ3in Gliomas Correlates with Tumor Grade and Is not Restricted to Tumor Vasculature. Brain Pathol. 2008, 18, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Arnaout, M.A. Leukocyte Adhesion Molecules Deficiency: Its Structural Basis, Pathophysiology and Implications for Modulating the Inflammatory Response. Immunol. Rev. 1990, 114, 145–180. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Richard, S.; Boucher, M.; Lalatonne, Y.; Mériaux, S.; Motte, L. Iron oxide nanoparticle surface decorated with cRGD peptides for magnetic resonance imaging of brain tumors. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Sonali, J.; Singh, R.P.; Sharma, G.; Kumari, L.; Koch, B.; Singh, S.; Bharti, S.; Rajinikanth, P.S.; Pandey, B.L.; Muthu, M.S. RGD-TPGS decorated theranostic liposomes for brain targeted delivery. Colloids Surf. B Biointerfaces 2016, 147, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Zhang, Q. Anti-tumor targeted drug delivery systems mediated by aminopeptidase N/CD13. Acta Pharm. Sin. B 2011, 1, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer Treatment by Targeted Drug Delivery to Tumor Vasculature in a Mouse Model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; Gao, X.; Hu, Q.; Jiang, D.; Feng, X.; Zhang, X.; Song, Q.; Yao, L.; Huang, M.; Jiang, X.; et al. iNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials 2014, 35, 4319–4332. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, F.; Brignole, C.; Di Paolo, D.; Nico, B.; Pezzolo, A.; Marimpietri, D.; Pagnan, G.; Piccardi, F.; Cilli, M.; Longhi, R.; et al. Targeting Liposomal Chemotherapy via Both Tumor Cell–Specific and Tumor Vasculature–Specific Ligands Potentiates Therapeutic Efficacy. Cancer Res. 2006, 66, 10073–10082. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Jiang, X.; Shi, J.; He, X.; Li, J.; Guo, Y.; Zhang, Y.; Ma, H.; Lu, Y.; Jiang, C. Single-component self-assembled RNAi nanoparticles functionalized with tumor-targeting iNGR delivering abundant siRNA for efficient glioma therapy. Biomaterials 2015, 53, 330–340. [Google Scholar] [CrossRef]
- Huang, N.; Cheng, S.; Zhang, X.; Tian, Q.; Pi, J.; Tang, J.; Wang, F.; Chen, J.; Xie, Z.; Xu, Z.; et al. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood–brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Sun, Y.; Zhao, H.; Lan, M.; Gao, F.; Song, C.; Lou, K.; Li, H.; Wang, W. A novel lactoferrin-modified β-cyclodextrin nanocarrier for brain-targeting drug delivery. Int. J. Pharm. 2013, 458, 110–117. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Doxorubicin-loaded Anti-EGFR-Immunoliposomes (C225-ILs-dox) in High-grade Gliomas—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03603379 (accessed on 1 October 2021).
- P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Parkinson’s Disease (REPAIR-PD)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03815916 (accessed on 1 October 2021).
- Study of APH-1105 in Patients with Mild to Moderate Alzheimer’s Disease—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03806478 (accessed on 1 October 2021).
- P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Amyotrophic Lateral Sclerosis (REPAIR-ALS) (REPAIR-ALS)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03843710 (accessed on 1 October 2021).
| Targeting Effector/Ligand | NPs Type | Composition | Therapeutic Agent | Size (nm) | Zeta Potential (mV) | EE (%) | Administration Route | In Vitro/In Vivo Results | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Adsortive-mediated transcytosis | |||||||||
| Lectin | Polymeric nanoparticles | PLA/PEG/WGA; PLA/PEG/STL; PLGA/PEG/STL; PLGA/PEG/OL | VIP, bFGF, HLP, UCN | 85 to 130 | −30 to −15 | 70 to 75 | Intranasal | - increased brain uptake in rats compared to NPs with no lectin; - improved spatial learning and memory in AD rats; - enhanced neuroprotection in schizophrenic or hemiparkinsonian rats. | [3,4,5,6,7] |
| Cardiolipin | Liposomes | SM/Chol/CL | n.i. | 100 to 150 | −38 to +50 | n.i. | intraperitoneal | - reduced cell viability and Tau phosphorilation in mouse neuroblastoma cell lines; - reduction of Aβ levels and Tau phosphorilation in plasma and brain of APP/PS1 transgenic mice; - high affinity for Aβ fibrils association. | [8,9,10,11] |
| Heparin | Magnetic nanoparticles | Fe3O4/Heparin | n.i. | 70 | −50 | n.i. | n.i. | - high affinity for Aβ fibrils association and protection of neuronal cells against Aβ toxicity. | [12] |
| CPPs | Polymeric nanoparticles; Polymeric micelles; Quantum dots | PLA/PEG/Penetratin; MPEG/PCL/TAT; ZnS:Mn/ZnS/TAT | siRNA for Raf-1/CPT | 60 to 200 | −5 to +15 | n.i. | intravenous, intranasal, intra-arterial | - enhanced brain uptake in SD rats; - inhibition of tumor growth in rat model of malignant glioma; - specific brain delivery for QDs theranostic. | [13,14,15] |
| Transporter-mediated transcytosis | |||||||||
| Mannose | Lipid nanoparticles; Cationic HSA nanoparticles | GMS/SA/SL/MAN; HSA/EDA/Mannose | DT, DOX | 90 to 100 | −15 to −10 | 75 to 85 | intravenous | - increase of DT brain uptake in mices; - higher DOX transport across bEnd.3 monolayer and uptake by U87MG glioblastoma cells; - reduction of tumor size of glioma-bearing mice. | [16,17] |
| Glutathione | Liposomes; Polymeric nanoparticles | EPC/CHOL/GSH; PLGA/GSH; PEI/GSH | RBV, PTX | 80 to 300 | −40 to −5 | 45 | intravenous, intranasal | - increase of RBV and PTX brain uptake in rats; - receptor-mediated uptake in human, bovine, and porcine cerebral microvascular endothelial cells; - enhanced passage across the hCMEC/D3 cells. | [18,19,20] |
| Amino acids | Liposomes; Lipid nanoparticles | SL/Chol/TPGS/GLU; DSPE/PEG/ PHE | DOX | 80 to 165 | −35 to −15 | 75 | intravenous | - higher uptake by C6 glioma cells and accumulation of DOX in brain of mice. | [21,22] |
| Receptor-mediated transcytosis | |||||||||
| Transferrin | Lipid nanoparticles; Polymeric nanoparticles; Carbon dots; Albumin nanoparticles; Liposomes; Silica/polymeric/magnetic; Dendrimers | Compritol/SA/Tf; PLGA/Tf; Carbon powder/Tf; HSA/Tf; DSPE/PEG/Tf; PLGA/TMOS/Fe3O4/Tf; DAB/Tf | SQV, LOP, α-M, DOX/PTX, pβ-Gal | 10 to 200 | −30 to −5 | 20 to 90 | intravenous | - Higher uptake and transcytosis across human brain-microvascular endothelial cell monolayers; - C-dots with Tf can enter easily in the CNS of injected zebrafish; - Higher LOP brain uptake and anti-nociceptive effects in the tail-flick test in ICR (CD-1) mice; - Tf-liposomes improved brain delivery of α-M in rats; - Strong anti-glioma activity of DOX/PTX Tf-NPs in mice; - higher β-Gal expression in the brain after Tf-dendriplex administration in mice. | [23,24,25,26,27,28,29] |
| OX26 mAb | Albumin nanoparticles; Lipid nanocapsules; Polymeric nanoparticles; Liposomes | HSA/OX26; SL/PC/PE/OX26; PLGA/ DE2B4/OX26; POPC/PEG/OX26; DSPC/Chol/DSPE-PEG/19B8Mab/OX26 | LOP, iAβ5, pTH | 115 to 170 | −35 to −3 | 60 | intravenous | - higher LOP brain uptake and anti-nociceptive effects in the tail-flick test in ICR (CD-1) mice; - increased brain uptake compared to non-targeted nanocapsules in rats; - increased uptake of iAβ5 in porcine brain capillary endothelial cells; - pTH-NPs enter CNS, rescue gene expression and revert motor impairment in PD rats. | [26,30,31,32,33] |
| Lactoferrin | Polymeric nanoparticles; Cyclodextrins; Dendrimers; Lipid nanoparticles; Liposomes | PLGA/PEG/Lf; PCL/PEG/Lf; PLGA/PEG/Lf; PLGA/FA/Lf; HA/Lf; β-CDs/PEG/Lf; DAB/Lf; PAMAM/PEG/Lf; BA/TP/Cac/TMX/Lf; EPC/CHOL/CHETA/Lf | UCN, ETP, NAP, DOX, pβ-Gal, GDNF, BCNU, DOX | 90 to 210 | −30 to +40 | 35 to 98 | intravenous, intranasal, | - enhanced brain uptake and bioavailability in mice; - neuroprotection and memory improvements in AD mice model after NAP-NPs administration; - attenuation of striatum lesion caused by 6-OHDA in PD rats after UCN-NPs administration; - improved locomotor activity and reduced neuronal cell loss in PD rats by GDNF-dendrimers; - higher β-Gal expression in the brain after Lf-dendrimers administration in mice; - enhanced permeation across BBB cell monolayer and inhibition of U87MG glioblastoma cell growth; - higher accumulation in tumor cells and extended survival time of glioma-bearing rats by DOX-NPs. | [34,35,36,37,38,39,40,41,42,43] |
| ApoE | Lipid nanoparticles; Albumin nanoparticles | CP/DSPE-PEG/ApoE; HSA/PEG/ApoE; SL/PC/mApoE | RSV | 120 to 250 | −55 to −15 | 85 to 98 | intravenous, intrapulmonary | - higher permeability of RSV-NPs through hCMEC/D3 monolayers; - only ApoE-NPs found in brain capillary endothelial cells and neurons of SV 129 mice; - enhanced brain target via pulmonary administration in mice. | [44,45,46] |
| Polysorbate 80 | Polymeric nanoparticles | PLA/PS80; PBCA/PS80; PLGA/PS80 | NTX-1, TC, RVT, BA, GEM | 35 to 210 | −40 to −10 | 50 to 60 | intravenous, intranasal | - only PS80-coated NPs found in brain tissues of mice; - antinociceptive effects after NTX-1-loaded NPs administration in mice; - higher accumulation of TC-NPs, RVT-NPs and BA-NPs than free forms in brain of rats; - GEM-NPs inhibit C6 glioma cells growth and increase survival time of rats. | [47,48,49,50,51,52] |
| Angiopep-2 | Polymeric nanoparticles; Micelles; Gold nanoparticles; Carbon nanotubes; Dendrimers | PCL/PEG/AP-2; PE/PEG/AP-2; Au/PEG/AP-2; MWNTs/PEG/AP-2; PAMAM/PEG/AP2 | AMB, DOX, TRAIL | 10 to 200 | −20 to +15 | 80 | intravenous | - higher accumulation of AP-2 NPs in the brain of SD rats, compared to non-functionalized ones; - enhanced permeation of AMB across BBB and brain uptake in SD rats for brain fungal burden; - increased survival time of glioma-bearing mice after DOX-loaded Au NPs and MWNTs; - enhanced brain uptake and survival time of tumor-bearing mices after TRAIL-dendrimers. | [53,54,55,56,57] |
| RVG29 | Polymeric nanoparticles; Dendrimers; | PLGA/RVG29; PAMAM/PEG/RVG29; DGL/PEG/RVG29; Pluronic/PEG/RVG29 | CPT, pLuc, shRNAs, β-Gal, | 110 to 150 | −3 to 12 | 40 to 80 | intravenous | - enhanced apparent brain delivery of NPs in the presence of RVG29 peptide; - increased expression of LUC gene in mice brain; - reduction of neurofibrillary tangles and rescue of memory loss in AD transgenic mice; - β-Gal delivered and accumulated efficiently in mice brain. | [58,59,60,61] |
| CDX | Liposomes; Polymeric nanoparticles | HSPC/DSPE-PEG/CDX; PLA/PEG/CDX | DOX, PTX | 95 | n.i | 95 | intravenous | - ability for crossing the BBB monolayer and enhanced median survival time in glioma-bearing mice after DOX and PTX-loaded NPs-CDX administration. | [62,63] |
| RGD | Magnetic nanoparticles; Liposomes | Fe2O3/PEG/RGD; DPPC/Chol/TPGS/RGD | DT/QDs | 40 to 180 | −20 to +1 | 70 | intravenous | - accumulation in the cancer site, generating a MRI contrast for mice brain tumor imaging; - enhanced brain uptake and imaging after DT/QDs-loaded liposomes administration in mice. | [64,65] |
| NGR | Polymeric nanoparticles; Liposomes; Quantum dots | PLGA/PEG/NGR; HSPC/CHOL/DSPE-PEG/NGR; OEI/PEG/NGR; CdSe/ZnS/PEG/NGR | DT, DOX, siRNA-Luc | 10 to 130 | 15 | 50 | intravenous | - anti-angiogenesis and prolonged survival time in mice bearing intracranial glioma by DT or DOX-NPs; - downregulation of reporter gene (Luciferase) in glioma cells in mice; - enhanced brain cancer imaging in rat glioma model after QDs-NGR administration. | [66,67,68,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, R.G.R.; Coutinho, A.J.; Pinheiro, M.; Neves, A.R. Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? Int. J. Mol. Sci. 2021, 22, 11654. https://doi.org/10.3390/ijms222111654
Pinheiro RGR, Coutinho AJ, Pinheiro M, Neves AR. Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? International Journal of Molecular Sciences. 2021; 22(21):11654. https://doi.org/10.3390/ijms222111654
Chicago/Turabian StylePinheiro, Rúben G. R., Ana Joyce Coutinho, Marina Pinheiro, and Ana Rute Neves. 2021. "Nanoparticles for Targeted Brain Drug Delivery: What Do We Know?" International Journal of Molecular Sciences 22, no. 21: 11654. https://doi.org/10.3390/ijms222111654
APA StylePinheiro, R. G. R., Coutinho, A. J., Pinheiro, M., & Neves, A. R. (2021). Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? International Journal of Molecular Sciences, 22(21), 11654. https://doi.org/10.3390/ijms222111654







