PR-619, a General Inhibitor of Deubiquitylating Enzymes, Diminishes Cisplatin Resistance in Urothelial Carcinoma Cells through the Suppression of c-Myc: An In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Results
2.1. The Cisplatin-Resistant Human UC Cell Line (T24/R) Was Resistant to Treatment with Cisplatin In Vitro and In Vivo
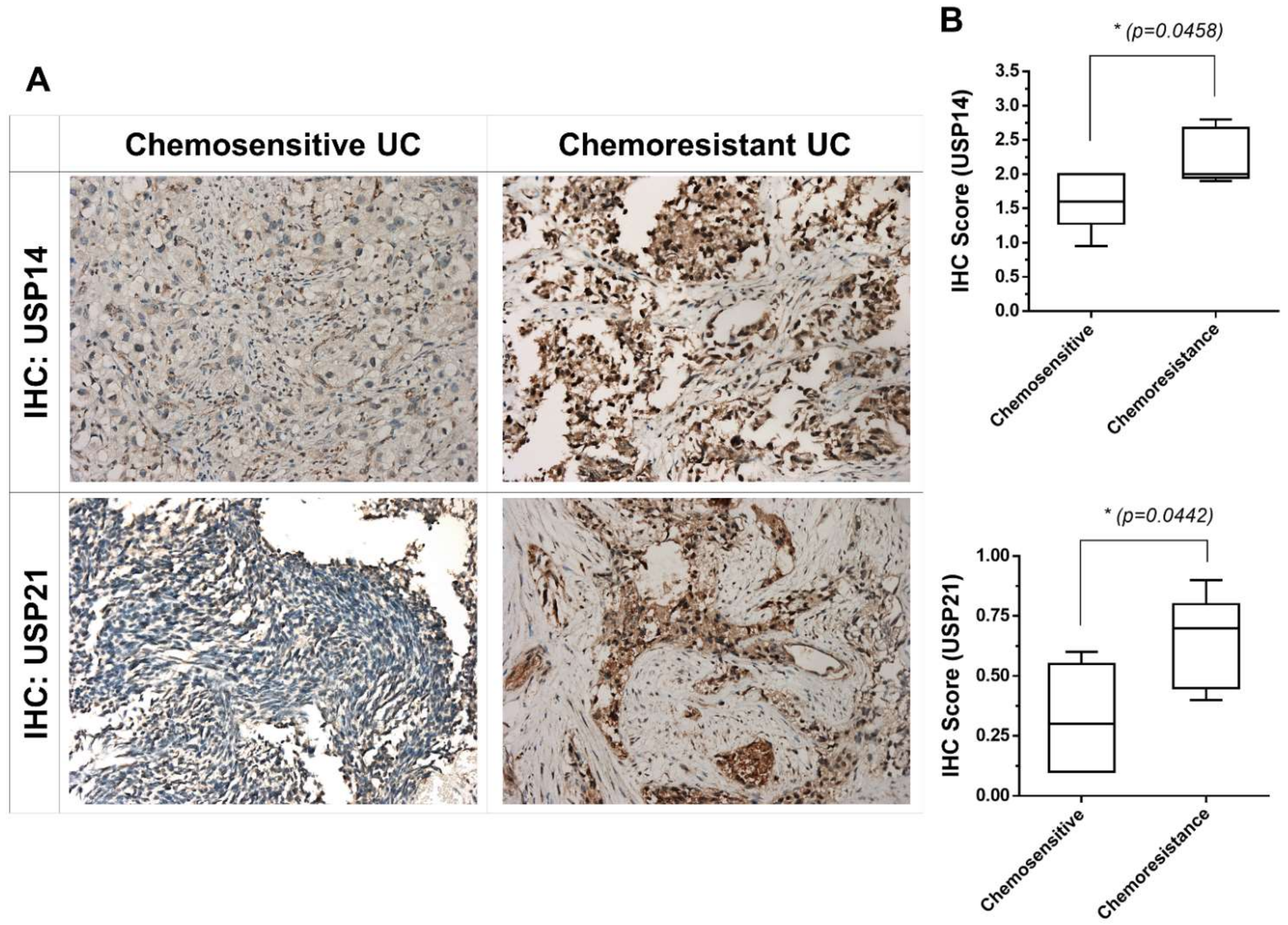
2.2. USP21 Overexpression Is Associated with Chemoresistance in Patients with Chemoresistant UC
2.3. PR-619 Effectively Induced Viability Inhibition, Apoptosis, and Endoplasmic Reticulum Stress-Related Apoptosis in T24/R Cells
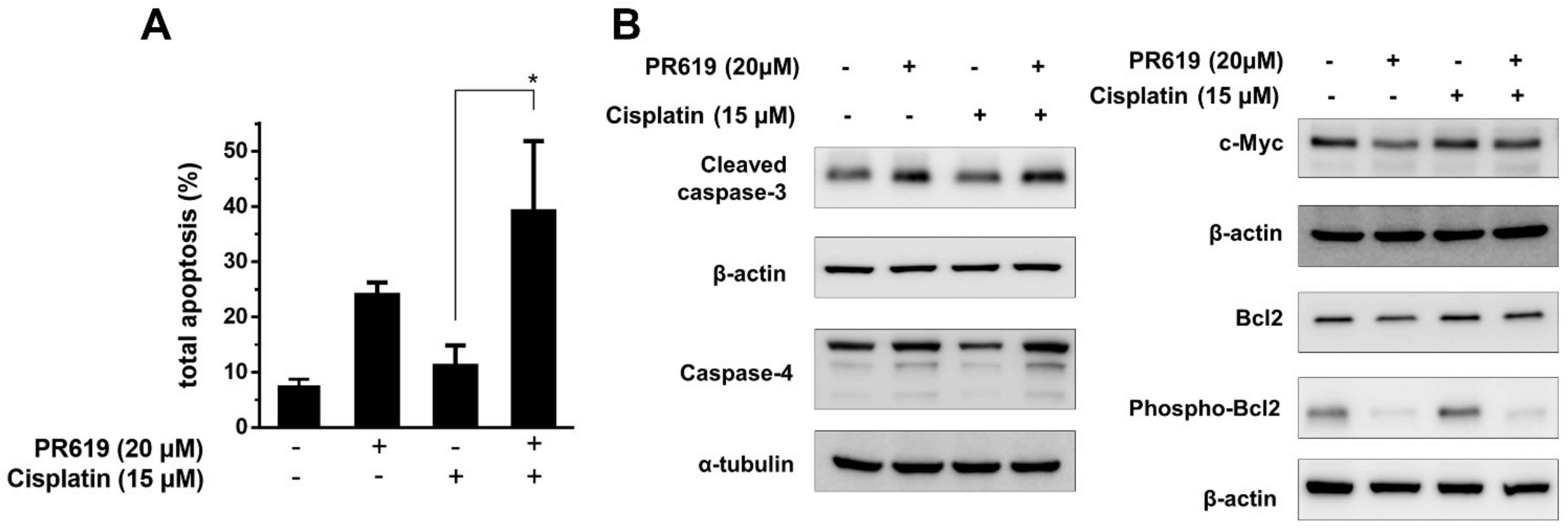
2.4. PR-619 Enhanced the Cytotoxic Effects of Cisplatin on T24/R Cells, and the Attenuation of Cisplatin Resistance Was Related to the Concurrent Suppression of c-Myc
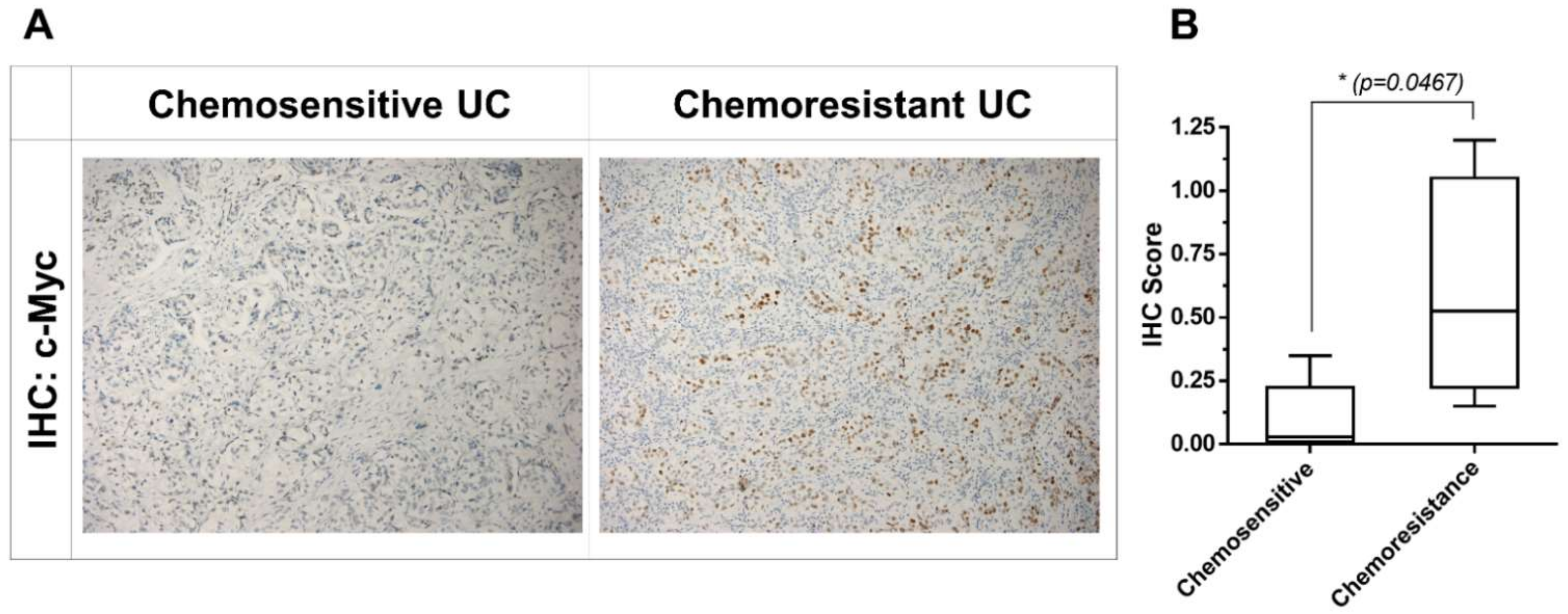
2.5. Upregulation of c-Myc Is Associated with Chemoresistance in Patients with Chemoresistant UC
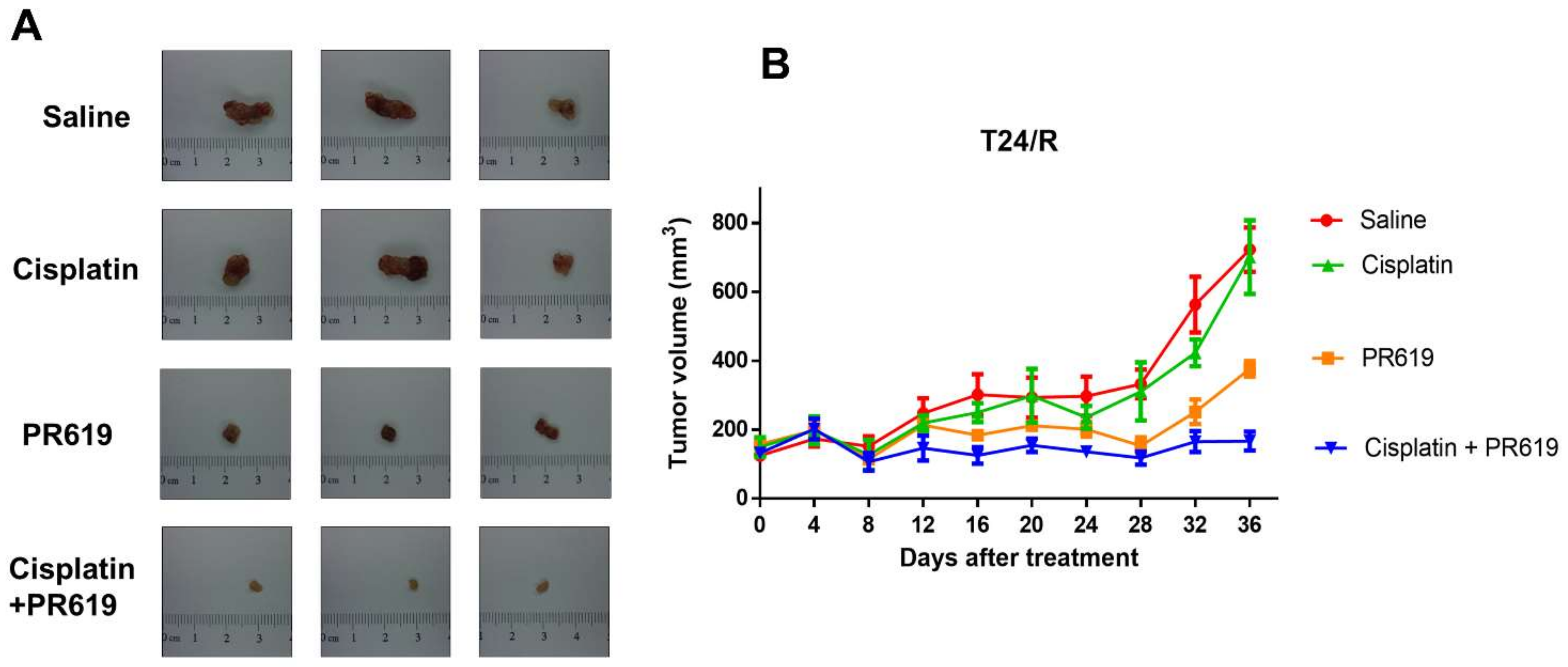
2.6. PR-619 Enhanced the Antitumor Effect of Cisplatin in a Xenograft Mouse Model of T24/R
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals and Antibodies
4.3. Measurement of Cell Viability
4.4. Western Blot Analysis
4.5. Apoptosis Assay
4.6. Cell Cycle Analysis
4.7. Immunohistochemical (IHC) Staining in Human UC Specimens
4.8. In Vivo Xenograft Experiments of PR-619 and Cisplatin Combination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rentsch, C.A.; Muller, D.C.; Ruiz, C.; Bubendorf, L. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma: A Step Closer to Clinical Translation? Eur. Urol. 2017, 72, 960–961. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nishiyama, H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017, 24, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt, J.; Albiol, S.; Suarez, C.; Albanell, J. Optimizing therapeutic strategies in advanced bladder cancer: Update on chemotherapy and the role of targeted agents. Crit. Rev. Oncol. Hematol. 2009, 69, 211–222. [Google Scholar] [CrossRef]
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.; Bajorin, D.F.; et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011, 12, 211–214. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, Z.; Liang, L.; Cheng, Y.; Zhou, J.; Li, J.; Xu, Y. NF-kappaB signaling plays irreplaceable roles in cisplatin-induced bladder cancer chemoresistance and tumor progression. Int. J. Oncol. 2016, 48, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Nunes, T.; Hamdan, D.; Leboeuf, C.; El Bouchtaoui, M.; Gapihan, G.; Nguyen, T.T.; Meles, S.; Angeli, E.; Ratajczak, P.; Lu, H.; et al. Targeting Cancer Stem Cells to Overcome Chemoresistance. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharm. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [Green Version]
- Stordal, B.; Davey, M. Understanding cisplatin resistance using cellular models. IUBMB Life 2007, 59, 696–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stordal, B.; Pavlakis, N.; Davey, R. A systematic review of platinum and taxane resistance from bench to clinic: An inverse relationship. Cancer Treat. Rev. 2007, 33, 688–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.H.; Chang, J.Y. New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madden, S.K.; de Araujo, A.D.; Gerhardt, M.; Fairlie, D.P.; Mason, J.M. Taking the Myc out of cancer: Toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer 2021, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Gabay, M.; Li, Y.; Felsher, D.W. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect Med. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.L.; Ciechanover, A. Targeting proteins for destruction by the ubiquitin system: Implications for human pathobiology. Annu. Rev. Pharm. Toxicol. 2009, 49, 73–96. [Google Scholar] [CrossRef] [Green Version]
- Nijman, S.M.; Luna-Vargas, M.P.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.; Sixma, T.K.; Bernards, R. A genomic and functional inventory of deubiquitinating enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Liu, Z.; Yang, Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol. Cancer 2020, 19, 146. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanguturi, P.; Kim, K.S.; Ramakrishna, S. The role of deubiquitinating enzymes in cancer drug resistance. Cancer Chemother. Pharm. 2020, 85, 627–639. [Google Scholar] [CrossRef]
- Tian, X.; Isamiddinova, N.S.; Peroutka, R.J.; Goldenberg, S.J.; Mattern, M.R.; Nicholson, B.; Leach, C. Characterization of selective ubiquitin and ubiquitin-like protease inhibitors using a fluorescence-based multiplex assay format. Assay. Drug Dev. Technol. 2011, 9, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Mullally, J.E.; Moos, P.J.; Edes, K.; Fitzpatrick, F.A. Cyclopentenone prostaglandins of the J series inhibit the ubiquitin isopeptidase activity of the proteasome pathway. J. Biol. Chem. 2001, 276, 30366–30373. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, M.; Sha, B.; Hu, X.; Sun, Y.; Zhu, M.; Xu, Y.; Li, P.; Wang, Y.; Guo, Y.; et al. Inhibition of deubiquitination by PR-619 induces apoptosis and autophagy via ubi-protein aggregation-activated ER stress in oesophageal squamous cell carcinoma. Cell Prolif. 2021, 54, e12919. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Liu, S.H.; Lin, W.C.; Hsu, F.S.; Chow, P.M.; Chang, Y.W.; Yang, S.P.; Shi, C.S.; Hsu, C.H.; Liao, S.M.; et al. Trifluoperazine, an Antipsychotic Drug, Effectively Reduces Drug Resistance in Cisplatin-Resistant Urothelial Carcinoma Cells via Suppressing Bcl-xL: An In Vitro and In Vivo Study. Int. J. Mol. Sci 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Xiao, H.; Yang, Q.; Hu, R.; Jiang, L.; Bi, R.; Jiang, X.; Wang, L.; Mei, J.; Ding, F.; et al. The USP21/YY1/SNHG16 axis contributes to tumor proliferation, migration, and invasion of non-small-cell lung cancer. Exp. Mol. Med. 2020, 52, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Song, T.; Sun, C.; He, N.; Cheng, Q.; Xiao, X.; Ran, J.; Liu, M.; Xie, S. USP21 upregulation in cholangiocarcinoma promotes cell proliferation and migration in a deubiquitinase-dependent manner. Asia Pac. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Lei, H.; Shan, H.; Wu, Y. Targeting deubiquitinating enzymes in cancer stem cells. Cancer Cell Int. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhou, B.; Chen, D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco. Targets Ther. 2017, 10, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Poondla, N.; Chandrasekaran, A.P.; Kim, K.S.; Ramakrishna, S. Deubiquitinating enzymes as cancer biomarkers: New therapeutic opportunities? BMB Rep. 2019, 52, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.S.; Wang, X.F.; Zhang, Y.J.; Luo, P.; Long, H.D.; Li, L.; Yang, H.Q.; Xie, R.T.; Jia, C.Y.; Lu, G.X.; et al. Inhibition of USP14 Deubiquitinating Activity as a Potential Therapy for Tumors with p53 Deficiency. Mol. Ther. Oncolytics 2020, 16, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Melo-Cardenas, J.; Zhang, Y.; Zhang, D.D.; Fang, D. Ubiquitin-specific peptidase 22 functions and its involvement in disease. Oncotarget 2016, 7, 44848–44856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, K.L.; Lin, W.C.; Liu, S.H.; Hsu, F.S.; Kuo, Y.; Liao, S.M.; Yang, S.P.; Wang, Z.H.; Hsu, C.H.; Huang, K.H. THZ1, a covalent CDK7 inhibitor, enhances gemcitabine-induced cytotoxicity via suppression of Bcl-2 in urothelial carcinoma. Am. J. Cancer Res. 2021, 11, 171–180. [Google Scholar] [PubMed]
- Check, W. How do things stand with cisplatin? JAMA J. Am. Med. Assoc. 1978, 240, 2521–2525. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, F.; Ma, X.; Cao, Z.; Li, Y.; Chen, Y.Z. Mechanisms of drug combinations: Interaction and network perspectives. Nat. Reviews. Drug Discov. 2009, 8, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C.; Gu, C.; Li, Q.; Wu, N. Function of Deubiquitinating Enzyme USP14 as Oncogene in Different Types of Cancer. Cell Physiol. Biochem. 2016, 38, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Ho, I.L.; Shi, C.S.; Wu, J.T.; Lin, W.C.; Tsai, Y.C.; Chang, H.C.; Chou, C.T.; Hsu, C.H.; Hsieh, J.T.; et al. MLN4924, a novel protein neddylation inhibitor, suppresses proliferation and migration of human urothelial carcinoma: In vitro and in vivo studies. Cancer Lett. 2015, 363, 127–136. [Google Scholar] [CrossRef]
- Tang, C.H.; Tan, T.W.; Fu, W.M.; Yang, R.S. Involvement of matrix metalloproteinase-9 in stromal cell-derived factor-1/CXCR4 pathway of lung cancer metastasis. Carcinogenesis 2008, 29, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Liu, S.H.; Lin, W.C.; Chow, P.M.; Chang, Y.W.; Yang, S.P.; Shi, C.S.; Hsu, C.H.; Liao, S.M.; Chang, H.C.; et al. The Deubiquitinating Enzyme Inhibitor PR-619 Enhances the Cytotoxicity of Cisplatin via the Suppression of Anti-Apoptotic Bcl-2 Protein: In Vitro and In Vivo Study. Cells 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.C.; Hsu, F.S.; Kuo, K.L.; Liu, S.H.; Shun, C.T.; Shi, C.S.; Chang, H.C.; Tsai, Y.C.; Lin, M.C.; Wu, J.T.; et al. Trichostatin A, a histone deacetylase inhibitor, induces synergistic cytotoxicity with chemotherapy via suppression of Raf/MEK/ERK pathway in urothelial carcinoma. J. Mol. Med. 2018, 96, 1307–1318. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, F.-S.; Lin, W.-C.; Kuo, K.-L.; Chiu, Y.-L.; Hsu, C.-H.; Liao, S.-M.; Dong, J.-R.; Liu, S.-H.; Chang, S.-C.; Yang, S.-P.; et al. PR-619, a General Inhibitor of Deubiquitylating Enzymes, Diminishes Cisplatin Resistance in Urothelial Carcinoma Cells through the Suppression of c-Myc: An In Vitro and In Vivo Study. Int. J. Mol. Sci. 2021, 22, 11706. https://doi.org/10.3390/ijms222111706
Hsu F-S, Lin W-C, Kuo K-L, Chiu Y-L, Hsu C-H, Liao S-M, Dong J-R, Liu S-H, Chang S-C, Yang S-P, et al. PR-619, a General Inhibitor of Deubiquitylating Enzymes, Diminishes Cisplatin Resistance in Urothelial Carcinoma Cells through the Suppression of c-Myc: An In Vitro and In Vivo Study. International Journal of Molecular Sciences. 2021; 22(21):11706. https://doi.org/10.3390/ijms222111706
Chicago/Turabian StyleHsu, Fu-Shun, Wei-Chou Lin, Kuan-Lin Kuo, Yen-Ling Chiu, Chen-Hsun Hsu, Shih-Ming Liao, Jun-Ren Dong, Shing-Hwa Liu, Shih-Chen Chang, Shao-Ping Yang, and et al. 2021. "PR-619, a General Inhibitor of Deubiquitylating Enzymes, Diminishes Cisplatin Resistance in Urothelial Carcinoma Cells through the Suppression of c-Myc: An In Vitro and In Vivo Study" International Journal of Molecular Sciences 22, no. 21: 11706. https://doi.org/10.3390/ijms222111706
APA StyleHsu, F.-S., Lin, W.-C., Kuo, K.-L., Chiu, Y.-L., Hsu, C.-H., Liao, S.-M., Dong, J.-R., Liu, S.-H., Chang, S.-C., Yang, S.-P., Chen, Y.-T., Chang, R.-J., & Huang, K.-H. (2021). PR-619, a General Inhibitor of Deubiquitylating Enzymes, Diminishes Cisplatin Resistance in Urothelial Carcinoma Cells through the Suppression of c-Myc: An In Vitro and In Vivo Study. International Journal of Molecular Sciences, 22(21), 11706. https://doi.org/10.3390/ijms222111706





