Collecting Duct-Specific CR6-Interacting Factor-1-Deletion Aggravates Renal Inflammation and Fibrosis Induced by Unilateral Ureteral Obstruction
Abstract
:1. Introduction
2. Results
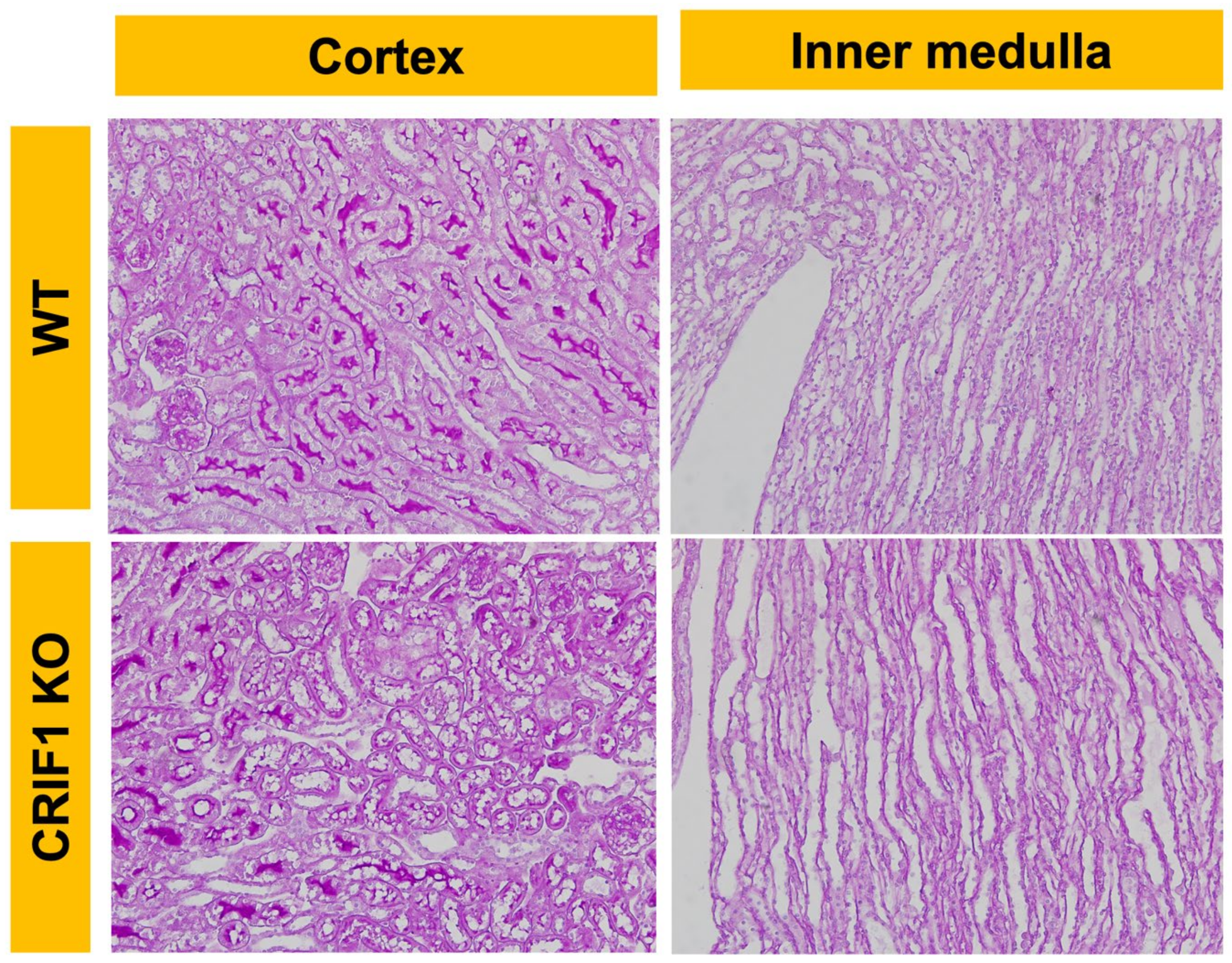
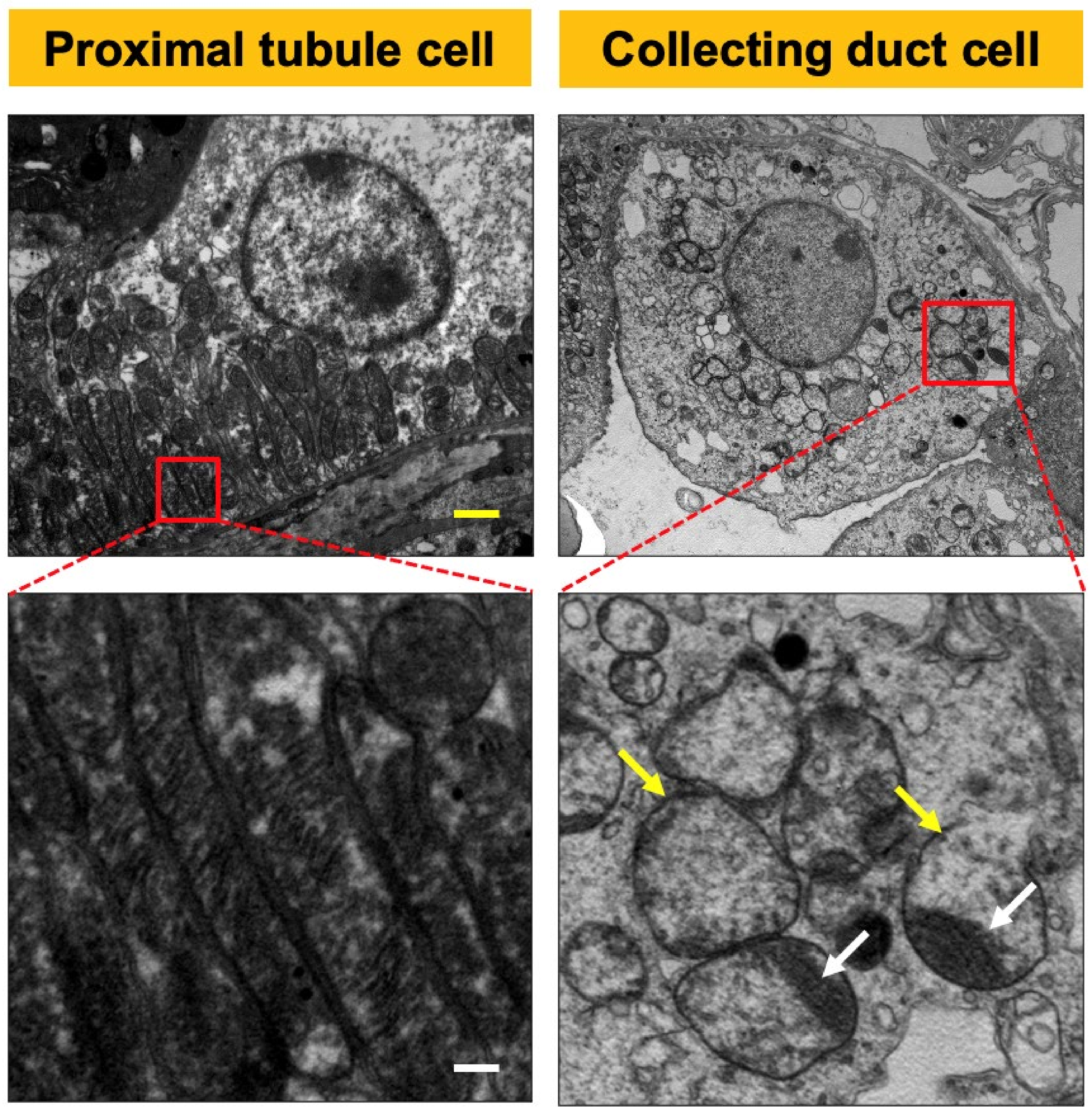
2.1. CRIF1-KO Mice Showed Extensive Mitochondrial Destruction of Collecting Duct Cells
2.2. Silencing RNA of CRIF1-Induced Mitochondrial Dysfunction in Inner Medullary Collecting Duct (mIMCD) Cells
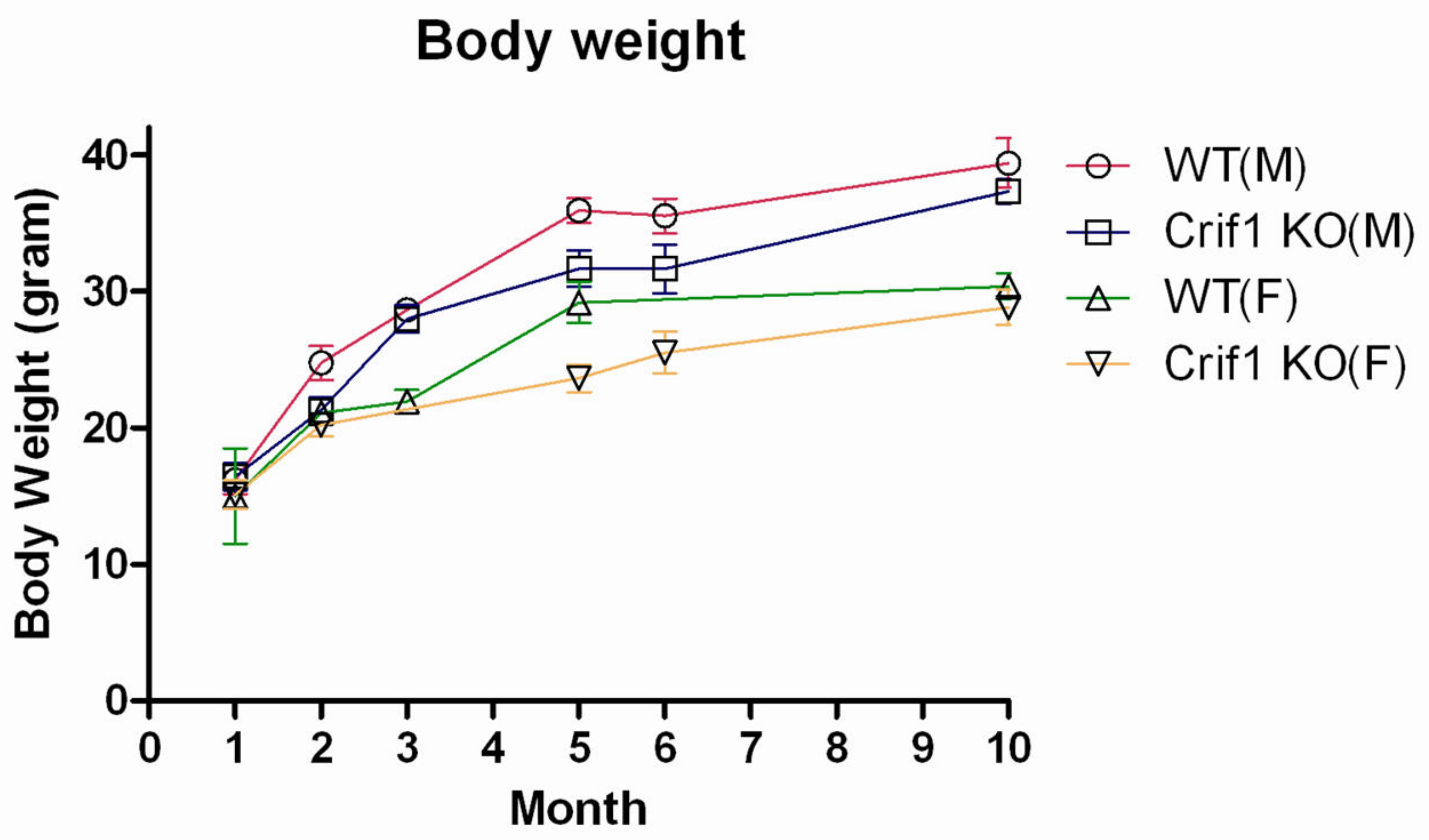
2.3. Phenotype of CRIF1-KO Mice
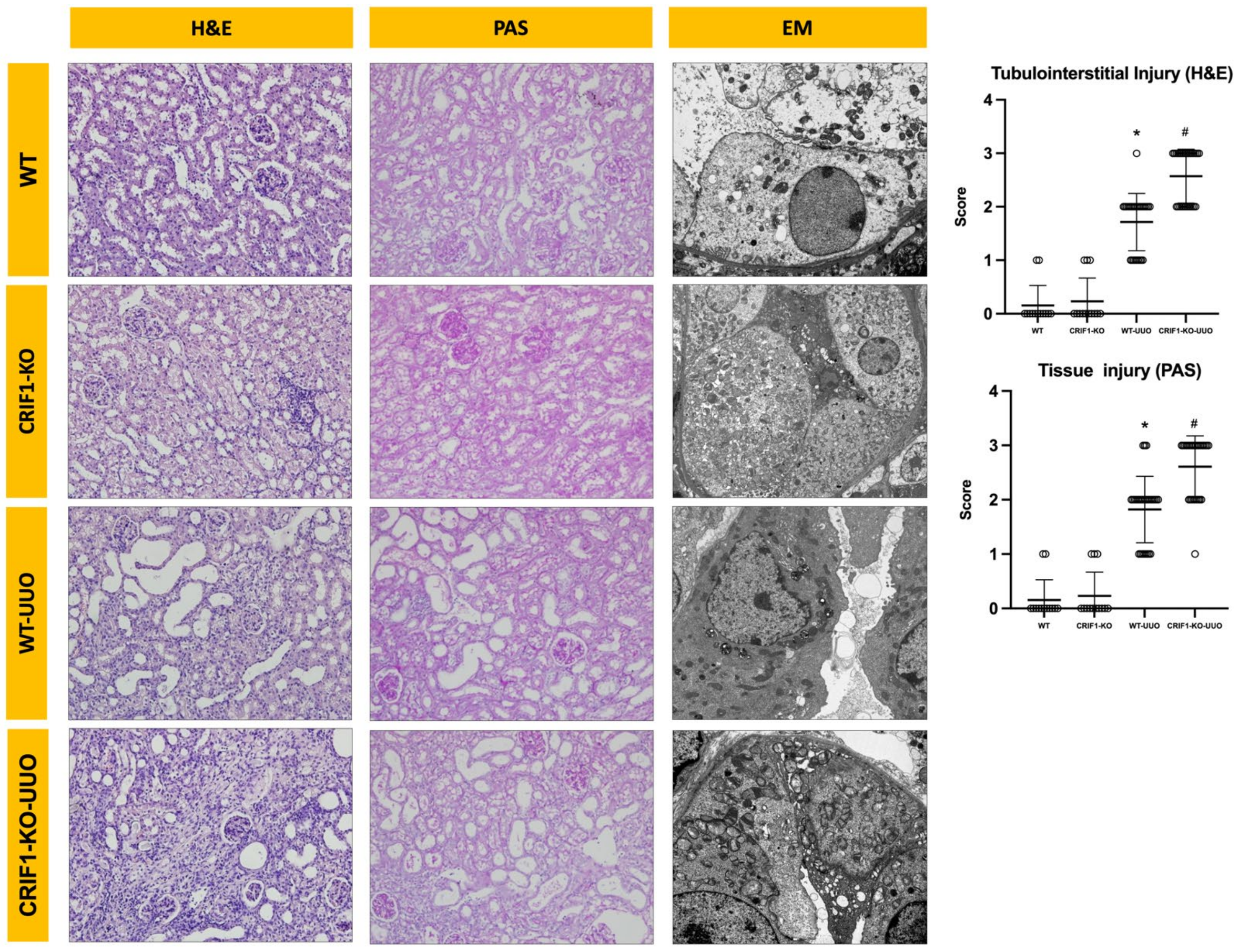
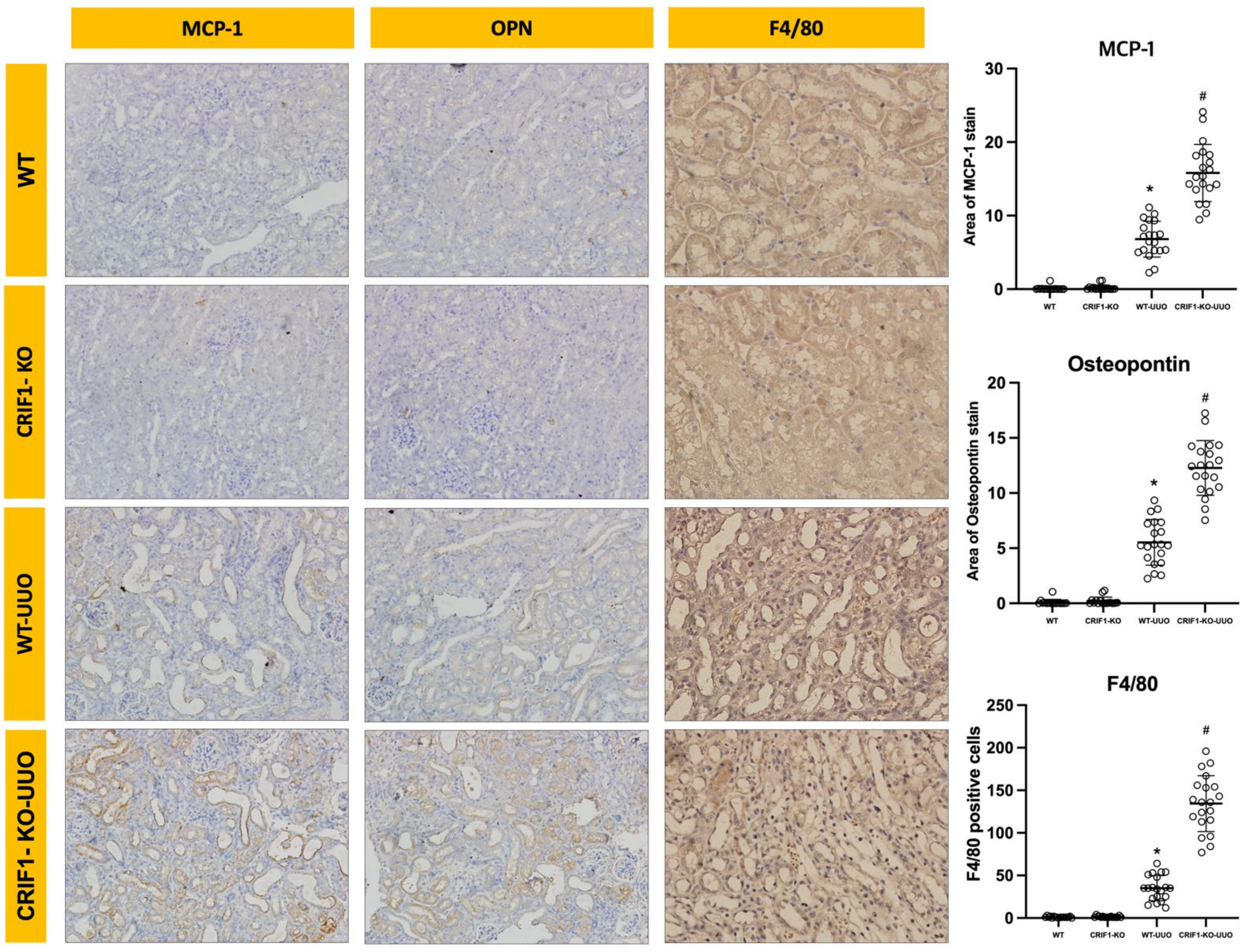
2.4. Effects of Mitochondrial Dysfunction of Collecting Duct Cells on UUO-Induced Renal Injury
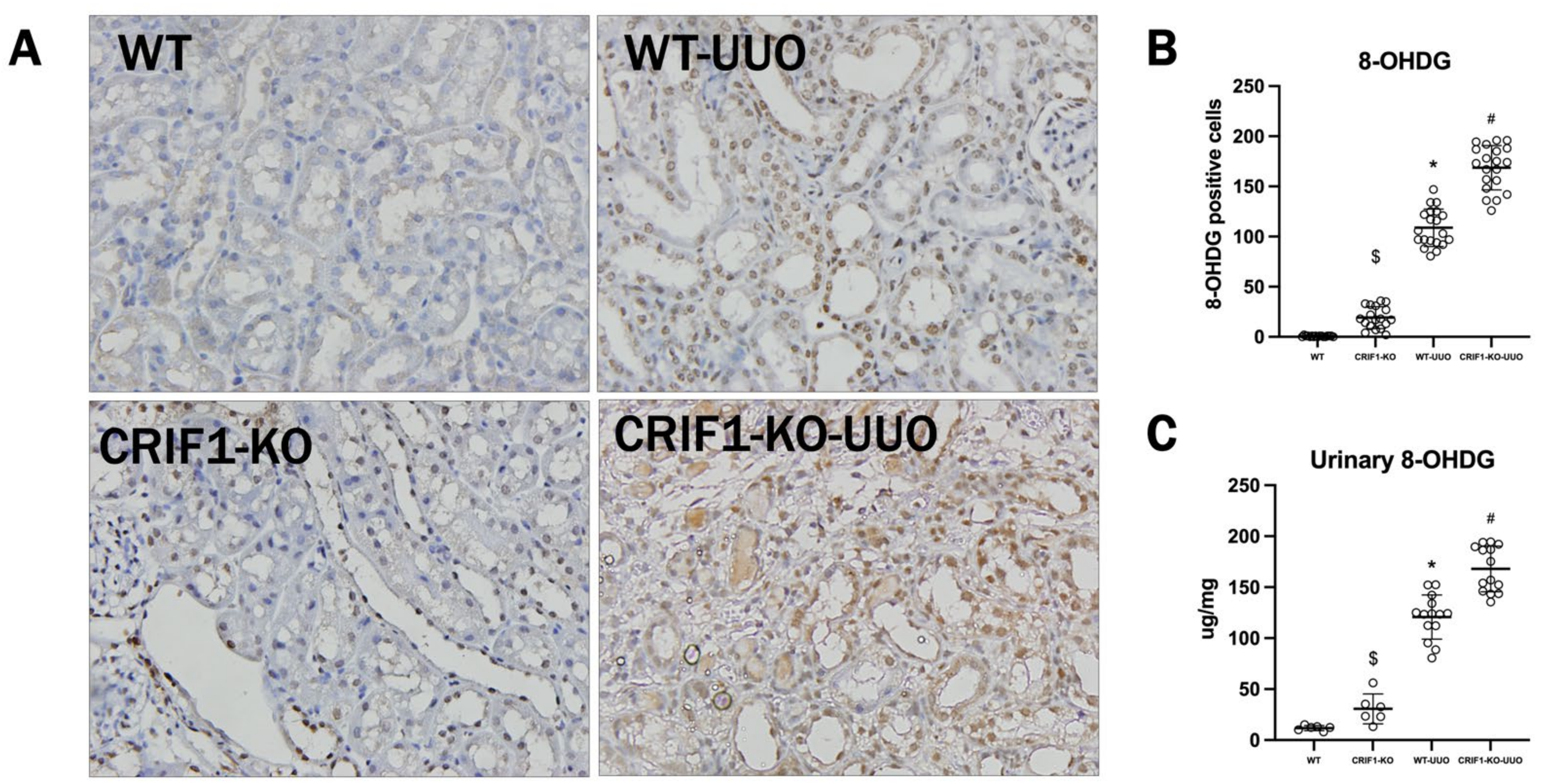
2.5. The Effect of Oxidative Stress on UUO-Induced Injury
3. Discussion
4. Materials and Methods
4.1. Animals and UUO Operation
4.2. Development of CRIF1-KO Mouse Models
4.3. Measurement of Blood and Urine Chemistries
4.4. Measurement of Urine 8-OHDG
4.5. Tissue Preparation
4.6. Light Microscopy Examination
4.7. Electron Microscopic Examination
4.8. Cell Culture
4.9. Small Interfering RNA (siRNA) Experiments
4.10. RNA Extraction, Semi-Quantitative Reverse Transcription PCR, and Real-Time PCR
4.11. Mitochondrial Function Test
4.12. Immunohistochemistry
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| UUO | Unilateral Ureteral Obstruction |
| ATP | Adenosine triphosphate |
| CRIF1 | CR6-interacting factor-1 |
| OCR | oxygen consumption rate |
| CCCP | chain decoupler carbonyl cyanide m-chlorophenylhydrazone |
| mIMCD | inner medullary collecting duct |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| OPN | osteopontin |
| CKD | chronic kidney disease |
References
- Parrish, A.R. Advances in chronic kidney disease. Int. J. Mol. Sci. 2016, 17, 1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomeru-lotubular junction. Am. J. Physiol.-Renal 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Fujiu, K.; Manabe, I.; Nagai, R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J. Clin. Investig. 2011, 121, 3425–3441. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.; Nam, S.A.; Kim, W.-Y.; Park, S.H.; Kim, H.; Yang, C.W.; Kim, J.; Kim, Y.K. Notch signaling in the collecting duct regulates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction in mice. Korean J. Intern. Med. 2018, 33, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Poyton, R.O.; Ball, K.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, H.-S.; Park, K.M. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 2010, 298, F158–F166. [Google Scholar] [CrossRef] [Green Version]
- Chacko, B.; Reily, C.; Srivastava, A.; Johnson, M.S.; Ulasova, E.; Agarwal, A.; Zinn, K.; Murphy, M.P.; Kalyanaraman, B.; Darley-Usmar, V. Prevention of diabetic nephropathy in Ins2+/-AkitaJ mice by the mitochondria-targeted therapy Mito Q. Biochem. J. 2010, 432, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Santos, N.A.; Catao, C.S.; Martins, N.M.; Curti, C.; Bianchi, M.L.; Santos, A.C. Cisplatin-induced nephrotoxicity is asso-ciated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mito-chondria. Arch. Toxicol. 2007, 81, 495–504. [Google Scholar] [CrossRef]
- Schwerdt, G.; Freudinger, R.; Schuster, C.; Weber, F.; Thews, O.; Gekle, M. Cisplatin-induced apoptosis is enhanced by hypoxia and by inhibition of mitochondria in renal collecting duct cells. Toxicol. Sci. 2005, 85, 735–742. [Google Scholar] [CrossRef]
- Wang, J.; Biju, M.P.; Wang, M.-H.; Haase, V.H.; Dong, Z. Cytoprotective Effects of hypoxia against cisplatin-induced tubular cell apoptosis: Involvement of mitochondrial inhibition and p53 suppression. J. Am. Soc. Nephrol. 2006, 17, 1875–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.K.; Yi, Y.W.; Jung, N.-C.; Kim, D.; Suh, J.M.; Kim, H.; Park, K.C.; Song, J.H.; Kim, D.W.; Hwang, E.S.; et al. CR6-interacting factor 1 interacts with gadd45 family proteins and modulates the cell cycle. J. Biol. Chem. 2003, 278, 28079–28088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, K.; Nakayama, N.; Wang, T.-L.; Shih, I.-M. NAC-1 Controls cell growth and survival by repressing transcription of gadd45gip1, a candidate tumor suppressor. Cancer Res. 2007, 67, 8058–8064. [Google Scholar] [CrossRef] [Green Version]
- Na, K.; Jeong, J.; Shin, J.; Chang, Y.-K.; Suh, K.-S.; Lee, K.; Choi, D. Mitochondrial dysfunction in podocytes caused by crif1 deficiency leads to progressive albuminuria and glomerular sclerosis in mice. Int. J. Mol. Sci. 2021, 22, 4827. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Al-Bataineh, M.M.; Pastor-Soler, N.M. Collecting Duct intercalated cell function and regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 305–324. [Google Scholar] [CrossRef]
- Lote, C.J.; Harper, L.; O Savage, C. Mechanisms of acute renal failure. Br. J. Anaesth. 1996, 77, 82–89. [Google Scholar] [CrossRef]
- Klahr, S.; Morrissey, J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Renal Physiol. 2002, 283, F861–F875. [Google Scholar] [CrossRef] [Green Version]
- Kinter, M.; Wolstenholme, J.T.; Thornhill, B.A.; Newton, E.A.; McCormick, M.L.; Chevalier, R.L. Unilateral ureteral ob-struction impairs renal antioxidant enzyme activation during sodium depletion. Kidney Int. 1999, 55, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.; Sun, L.; Nayak, B.; Haruna, Y.; Liu, F.Y.; Kashihara, N.; Kanwar, Y.S. C/EBP-beta modulates transcription of tubu-lointerstitial nephritis antigen in obstructive uropathy. J. Am. Soc. Nephrol. 2009, 20, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nat. Cell Biol. 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Dicus, M.; Ho, N.D.; Boroujerdi-Rad, L.; Sindhu, R.K. Oxidative stress and dysregulation of superoxide dis-mutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003, 63, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and ther-apeutic possibilities. Diabetes Care 2008, 31 (Suppl. 2), S170–S180. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.; Fujimoto, S.; Haruna, Y.; Arakawa, S.; Horike, H.; Komai, N.; Sasaki, T.; Tsujioka, K.; Makino, H.; Kashihara, N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am. J. Physiol. Physiol. 2005, 288, F1144–F1152. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2008, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skulachev, V.P. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem. Biophys. Res. Commun. 2013, 441, 275–279. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2011, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, Y.; Tanaka, T.; Yoshida, Y.; Inagi, R. Physiological and pathophysiological role of reactive oxygen species and reactive nitrogen species in the kidney. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1097–1105. [Google Scholar] [CrossRef]



| WT | CRIF1-KO | p Value | |
|---|---|---|---|
| Arterial blood | |||
| pH | 7.22 (±0.06) | 7.18 (±0.01) | ns |
| Pco2(mmHg) | 47.80 (±11.48) | 54.33 (±1.53) | ns |
| HCO3-(mEq/L) | 19.26 (2.29) | 20.47 (±0.83) | ns |
| Serum | |||
| Urea Nitrogen (mg/dL) | 12.10 (±1.29) | 10.90 (±1.43) | ns |
| Creatinine (mg/dL) | 0.04 (±0.02) | 0.03 (±0.03) | ns |
| Na (mEq/L) | 142.8 (±2.37) | 150.6(4±8.82) | ns |
| K (mEq/L) | 3.6 (±0.31) | 3.9 (±0.63) | ns |
| Cl (mEq/L) | 93.1 (±2.04) | 98 (±6.34) | ns |
| Urine | |||
| 24 h Urine Volume(ml/g.bwt) | 0.059 (± 0.021) | 0.065 (±0.017) | ns |
| Urea Nitrogen (mg/dL) | 522.44 (±207.0) | 298.04 (±173.69) | ns |
| Creatinine (mg/dL) | 9.65 (±40.77) | 6.10 (±3.24) | ns |
| Na (mEq/L) | 32.04 (±7.51) | 21.82 (±11.95) | ns |
| K (mEq/L) | 35.68 (±14.87) | 17.34 (±12.42) | ns |
| Cl (mEq/L) | 32.52 (±10.11) | 18.82 (±15.53) | ns |
| ACR | 60.1 (±30.08) | 81.3 (±16.53) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.Y.; Na, K.R.; Shin, J.A.; Suh, K.-S.; Kim, J.-J.; Lee, K.W.; Choi, D.E. Collecting Duct-Specific CR6-Interacting Factor-1-Deletion Aggravates Renal Inflammation and Fibrosis Induced by Unilateral Ureteral Obstruction. Int. J. Mol. Sci. 2021, 22, 11699. https://doi.org/10.3390/ijms222111699
Jeong JY, Na KR, Shin JA, Suh K-S, Kim J-J, Lee KW, Choi DE. Collecting Duct-Specific CR6-Interacting Factor-1-Deletion Aggravates Renal Inflammation and Fibrosis Induced by Unilateral Ureteral Obstruction. International Journal of Molecular Sciences. 2021; 22(21):11699. https://doi.org/10.3390/ijms222111699
Chicago/Turabian StyleJeong, Jin Young, Ki Ryang Na, Jin Ah Shin, Kwang-Sun Suh, Jwa-Jin Kim, Kang Wook Lee, and Dae Eun Choi. 2021. "Collecting Duct-Specific CR6-Interacting Factor-1-Deletion Aggravates Renal Inflammation and Fibrosis Induced by Unilateral Ureteral Obstruction" International Journal of Molecular Sciences 22, no. 21: 11699. https://doi.org/10.3390/ijms222111699
APA StyleJeong, J. Y., Na, K. R., Shin, J. A., Suh, K.-S., Kim, J.-J., Lee, K. W., & Choi, D. E. (2021). Collecting Duct-Specific CR6-Interacting Factor-1-Deletion Aggravates Renal Inflammation and Fibrosis Induced by Unilateral Ureteral Obstruction. International Journal of Molecular Sciences, 22(21), 11699. https://doi.org/10.3390/ijms222111699






