Decline in Constitutive Proliferative Activity in the Zebrafish Retina with Ageing
Abstract
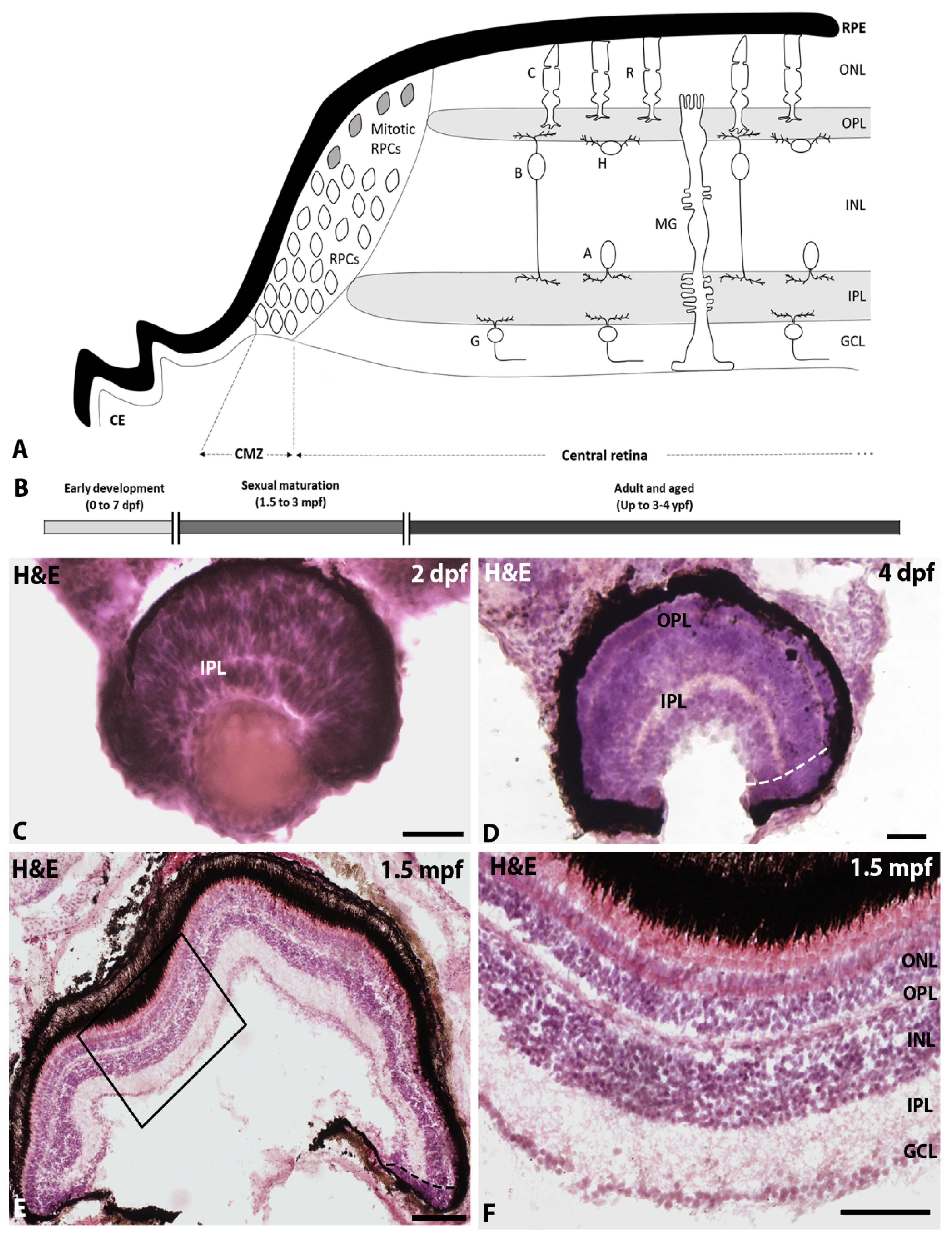
:1. Introduction
2. Results
2.1. Changes in Proliferative Activity with Ageing
2.2. Changes in the Location of Proliferating Cells of the Central Retina with Age
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation for Histology
4.3. Haematoxylin–Eosin Staining
4.4. Immunofluorescence
4.5. Specificity of Antibodies
4.6. Image Acquisition
4.7. Cell Quantifications and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Doetsch, F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003, 13, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, P. Is there a relationship between adult neurogenesis and neuron generation following injury across evolution? Eur. J. Neurosci. 2011, 34, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.; Sîrbulescu, R.F. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Eur. J. Neurosci. 2011, 34, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Grandel, H.; Brand, M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev Genes Evol. 2013, 223, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Than-Trong, E.; Bally-Cuif, L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 2015, 63, 1406–1428. [Google Scholar] [CrossRef]
- Alunni, A.; Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development 2016, 143, 741–753. [Google Scholar] [CrossRef] [Green Version]
- Zupanc, G.K.H. Adult neurogenesis in the central nervous system of teleost fish: From stem cells to function and evolution. J. Exp. Biol. 2021, 224, jeb226357. [Google Scholar] [CrossRef]
- Miles, A.; Tropepe, V. Retinal stem cell ‘retirement plans’: Growth, regulation and species adaptations in the retinal ciliary marginal zone. Int. J. Mol. Sci. 2021, 22, 6528. [Google Scholar] [CrossRef]
- Harris, W.A.; Perron, M. Molecular recapitulation: The growth of the vertebrate retina. Int. J. Dev. Biol. 1998, 42, 299–304. [Google Scholar]
- Fischer, A.J. Neural regeneration in the chick retina. Prog. Retin. Eye Res. 2005, 24, 161–182. [Google Scholar] [CrossRef]
- Raymond, P.A.; Barthel, L.K.; Bernardos, R.I.; Perkowski, J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Develop. Biol. 2006, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.J.; Bosse, J.L.; El-Hodiri, M. The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp. Eye Res. 2013, 116, 199–204. [Google Scholar] [CrossRef]
- Marcucci, F.; Murcia-Belmonte, V.; Wang, Q.; Coca, Y.; Ferreiro-Galve, S.; Kuwajima, T.; Khalid, S.; Ross, M.E.; Mason, C.; Herrera, E. The ciliary margin zone of the mammalian retina generates retinal ganglion cells. Cell Rep. 2016, 17, 3153–3164. [Google Scholar] [CrossRef]
- Bélanger, M.C.; Robert, B.; Cayouette, M. Msx1-positive progenitors in the retinal ciliary margin give rise to both neural and non-neural progenies in mammals. Dev. Cell. 2017, 40, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Fausett, B.V.; Goldman, D. A role for α1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006, 26, 6303–6313. [Google Scholar] [CrossRef] [Green Version]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilken, M.S.; Reh, T.A. Retinal regeneration in birds and mice. Curr. Opin. Genet. Dev. 2016, 40, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, T.S. Cellular metaplasia or transdifferentiation as a model for retinal cell differentiation. Curr. Top. Dev. Biol. 1980, 16, 349–380. [Google Scholar]
- Engelhardt, M.; Bogdahn, U.; Aigner, L. Adult retinal pigment epithelium cells express neural progenitor properties and the neuronal precursor protein doublecortin. Brain Res. 2005, 1040, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.T.Y.; Li, X.; Wang, S.Z. Reprogramming RPE to differentiate towards retinal neurons with Sox2. Stem Cells 2009, 27, 1376–1387. [Google Scholar] [CrossRef] [Green Version]
- Eymann, J.; Salomies, L.; Macrì, S.; Di-Poï, N. Variations in the proliferative activity of the peripheral retina correlate with postnatal ocular growth in squamate reptiles. J. Comp. Neurol. 2019, 527, 2356–2370. [Google Scholar] [CrossRef] [Green Version]
- Tropepe, V.; Coles, B.L.; Chiasson, B.J.; Horsford, D.J.; Elia, A.J.; McInnes, R.R.; van der Kooy, D. Retinal stem cells in the adult mammalian eye. Science 2000, 287, 2032–2036. [Google Scholar] [CrossRef]
- Fischer, A.J.; Reh, T.A. Transdifferentiation of pigmented epithelial cells: A source of retinal stem cells? Dev. Neurosci. 2001, 23, 268–276. [Google Scholar] [CrossRef]
- Fischer, A.J.; Reh, T.A. Growth factors induce neurogenesis in the ciliary body. Dev. Biol. 2003, 259, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Das, A.V.; James, J.; Rahnenführer, J.; Thoreson, W.B.; Bhattacharya, S.; Zhao, X.; Ahmad, I. Retinal properties and po-tential of the adult mammalian ciliary epithelium stem cells. Vision Res. 2005, 45, 1653–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.V.; Zhao, X.; James, J.; Kim, M.; Cowan, K.H.; Ahmad, I. Neural stem cells in the adult ciliary epithelium express GFAP and are regulated by Wnt signaling. Biochem. Biophys. Res. Commun. 2006, 339, 708–716. [Google Scholar] [CrossRef]
- Reh, T.A.; Fischer, A.J. Stem cells in the vertebrate retina. Brain Behav. Evol. 2001, 58, 296–305. [Google Scholar] [CrossRef]
- Amato, M.A.; Arnault, E.; Perron, M. Retinal stem cells in vertebrates: Parallels and divergences. Int. J. Dev. Biol. 2004, 48, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Moshiri, A.; Close, J.; Reh, T.A. Retinal stem cells and regeneration. Int. J. Dev. Biol. 2004, 48, 1003–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernald, R.D. Teleost vision: Seeing while growing. J. Exp. Zool. 1991, 5, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Perron, M.; Harris, W.A. Retinal stem cells in vertebrates. Bioessays 2000, 22, 685–688. [Google Scholar] [CrossRef]
- Kubota, R.; Hokoc, J.N.; Moshiri, A.; McGuire, C.; Reh, T.A. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Dev. Brain Res. 2002, 134, 31–41. [Google Scholar] [CrossRef]
- Wan, Y.; Almeida, A.D.; Rulands, S.; Chalour, N.; Muresan, L.; Wu, Y.; Simons, B.D.; He, J.; Harris, W.A. The ciliary marginal zone of the zebrafish retina: Clonal and time-lapse analysis of a continuously growing tissue. Development 2016, 143, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- García-Pradas, L.; Gleiser, C.; Wizenmann, A.; Wolburg, H.; Mack, A.F. Glial cells in the fish retinal nerve fiber layer form tight junctions, separating and surrounding axons. Front. Mol. Neurosci. 2018, 11, 367. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Abalo, X.M.; Villar-Cerviño, V.; Barreiro-Iglesias, A.; Anadón, R.; Rodicio, M.C. Late proliferation and photoreceptor differentiation in the transforming lamprey retina. Brain Res. 2008, 1201, 60–67. [Google Scholar] [CrossRef]
- Hernández-Núñez, I.; Robledo, D.; Mayeur, H.; Mazan, S.; Sánchez, L.; Adrio, F.; Barreiro-Iglesias, A.; Candal, E. Loss of active neurogenesis in the adult shark retina. Front. Cell Dev. Biol. 2021, 9, 628721. [Google Scholar] [CrossRef]
- Marcus, R.C.; Delaney, C.L.; Easter, S.S., Jr. Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio). off. Vis. Neurosci. 1999, 16, 417–424. [Google Scholar] [CrossRef]
- Van Houcke, J.; Geeraerts, E.; Vanhunsel, S.; Beckers, A.; Noterdaeme, L.; Christiaens, M.; Bollaerts, I.; De Groef, L.; Moons, L. Extensive growth is followed by neurodegenerative pathology in the continuously expanding adult zebrafish retina. Biogerontology 2019, 20, 109–125. [Google Scholar] [CrossRef]
- Mandyam, C.D.; Harburg, G.C.; Eisch, A.J. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 2007, 146, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Malicki, J.; Neuhauss, S.C.; Schier, A.F.; Solnica-Krezel, L.; Stemple, D.L.; Stainier, D.Y.; Abdelilah, S.; Zwartkruis, F.; Rangini, Z.; Driever, W. Mutations affecting development of the zebrafish retina. Development 1996, 123, 263–273. [Google Scholar] [CrossRef]
- Easter, S.S., Jr.; Nicola, G.N. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef] [Green Version]
- Zerjatke, T.; Gak, I.A.; Kirova, D.; Fuhrmann, M.; Daniel, K.; Gonciarz, M.; Müller, D.; Glauche, I.; Mansfeld, J. Quantitative cell cycle analysis based on an endogenous all-in-one reporter for cell tracking and classification. Cell Rep. 2017, 19, 1953–1966. [Google Scholar] [CrossRef] [Green Version]
- Johns, P.R.; Fernald, R.D. Genesis of rods in teleost fish retina. Nature 1981, 293, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Biehlmaier, O.; Neuhauss, S.C.; Kohler, K. Onset and time course of apoptosis in the developing zebrafish retina. Cell Tissue Res. 2001, 306, 199–207. [Google Scholar] [CrossRef]
- Li, L.; Wojtowicz, J.L.; Malin, J.H.; Huang, T.; Lee, E.B.; Chen, Z. GnRH-mediated olfactory and visual inputs promote mating-like behaviors in male zebrafish. PLoS ONE 2017, 12, e0174143. [Google Scholar] [CrossRef] [Green Version]
- Johns, P.R. Formation of photoreceptors in larval and adult goldfish. J. Neurosci. 1982, 2, 178–198. [Google Scholar] [CrossRef] [Green Version]
- Otteson, D.C.; D’Costa, A.R.; Hitchcock, P.F. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev. Biol. 2001, 232, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, A.C.; Scholz, T.L.; Brockerhoff, S.E.; Fadool, J.M. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Dev. Neurobiol. 2008, 68, 605–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, A.C.; Scholz, T.; Fadool, J.M. Rod progenitor cells in the mature zebrafish retina. Adv. Exp. Med. Biol. 2008, 613, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Lenkowski, J.R.; Raymond, P.A. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 2014, 40, 94–123. [Google Scholar] [CrossRef]
- Stenkamp, D.L. The rod photoreceptor lineage of teleost fish. Prog. Retin. Eye Res. 2011, 30, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Crespo, C.; Knust, E. Characterisation of maturation of photoreceptor cell subtypes during zebrafish retinal development. Biol. Open. 2018, 7, bio036632. [Google Scholar] [CrossRef] [Green Version]
- Hutter, S.; Hettyey, A.; Penn, D.J.; Zala, S.M. Ephemeral sexual dichromatism in zebrafish (Danio rerio). Ethology 2012, 118, 1208–1218. [Google Scholar] [CrossRef]
- Negishi, K.; Stell, W.K.; Takasaki, Y. Early histogenesis of the teleostean retina: Studies using a novel immunochemical marker, proliferating cell nuclear antigen (PCNA/cyclin). Brain Res. Dev. 1990, 55, 121–125. [Google Scholar] [CrossRef]
- Mack, A.F.; Fernald, R.D. New rods move before differentiating in adult teleost retina. Dev. Biol. 1995, 170, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Mack, A.F.; Fernald, R.D. Cell movement and cell cycle dynamics in the retina of the adult teleost Haplochromis burtoni. J. Comp. Neurol. 1997, 388, 435–443. [Google Scholar] [CrossRef]
- Julian, D.; Ennis, K.; Korenbrot, J.I. Birth and fate of proliferative cells in the inner nuclear layer of the mature fish retina. J. Comp. Neurol. 1998, 394, 271–282. [Google Scholar] [CrossRef]
- Velasco, A.; Cid, E.; Ciudad, J.; Orfao, A.; Aijón, J.; Lara, J.M. Temperature induces variations in the retinal cell proliferation rate in a cyprinid. Brain Res. 2001, 913, 190–194. [Google Scholar] [CrossRef]
- Cid, E.; Velasco, A.; Ciudad, J.; Orfao, A.; Aijón, J.; Lara, J.M. Quantitative evaluation of the distribution of proliferating cells in the adult retina in three cyprinid species. Cell Tissue Res. 2002, 308, 47–59. [Google Scholar] [CrossRef]
- Jimeno, D.; Lillo, C.; Cid, E.; Aijón, J.; Velasco, A.; Lara, J.M. The degenerative and regenerative processes after the elim-ination of the proliferative peripheral retina of fish. Exp. Neurol. 2003, 179, 210–228. [Google Scholar] [CrossRef]
- Candal, E.; Anadón, R.; DeGrip, W.J.; Rodríguez-Moldes, I. Patterns of cell proliferation and cell death in the developing retina and optic tectum of the brown trout. Brain Res. Dev. 2005, 154, 101–119. [Google Scholar] [CrossRef]
- Amini, R.; Labudina, A.A.; Norden, C. Stochastic single cell migration leads to robust horizontal cell layer formation in the vertebrate retina. Development 2019, 146, dev173450. [Google Scholar] [CrossRef] [Green Version]
- Ferreiro-Galve, S.; Rodríguez-Moldes, I.; Anadón, R.; Candal, E. Patterns of cell proliferation and rod photoreceptor differentiation in shark retinas. J. Chem. Neuroanat. 2010, 39, 1–14. [Google Scholar] [CrossRef]
- Ferreiro-Galve, S.; Rodríguez-Moldes, I.; Candal, E. Calretinin immunoreactivity in the developing retina of sharks: Comparison with cell proliferation and GABAergic system markers. Exp. Eye Res. 2010, 91, 378–386. [Google Scholar] [CrossRef]
- Ferreiro-Galve, S.; Rodríguez-Moldes, I.; Candal, E. Pax6 expression during retinogenesis in sharks: Comparison with markers of cell proliferation and neuronal differentiation. J. Exp. Zool. B Mol. Dev. Evol. 2012, 318, 91–108. [Google Scholar] [CrossRef]
- Sánchez-Farías, N.; Candal, E. Doublecortin widely expressed in the developing and adult retina of sharks. Exp. Eye Res. 2015, 134, 90–100. [Google Scholar] [CrossRef]
- Sánchez-Farías, N.; Candal, E. Identification of radial glia progenitors in the developing and adult retina of sharks. Front. Neuroanat. 2016, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Bejarano-Escobar, R.; Blasco, M.; Durán, A.C.; Rodríguez, C.; Martín-Partido, G.; Francisco-Morcillo, J. Retinal histogenesis and cell differentiation in an elasmobranch species, the small-spotted catshark Scyliorhinus canicula. J. Anat. 2012, 220, 318–335. [Google Scholar] [CrossRef]
- Jensen, A.M.; Walker, C.; Westerfield, M. Mosaic eyes: A zebrafish gene required in pigmented epithelium for apical localization of retinal cell division and lamination. Development 2001, 128, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Godinho, L.; Williams, P.R.; Claassen, Y.; Provost, E.; Leach, S.D.; Kamermans, M.; Wong, R.O. Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron 2007, 56, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Weber, I.P.; Ramos, A.P.; Strzyz, P.J.; Leung, L.C.; Young, S.; Norden, C. Mitotic position and morphology of committed precursor cells in the zebrafish retina adapt to architectural changes upon tissue maturation. Cell Rep. 2014, 7, 386–397. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Núñez, I.; Quelle-Regaldie, A.; Sánchez, L.; Adrio, F.; Candal, E.; Barreiro-Iglesias, A. Decline in Constitutive Proliferative Activity in the Zebrafish Retina with Ageing. Int. J. Mol. Sci. 2021, 22, 11715. https://doi.org/10.3390/ijms222111715
Hernández-Núñez I, Quelle-Regaldie A, Sánchez L, Adrio F, Candal E, Barreiro-Iglesias A. Decline in Constitutive Proliferative Activity in the Zebrafish Retina with Ageing. International Journal of Molecular Sciences. 2021; 22(21):11715. https://doi.org/10.3390/ijms222111715
Chicago/Turabian StyleHernández-Núñez, Ismael, Ana Quelle-Regaldie, Laura Sánchez, Fátima Adrio, Eva Candal, and Antón Barreiro-Iglesias. 2021. "Decline in Constitutive Proliferative Activity in the Zebrafish Retina with Ageing" International Journal of Molecular Sciences 22, no. 21: 11715. https://doi.org/10.3390/ijms222111715
APA StyleHernández-Núñez, I., Quelle-Regaldie, A., Sánchez, L., Adrio, F., Candal, E., & Barreiro-Iglesias, A. (2021). Decline in Constitutive Proliferative Activity in the Zebrafish Retina with Ageing. International Journal of Molecular Sciences, 22(21), 11715. https://doi.org/10.3390/ijms222111715






