Regulation of p27 and Cdk2 Expression in Different Adipose Tissue Depots in Aging and Obesity
Abstract
:1. Introduction
2. Results
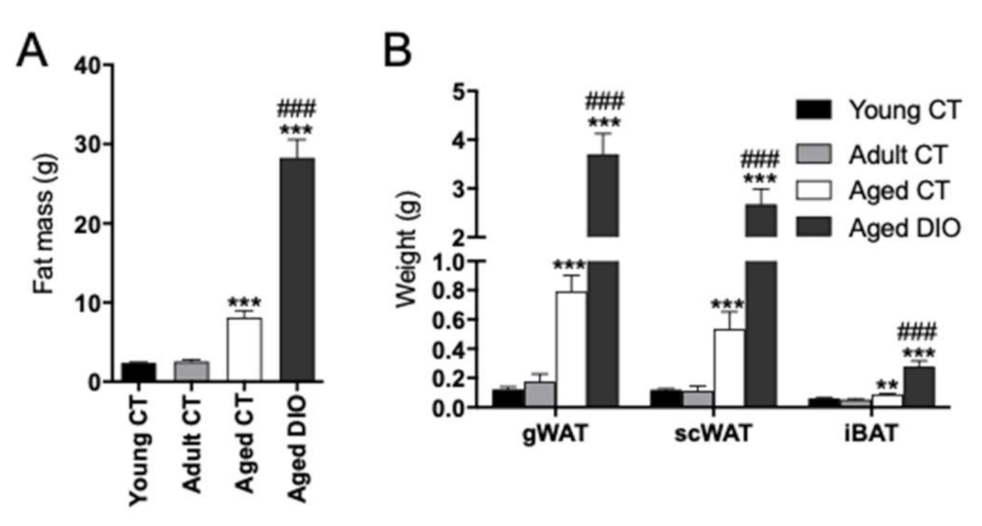
2.1. Effects of Aging and Long-Term High Fat Diet (HFD) on Body Fatness and Weights of Different Fat Depots in Female Mice
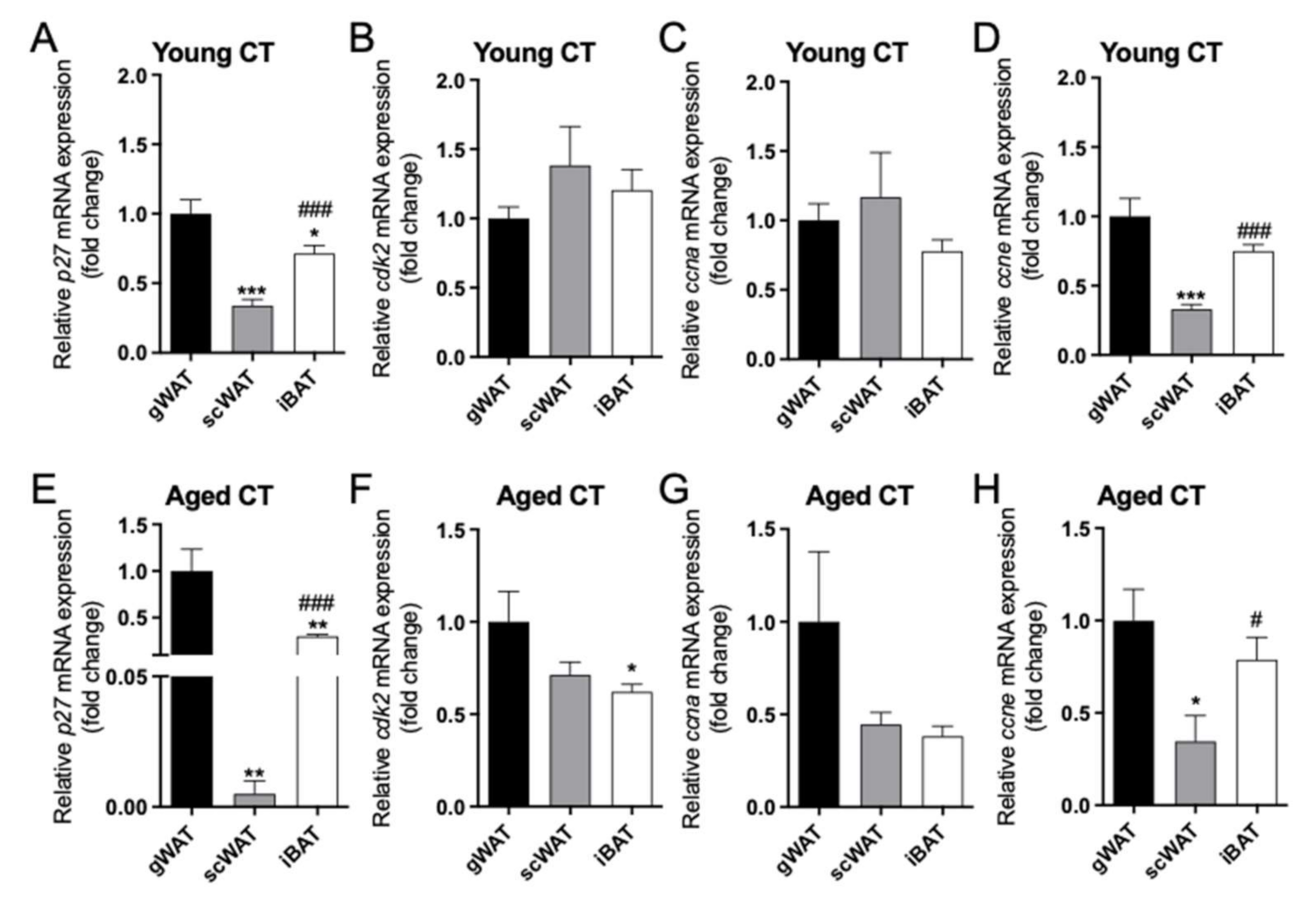
2.2. Differential Expression of p27/cdk2/ccna/ccne between Adipose Tissue Depots in Female Mice
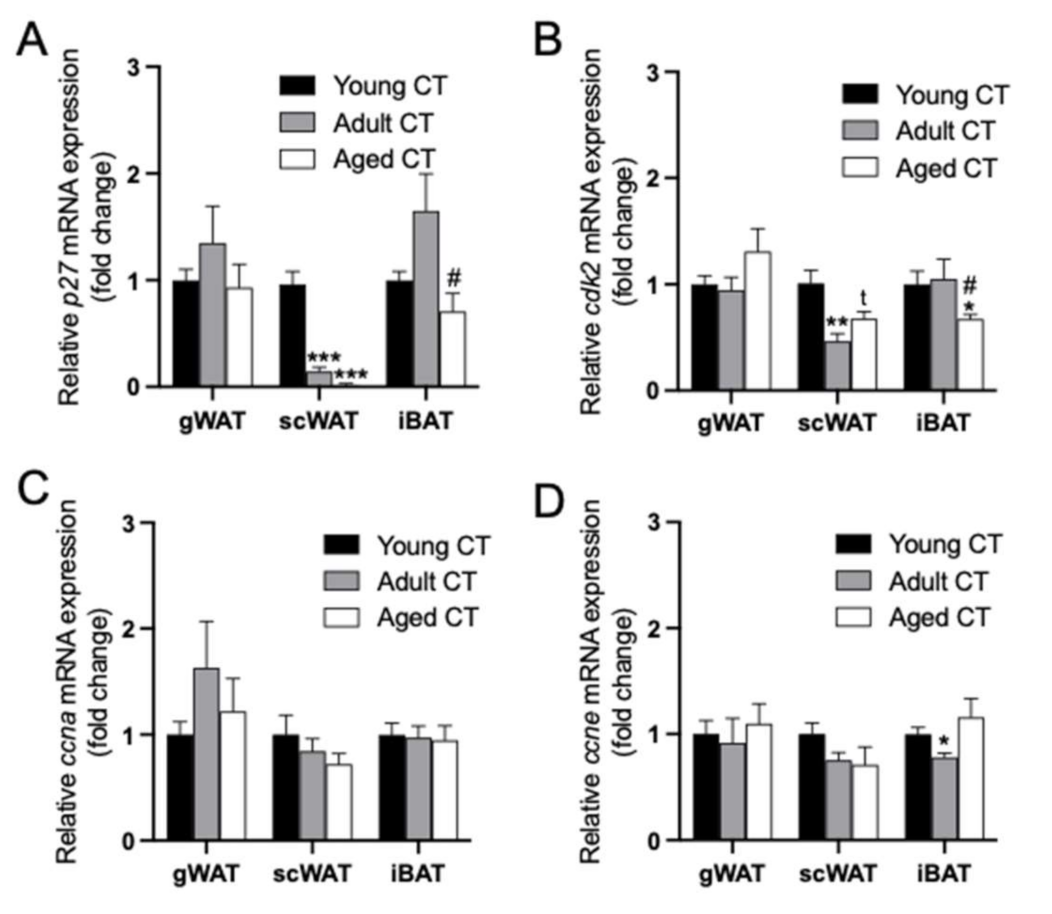
2.3. Changes in the Expression of p27/cdk2/ccna/ccne within each Adipose Tissue Depot during Aging in Female Mice
2.4. Effects of Diet-Induced Obesity on the Expression of p27/cdk2/ccna/ccne within each Adipose Tissue Depot in Aged Female Mice
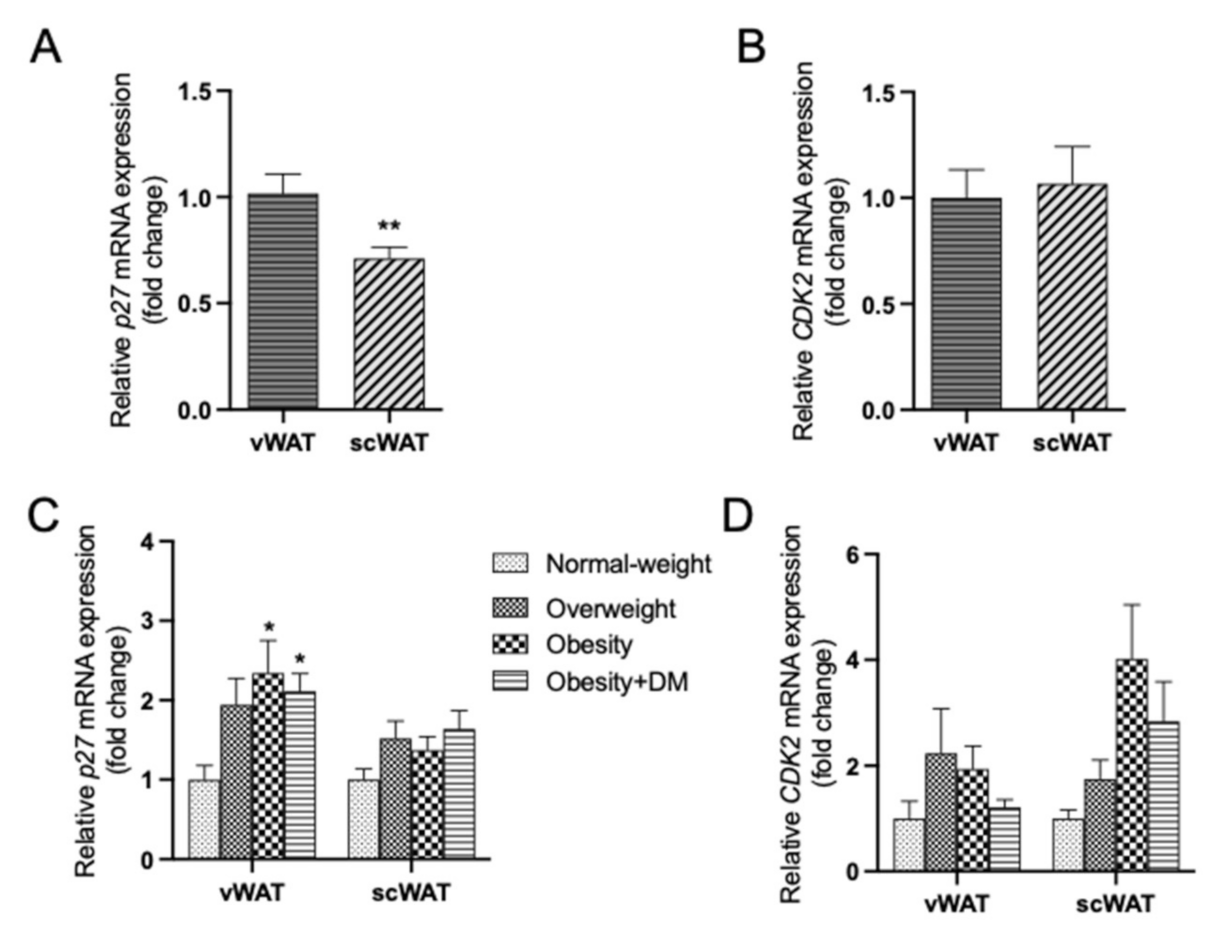
2.5. Expression of p27 and CDK2 mRNA in vWAT and scWAT of Men and Women: Regulation by Obesity and Type 2 DM and Correlation Analyses with Age, BMI, vWAT and scWAT Area, Serum Glucose and Triglycerides
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Human Samples
4.3. Blood Analyses
4.4. Determination of Body Fat Distribution and Content in Humans
4.5. RT-PCR
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jura, M.; Kozak, L.P. Obesity and Related Consequences to Ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Miard, S.; Picard, F. Obesity and Aging Have Divergent Genomic Fingerprints. Int. J. Obes. 2008, 32, 1873–1874. [Google Scholar] [CrossRef] [Green Version]
- Batsis, J.A.; Zagaria, A.B. Addressing Obesity in Aging Patients. Med. Clin. N. Am. 2018, 102, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [Green Version]
- Güngör, N.K. Overweight and Obesity in Children and Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 129–143. [Google Scholar] [CrossRef]
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Al Mamun, A.; Bonneux, L. Epidemiology and Demography Compression of Morbidity Research Group Obesity in Adulthood and Its Consequences for Life Expectancy: A Life-Table Analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef]
- Mau, T.; Yung, R. Adipose Tissue Inflammation in Aging. Exp. Gerontol. 2018, 105, 27–31. [Google Scholar] [CrossRef]
- Kwok, K.H.M.; Lam, K.S.L.; Xu, A. Heterogeneity of White Adipose Tissue: Molecular Basis and Clinical Implications. Exp. Mol. Med. 2016, 48, e215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered Adipose Tissue and Adipocyte Function in the Pathogenesis of Metabolic Syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Hirsch, J.; Batchelor, B. Adipose Tissue Cellularity in Human Obesity. Clin. Endocrinol. Metab. 1976, 5, 299–311. [Google Scholar] [CrossRef]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose Tissue Inflammation and Metabolic Syndrome. The Proactive Role of Probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Brown, C.M.; Layman, D.K. Lipoprotein Lipase Activity and Chylomicron Clearance in Rats Fed a High Fat Diet. J. Nutr. 1988, 118, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Bova, R.; Schofield, L.; Bryant, W.; Dieckmann, W.; Slattery, A.; Govendir, M.A.; Emmett, L.; Greenfield, J.R. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab. 2016, 23, 602–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trayhurn, P. Recruiting Brown Adipose Tissue in Human Obesity. Diabetes 2016, 65, 1158–1160. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [Green Version]
- Schosserer, M.; Grillari, J.; Wolfrum, C.; Scheideler, M. Age-Induced Changes in White, Brite, and Brown Adipose Depots: A Mini-Review. Gerontology 2018, 64, 229–236. [Google Scholar] [CrossRef]
- Smith, U.; Li, Q.; Rydén, M.; Spalding, K.L. Cellular Senescence and Its Role in White Adipose Tissue. Int. J. Obes. 2021. [Google Scholar] [CrossRef]
- Solaki, M.; Ewald, J.C. Fueling the Cycle: CDKs in Carbon and Energy Metabolism. Front. Cell. Dev. Biol. 2018, 6, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaldis, P.; Cheng, A.; Solomon, M.J. The Effects of Changing the Site of Activating Phosphorylation in CDK2 from Threonine to Serine. J. Biol. Chem. 2000, 275, 32578–32584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viallard, J.F.; Lacombe, F.; Belloc, F.; Pellegrin, J.L.; Reiffers, J. [Molecular mechanisms controlling the cell cycle: Fundamental aspects and implications for oncology]. Cancer Radiother. 2001, 5, 109–129. [Google Scholar] [CrossRef]
- Denicourt, C.; Dowdy, S.F. Another Twist in the Transforming Growth Factor Beta-Induced Cell-Cycle Arrest Chronicle. Proc. Natl. Acad. Sci. USA 2003, 100, 15290–15291. [Google Scholar] [CrossRef] [Green Version]
- Lagarrigue, S.; Lopez-Mejia, I.C.; Denechaud, P.-D.; Escoté, X.; Castillo-Armengol, J.; Jimenez, V.; Chavey, C.; Giralt, A.; Lai, Q.; Zhang, L.; et al. CDK4 Is an Essential Insulin Effector in Adipocytes. J. Clin. Investig. 2016, 126, 335–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajas, L.; Lopez-Mejia, I.C. CDK4, a New Metabolic Sensor That Antagonizes AMPK. Mol. Cell Oncol. 2018, 5, e1409862. [Google Scholar] [CrossRef]
- Banks, A.S.; McAllister, F.E.; Camporez, J.P.G.; Zushin, P.-J.H.; Jurczak, M.J.; Laznik-Bogoslavski, D.; Shulman, G.I.; Gygi, S.P.; Spiegelman, B.M. An ERK/Cdk5 Axis Controls the Diabetogenic Actions of PPARγ. Nature 2015, 517, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Chen, Y.; Castillo-Armengol, J.; Dreos, R.; Moret, C.; Niederhäuser, G.; Delacuisine, B.; Lopez-Mejia, I.C.; Denechaud, P.-D.; Fajas, L. CDK7 Mediates the Beta-Adrenergic Signaling in Thermogenic Brown and White Adipose Tissues. iScience 2020, 23, 101163. [Google Scholar] [CrossRef]
- Naaz, A.; Holsberger, D.R.; Iwamoto, G.A.; Nelson, A.; Kiyokawa, H.; Cooke, P.S. Loss of Cyclin-Dependent Kinase Inhibitors Produces Adipocyte Hyperplasia and Obesity. FASEB J. 2004, 18, 1925–1927. [Google Scholar] [CrossRef]
- Lin, J.; Della-Fera, M.A.; Li, C.; Page, K.; Choi, Y.H.; Hartzell, D.L.; Baile, C.A. p27 Knockout Mice: Reduced Myostatin in Muscle and Altered Adipogenesis. Biochem. Biophys. Res. Commun. 2003, 300, 938–942. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.W.; Grønborg, M.; Urlaub, H.; Lane, M.D.; Tang, Q.-Q. Role of Cdk2 in the Sequential Phosphorylation/Activation of C/EBPbeta during Adipocyte Differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 11597–11602. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional Regulation of Adipocyte Differentiation: A Central Role for CCAAT/Enhancer-Binding Protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between Mouse Age and Human Age in Anti-Tumor Research: Significance and Method Establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Manabe, I.; Nagai, R. Adipose Tissue Inflammation in Obesity and Metabolic Syndrome. Discov. Med. 2009, 8, 55–60. [Google Scholar]
- Morrison, R.F.; Farmer, S.R. Role of PPARgamma in Regulating a Cascade Expression of Cyclin-Dependent Kinase Inhibitors, P18(INK4c) and P21(Waf1/Cip1), during Adipogenesis. J. Biol. Chem. 1999, 274, 17088–17097. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Cho, J.A.; Lim, E.H.; Lee, C.W.; Lee, S.H.; Won Seo, S.; Yang, D.Y.; Lee, K.W. Cell Cycle Regulators Are Critical for Maintaining the Differentiation Potential and Immaturity in Adipogenesis of Adipose-Derived Stem Cells. Differentiation 2011, 82, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Auld, C.A.; Morrison, R.F. Evidence for Cytosolic p27(Kip1) Ubiquitylation and Degradation during Adipocyte Hyperplasia. Obesity 2006, 14, 2136–2144. [Google Scholar] [CrossRef] [Green Version]
- Mathus-Vliegen, E.M.H. Obesity and the Elderly. J. Clin. Gastroenterol. 2012, 46, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-Related Changes in Total and Regional Fat Distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and Functions of Cellular Senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Wang, L.; Jin, S.; Dai, P.; Zhang, T.; Shi, Y.; Ai, G.; Shao, X.; Xie, Y.; Xu, J.; Chen, Z.; et al. P57Kip2 Is a Master Regulator of Human Adipose Derived Stem Cell Quiescence and Senescence. Stem Cell Res. 2020, 44, 101759. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.; Wing, A.; Holtrup, B.; Sebo, Z.; Kaplan, J.L.; Saavedra-Peña, R.; Church, C.D.; Colman, L.; Berry, R.; Rodeheffer, M.S. The Adipose Tissue Microenvironment Regulates Depot-Specific Adipogenesis in Obesity. Cell Metab. 2016, 24, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, J.M.; Martín-Caballero, J.; García-Fernández, R.A. P21 and p27 a Shared Senescence History. Cell Cycle 2014, 13, 1655–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, L.K.; White, J.P. The AMPK/p27Kip1 Pathway as a Novel Target to Promote Autophagy and Resilience in Aged Cells. Cells 2021, 10, 1430. [Google Scholar] [CrossRef]
- Dotto, G.P. P21(WAF1/Cip1): More than a Break to the Cell Cycle? Biochim. Biophys. Acta 2000, 1471, M43–M56. [Google Scholar] [CrossRef]
- Schwarze, S.R.; Shi, Y.; Fu, V.X.; Watson, P.A.; Jarrard, D.F. Role of Cyclin-Dependent Kinase Inhibitors in the Growth Arrest at Senescence in Human Prostate Epithelial and Uroepithelial Cells. Oncogene 2001, 20, 8184–8192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, M.; Griffin, C.; McKernan, K.; Eter, L.; Abrishami, S.; Singer, K. Female Adipose Tissue Has Improved Adaptability and Metabolic Health Compared to Males in Aged Obesity. Aging 2020, 12, 1725–1746. [Google Scholar] [CrossRef]
- Inoue, N.; Yahagi, N.; Yamamoto, T.; Ishikawa, M.; Watanabe, K.; Matsuzaka, T.; Nakagawa, Y.; Takeuchi, Y.; Kobayashi, K.; Takahashi, A.; et al. Cyclin-Dependent Kinase Inhibitor, P21WAF1/CIP1, Is Involved in Adipocyte Differentiation and Hypertrophy, Linking to Obesity, and Insulin Resistance. J. Biol. Chem. 2008, 283, 21220–21229. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Mejia, I.C.; Castillo-Armengol, J.; Lagarrigue, S.; Fajas, L. Role of Cell Cycle Regulators in Adipose Tissue and Whole Body Energy Homeostasis. Cell Mol. Life Sci. 2018, 75, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Marcadenti, A.; de Abreu-Silva, E.O. Different Adipose Tissue Depots: Metabolic Implications and Effects of Surgical Removal. Endocrinol. Nutr. 2015, 62, 458–464. [Google Scholar] [CrossRef]
- Sakai, T.; Sakaue, H.; Nakamura, T.; Okada, M.; Matsuki, Y.; Watanabe, E.; Hiramatsu, R.; Nakayama, K.; Nakayama, K.I.; Kasuga, M. Skp2 Controls Adipocyte Proliferation during the Development of Obesity. J. Biol. Chem. 2007, 282, 2038–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Pette, M.; Tunster, S.J.; John, R.M. Loss of Imprinting of Cdkn1c Protects against Age and Diet-Induced Obesity. Int. J. Mol. Sci. 2018, 19, E2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, M.; Lin, J.; Lim, W.; Jin, W.; Lee, H.J. Role of Brown Adipose Tissue in Metabolic Syndrome, Aging, and Cancer Cachexia. Front. Med. 2018, 12, 130–138. [Google Scholar] [CrossRef]
- Lecoultre, V.; Ravussin, E. Brown Adipose Tissue and Aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 1–6. [Google Scholar] [CrossRef]
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of Brown/Beige Adipose Tissues in Obesity and Metabolic Disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Alcalá, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Viana, M. Mechanisms of Impaired Brown Adipose Tissue Recruitment in Obesity. Front. Physiol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Félix-Soriano, E.; Sáinz, N.; Gil-Iturbe, E.; Collantes, M.; Fernández-Galilea, M.; Castilla-Madrigal, R.; Ly, L.; Dalli, J.; Moreno-Aliaga, M.J. Changes in Brown Adipose Tissue Lipid Mediator Signatures with Aging, Obesity, and DHA Supplementation in Female Mice. FASEB J. 2021. [Google Scholar] [CrossRef]
- Darcy, J.; Tseng, Y.-H. ComBATing Aging-Does Increased Brown Adipose Tissue Activity Confer Longevity? Geroscience 2019, 41, 285–296. [Google Scholar] [CrossRef]
- Okamatsu-Ogura, Y.; Fukano, K.; Tsubota, A.; Nio-Kobayashi, J.; Nakamura, K.; Morimatsu, M.; Sakaue, H.; Saito, M.; Kimura, K. Cell-Cycle Arrest in Mature Adipocytes Impairs BAT Development but Not WAT Browning, and Reduces Adaptive Thermogenesis in Mice. Sci. Rep. 2017, 7, 6648. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Z.; Jin, W.; Zhou, Z.; Sun, C. Foxc2 Enhances Proliferation and Inhibits Apoptosis through Activating Akt/MTORC1 Signaling Pathway in Mouse Preadipocytes. J. Lipid Res. 2015, 56, 1471–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, K.; Tamura, M.; Tanaka, N.; Tomonaga, T.; Matsubara, H.; Shimada, H.; Levens, D.; He, L.; Liu, J.; Yoshida, M.; et al. Interactions between SAP155 and FUSE-Binding Protein-Interacting Repressor Bridges c-Myc and p27Kip1 Expression. Mol. Cancer Res. 2013, 11, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebisch, M.; Bondeva, T.; Franke, S.; Daniel, C.; Amann, K.; Wolf, G. Activation of the Receptor for Advanced Glycation End Products Induces Nuclear Inhibitor of Protein Phosphatase-1 Suppression. Kidney Int. 2014, 86, 103–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviner, R.; Shenoy, A.; Elroy-Stein, O.; Geiger, T. Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-Omic Analysis. PLoS Genet. 2015, 11, e1005554. [Google Scholar] [CrossRef]
- Birsoy, K.; Festuccia, W.T.; Laplante, M. A Comparative Perspective on Lipid Storage in Animals. J. Cell Sci. 2013, 126, 1541–1552. [Google Scholar] [CrossRef] [Green Version]
- Zuriaga, M.A.; Fuster, J.J.; Gokce, N.; Walsh, K. Humans and Mice Display Opposing Patterns of “Browning” Gene Expression in Visceral and Subcutaneous White Adipose Tissue Depots. Front. Cardiovasc. Med. 2017, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Herz, C.T.; Kiefer, F.W. Adipose Tissue Browning in Mice and Humans. J. Endocrinol. 2019, 241, R97–R109. [Google Scholar] [CrossRef]
- Gordon-Larsen, P.; French, J.E.; Moustaid-Moussa, N.; Voruganti, V.S.; Mayer-Davis, E.J.; Bizon, C.A.; Cheng, Z.; Stewart, D.A.; Easterbrook, J.W.; Shaikh, S.R. Synergizing Mouse and Human Studies to Understand the Heterogeneity of Obesity. Adv. Nutr. 2021, nmab040. [Google Scholar] [CrossRef]
- Yazdi, F.T.; Clee, S.M.; Meyre, D. Obesity Genetics in Mouse and Human: Back and Forth, and Back Again. PeerJ 2015, 3, e856. [Google Scholar] [CrossRef] [Green Version]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracken, M.B. Why Animal Studies Are Often Poor Predictors of Human Reactions to Exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef] [Green Version]
- Takao, K.; Miyakawa, T. Genomic Responses in Mouse Models Greatly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Justice, M.J.; Dhillon, P. Using the Mouse to Model Human Disease: Increasing Validity and Reproducibility. Dis. Model Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef] [Green Version]
- Garrow, J.S.; Webster, J. Quetelet’s Index (W/H2) as a Measure of Fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Buss, J. Limitations of Body Mass Index to Assess Body Fat. Workplace Health Saf. 2014, 62, 264. [Google Scholar] [CrossRef]
- Sato, F.; Maeda, N.; Yamada, T.; Namazui, H.; Fukuda, S.; Natsukawa, T.; Nagao, H.; Murai, J.; Masuda, S.; Tanaka, Y.; et al. Association of Epicardial, Visceral, and Subcutaneous Fat With Cardiometabolic Diseases. Circ. J. 2018, 82, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, G.H.; Lee, S.; Park, S.H.; Pyo, H.B.; Cho, J.S. Body Fat Measurement in Computed Tomography Image. Biomed. Sci. Instrum. 1999, 35, 303–308. [Google Scholar] [PubMed]
- Goodpaster, B.H. Measuring Body Fat Distribution and Content in Humans. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 481–487. [Google Scholar] [CrossRef]
- Denton, N.; Karpe, F. Measuring Body Composition and Regional Fat Mass Accurately. Pract. Diab. 2016, 33, 224–226. [Google Scholar] [CrossRef]
- Hong, J.; Stubbins, R.E.; Smith, R.R.; Harvey, A.E.; Núñez, N.P. Differential Susceptibility to Obesity between Male, Female and Ovariectomized Female Mice. Nutr. J. 2009, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Medrikova, D.; Jilkova, Z.M.; Bardova, K.; Janovska, P.; Rossmeisl, M.; Kopecky, J. Sex Differences during the Course of Diet-Induced Obesity in Mice: Adipose Tissue Expandability and Glycemic Control. Int. J. Obes. 2012, 36, 262–272. [Google Scholar] [CrossRef]
- Fuente-Martín, E.; Argente-Arizón, P.; Ros, P.; Argente, J.; Chowen, J.A. Sex Differences in Adipose Tissue: It Is Not Only a Question of Quantity and Distribution. Adipocyte 2013, 2, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Keuper, M.; Jastroch, M. The Good and the BAT of Metabolic Sex Differences in Thermogenic Human Adipose Tissue. Mol. Cell Endocrinol. 2021, 533, 111337. [Google Scholar] [CrossRef]
- Torres-Perez, E.; Valero, M.; Garcia-Rodriguez, B.; Gonzalez-Irazabal, Y.; Calmarza, P.; Calvo-Ruata, L.; Ortega, C.; Garcia-Sobreviela, M.P.; Sanz-Paris, A.; Artigas, J.M.; et al. The FAT Expandability (FATe) Project: Biomarkers to Determine the Limit of Expansion and the Complications of Obesity. Cardiovasc. Diabetol. 2015, 14, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Mokbel, N.; Cheng, K.; Gunton, J.E. Vitamin D Signaling Regulates Proliferation, Differentiation, and Myotube Size in C2C12 Skeletal Muscle Cells. Endocrinology 2014, 155, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Watts, R.; Johnsen, V.L.; Shearer, J.; Hittel, D.S. Myostatin-Induced Inhibition of the Long Noncoding RNA Malat1 Is Associated with Decreased Myogenesis. Am. J. Physiol. Cell Physiol. 2013, 304, C995–C1001. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Ohsugi, M.; Sasako, T.; Awazawa, M.; Umehara, T.; Iwane, A.; Kobayashi, N.; Okazaki, Y.; Kubota, N.; Suzuki, R.; et al. The RNA Methyltransferase Complex of WTAP, METTL3, and METTL14 Regulates Mitotic Clonal Expansion in Adipogenesis. Mol. Cell Biol. 2018, 38, e00116-18. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhou, X.; Xia, Q.; Shen, J.; Yan, J.; Zhu, J.; Li, X.; Shu, M. Long Non-Coding RNA CCAT1 That Can Be Activated by c-Myc Promotes Pancreatic Cancer Cell Proliferation and Migration. Am. J. Transl. Res. 2016, 8, 5444–5454. [Google Scholar]
- Teratake, Y.; Kuga, C.; Hasegawa, Y.; Sato, Y.; Kitahashi, M.; Fujimura, L.; Watanabe-Takano, H.; Sakamoto, A.; Arima, M.; Tokuhisa, T.; et al. Transcriptional Repression of p27 Is Essential for Murine Embryonic Development. Sci. Rep. 2016, 6, 26244. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.H.H.; Wintch, H.D.; Capecchi, M.R. Hoxa11 Regulates Stromal Cell Death and Proliferation during Neonatal Uterine Development. Mol. Endocrinol. 2004, 18, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Gupta, A.; Yu, J.; Granados, J.Z.; Gandhi, A.Y.; Evers, B.M.; Iyengar, P.; Infante, R.E. LIFR-α-Dependent Adipocyte Signaling in Obesity Limits Adipose Expansion Contributing to Fatty Liver Disease. iScience 2021, 24, 102227. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sáinz, N.; Félix-Soriano, E.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Fernández-Galilea, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of Long-Term DHA Supplementation and Physical Exercise on Non-Alcoholic Fatty Liver Development in Obese Aged Female Mice. Nutrients 2021, 13, 501. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Wang, Z.; Banerjee, S.; Yang, J.; Huang, L.; Dixon, J.E. Site-Specific Proteasome Phosphorylation Controls Cell Proliferation and Tumorigenesis. Nat. Cell Biol. 2016, 18, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Gehring, M.P.; Pereira, T.C.B.; Zanin, R.F.; Borges, M.C.; Braga Filho, A.; Battastini, A.M.O.; Bogo, M.R.; Lenz, G.; Campos, M.M.; Morrone, F.B. P2X7 Receptor Activation Leads to Increased Cell Death in a Radiosensitive Human Glioma Cell Line. Purinergic Signal 2012, 8, 729–739. [Google Scholar] [CrossRef] [Green Version]

| gWAT (g) | scWAT (g) | iBAT (g) | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| p27 | 0.15 a | 0.63 | 0.77 b | <0.05 | −0.21 b | 0.48 |
| cdk2 | 0.09 a | 0.75 | 0.73 a | <0.05 | 0.62 b | <0.05 |
| ccna | 0.85 a | <0.001 | 0.05 a | 0.88 | 0.69 a | <0.05 |
| ccne | −0.07 a | 0.81 | 0.09 a | 0.83 | 0.33 a | 0.25 |
| Normal-Weight | Overweight | Obesity | Obesity + DM | |
|---|---|---|---|---|
| Sex (Men/Women) | 5/5 | 5/5 | 10/10 | 10/10 |
| Age (yr) | 47.90 ± 10.56 | 53.89 ± 14.01 | 47.75 ± 10.86 | 56.05 ± 10.66 |
| BMI (kg/m2) | 22.81 ± 1.70 | 28.51 ± 0.87 ** | 36.58 ± 4.88 *** | 37.43 ± 4.16 *** |
| vWAT area (cm2) a | 88.38 ± 56.38 | 121.50 ± 60.86 | 182.60 ± 113.80 | 326.90 ± 79.11 ***$$ |
| scWAT area (cm2) a | 103.60 ± 62.82 | 208.40 ± 88.70 | 320.80 ± 126.90 ** | 347.50 ± 137.70 ** |
| Glucose (mg/dl) | 74.00 ± 5.03 | 77.20 ± 6.97 | 90.63 ± 17.70 | 135.00 ± 35.06 ***$$$ |
| Triglycerides (mg/dl) | 91.74 ± 27.47 | 108.40 ± 46.66 | 123.08 ± 49.41 | 122.50 ± 36.54 |
| Cholesterol (mg/dl) | 217.80 ± 28.98 | 215.40 ± 22.31 | 202.60 ± 38.71 | 176.50 ± 29.37 * |
| HDL-cholesterol (mg/dl) | 58.00 ± 13.13 | 54.10 ± 12.20 | 48.58 ± 10.96 | 48.71 ± 12.07 |
| LDL-cholesterol (mg/dl) | 138.20 ± 22.29 | 139.1 ± 25.07 | 131.10 ± 36.92 | 91.43 ± 26.29 **$$ |
| Insulin (mU/L) | 4.23 ± 1.76 | 4.67 ± 1.95 | 5.94 ± 4.10 | 10.45 ± 6.13 **$ |
| HbA1c (%) | 5.32 ± 0.27 | 5.38 ± 0.31 | 5.43 ± 0.29 | 7.47 ± 1.59 ***$$$ |
| vWAT | scWAT | |||||||
|---|---|---|---|---|---|---|---|---|
| p27 | CDK2 | p27 | CDK2 | |||||
| r | p | r | p | r | p | r | p | |
| Age (yr) | 0.20 | 0.13 | 0.13 | 0.35 | 0.04 | 0.75 | −0.09 | 0.54 |
| BMI (kg/m2) | 0.22 | 0.10 | 0.06 | 0.68 | 0.15 | 0.26 | 0.24 | 0.08 |
| vWAT area (cm2) | 0.09 | 0.62 | 0.09 | 0.60 | 0.05 | 0.78 | 0.11 | 0.56 |
| scWAT area (cm2) | 0.19 | 0.31 | 0.16 | 0.38 | 0.38 | <0.05 | 0.44 | <0.05 |
| Glucose (mg/dL) | 0.20 | 0.14 | 0.01 | 0.95 | 0.04 | 0.74 | 0.19 | 0.16 |
| Triglycerides (mg/dL) | −0.08 | 0.57 | −0.10 | 0.48 | 0.13 | 0.37 | 0.25 | 0.09 |
| Primer | Species | Sequence | Reference |
|---|---|---|---|
| p27 | Mus musculus | Fw: CAGAATCATAAGCCCCTGGA | [89] |
| Rv: TCTGACGAGTCAGGCATTTG | |||
| cdk2 | Mus musculus | Fw: CATTCCTCTTCCCCTCATCA | [90] |
| Rv: GCAGCCCAGAAGAATTTCAG | |||
| ccna | Mus musculus | Fw: CTTGGCTGCACCAACAGTAA | [91] |
| Rv: ATGACTCAGGCCAGCTCTGT | |||
| ccne | Mus musculus | Fw: CCTCCAAAGTTGCACCAGTT | [92] |
| Rv: GGACGCACAGGTCTAGAAGC | |||
| p21 | Mus musculus | Fw: CTGTCTTGCACTCTGGTGTCTGA | [93] |
| Rv: CCAATCTGCGCTTGGAGTGA | |||
| p57 | Mus musculus | Fw: GCCGGGTGATGAGCTGGGAA | [94] |
| Rv: AGAGAGGCTGGTCCTTCAGC | |||
| Lep | Mus musculus | Fw: CTCCATCTGCTGGCCTTCTC | [95] |
| Rv: CATCCAGGCTCTCTGGCTTCT | |||
| 36b4 | Mus musculus | Fw: CACTGGTCTAGGACCCGAGAAG | [96] |
| Rv: GGTGCCTCTGGAGATTTTCG | |||
| p27 | Homo sapiens | Fw: TCTGAGGACACGCATTTGG | [97] |
| Rv: TGTTCTGTTGGCTCTTTTGTTT | |||
| CDK2 | Homo sapiens | Hs01548894_m1 | - |
| Β-ACTIN | Homo sapiens | Fw: CATCGAGCACGGCATCGTCA | [98] |
| Rv: TAGCACAGCCTGGATAGCAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colón-Mesa, I.; Fernández-Galilea, M.; Sáinz, N.; Lopez-Yus, M.; Artigas, J.M.; Arbonés-Mainar, J.M.; Félix-Soriano, E.; Escoté, X.; Moreno-Aliaga, M.J. Regulation of p27 and Cdk2 Expression in Different Adipose Tissue Depots in Aging and Obesity. Int. J. Mol. Sci. 2021, 22, 11745. https://doi.org/10.3390/ijms222111745
Colón-Mesa I, Fernández-Galilea M, Sáinz N, Lopez-Yus M, Artigas JM, Arbonés-Mainar JM, Félix-Soriano E, Escoté X, Moreno-Aliaga MJ. Regulation of p27 and Cdk2 Expression in Different Adipose Tissue Depots in Aging and Obesity. International Journal of Molecular Sciences. 2021; 22(21):11745. https://doi.org/10.3390/ijms222111745
Chicago/Turabian StyleColón-Mesa, Ignacio, Marta Fernández-Galilea, Neira Sáinz, Marta Lopez-Yus, Jose M. Artigas, José Miguel Arbonés-Mainar, Elisa Félix-Soriano, Xavier Escoté, and María Jesús Moreno-Aliaga. 2021. "Regulation of p27 and Cdk2 Expression in Different Adipose Tissue Depots in Aging and Obesity" International Journal of Molecular Sciences 22, no. 21: 11745. https://doi.org/10.3390/ijms222111745







