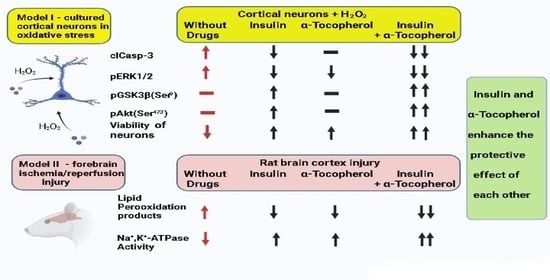
Insulin and α-Tocopherol Enhance the Protective Effect of Each Other on Brain Cortical Neurons under Oxidative Stress Conditions and in Rat Two-Vessel Forebrain Ischemia/Reperfusion Injury
Abstract
:1. Introduction
2. Results
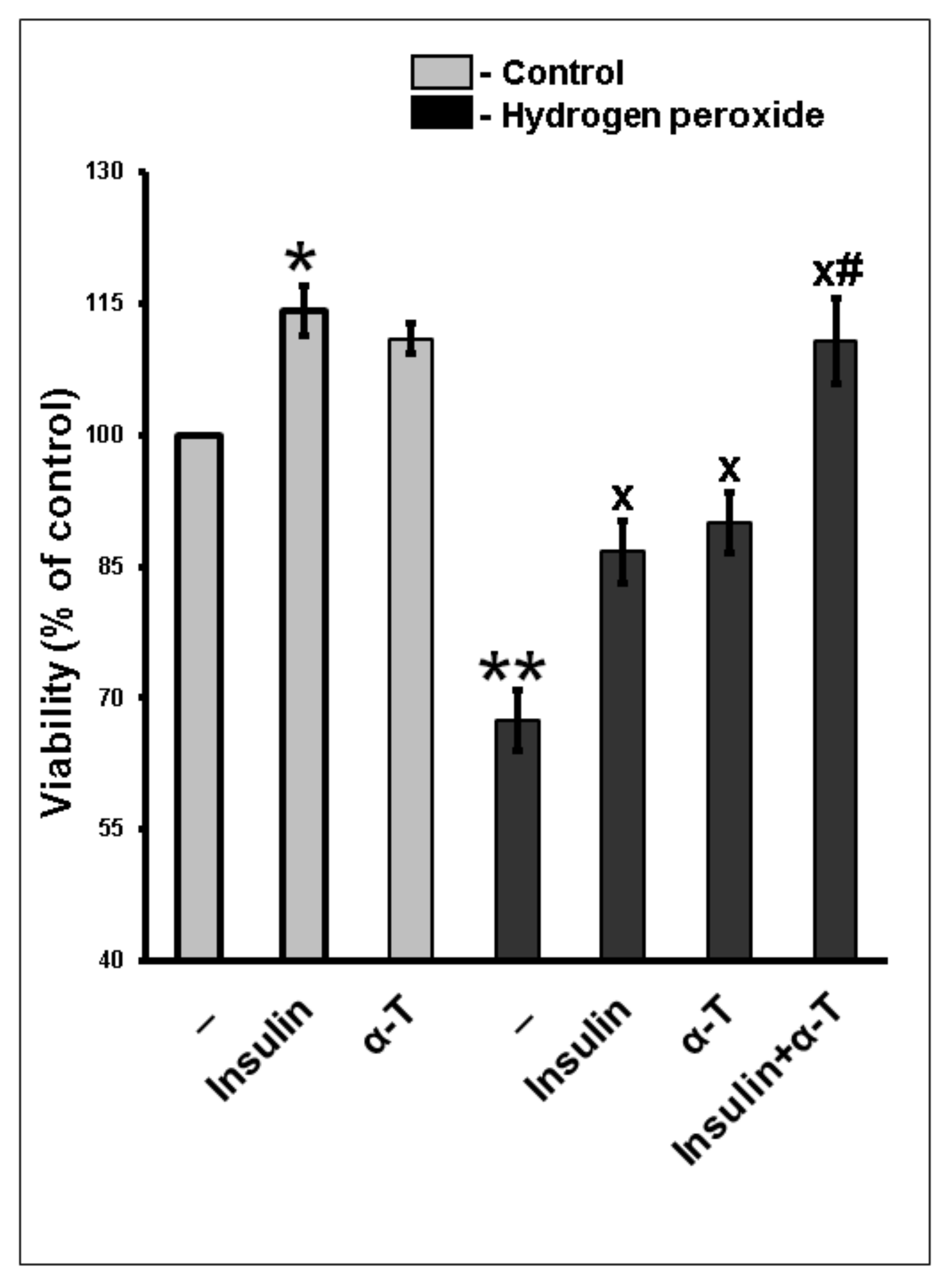
2.1. Additivity of Protective Effects of Insulin and α-Tocopherol on Viability of Rat Brain Cortical Neurons Exposed to Hydrogen Peroxide
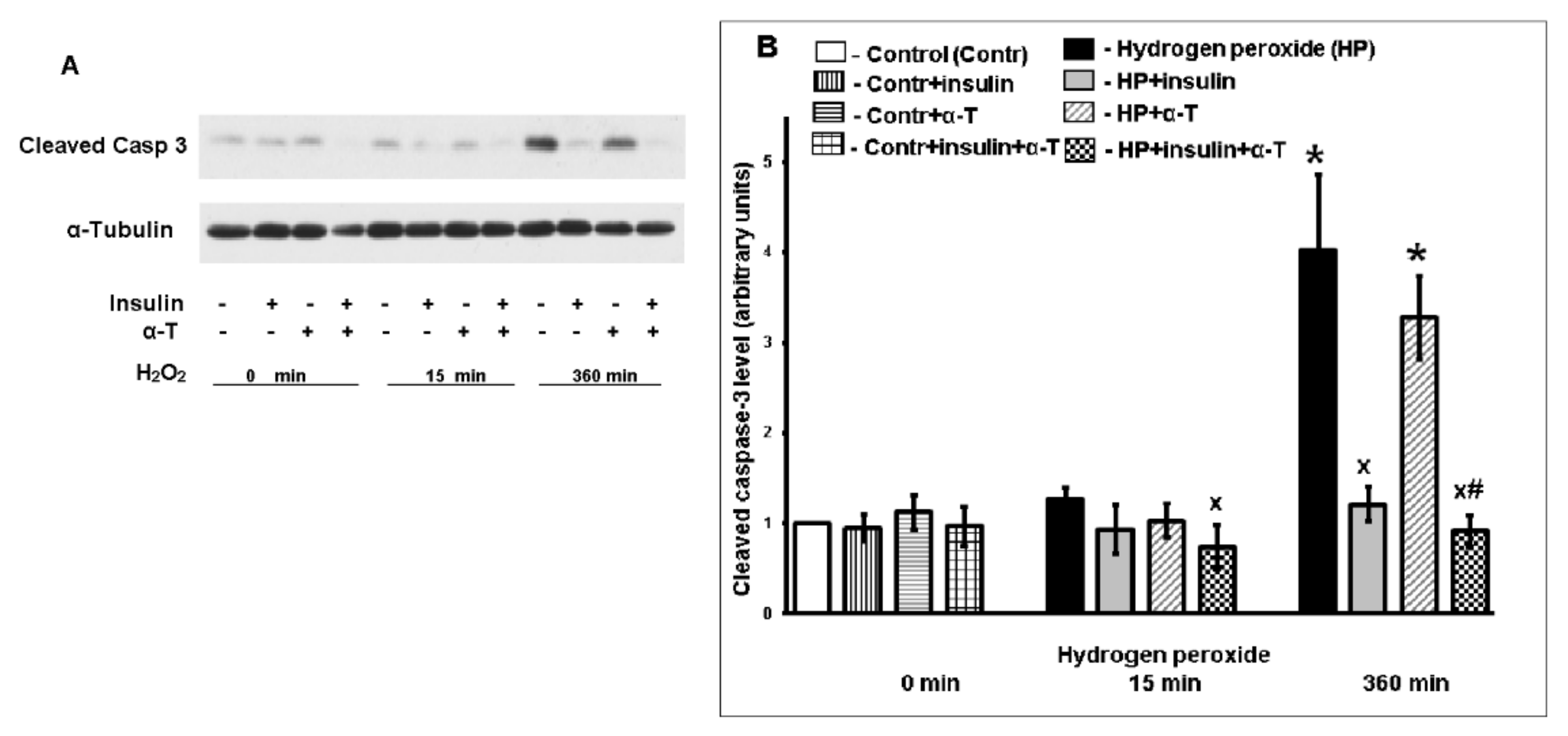
2.2. Antiapoptotic Effect of Insulin Plus α-T on Rat Brain Cortical Neurons Exposed to Hydrogen Peroxide Is Higher than the Effects of Each Drug
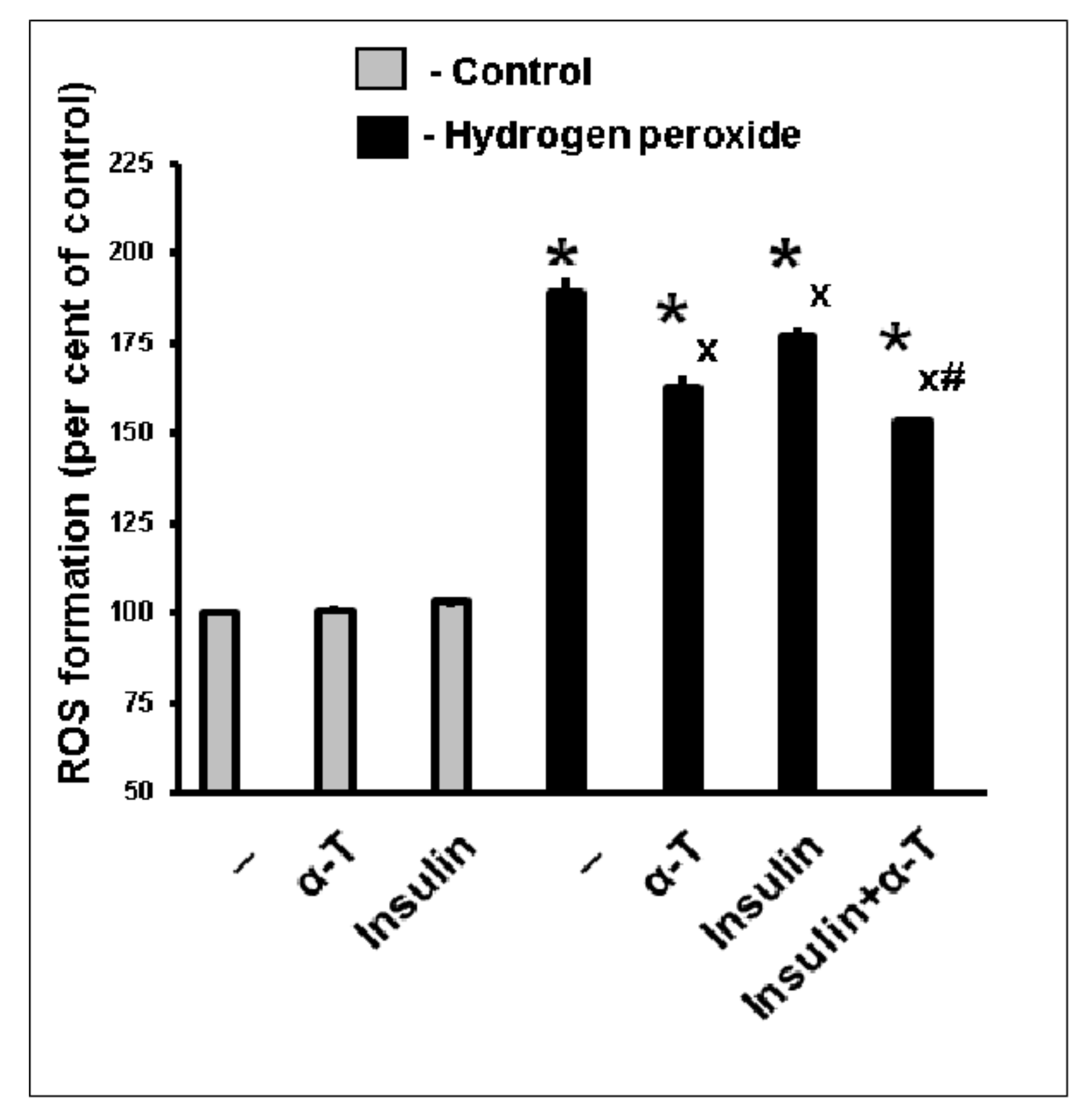
2.3. Antioxidative Effect of Insulin Plus α-T on Rat Brain Cortical Neurons Exposed to Hydrogen Peroxide Is Higher than the Effects of Each Drug
2.4. Effect of Insulin and α-T on Activity of Protein Kinase B (Akt) in Control and Hydrogen Peroxide-Exposed Brain Cortical Neurons
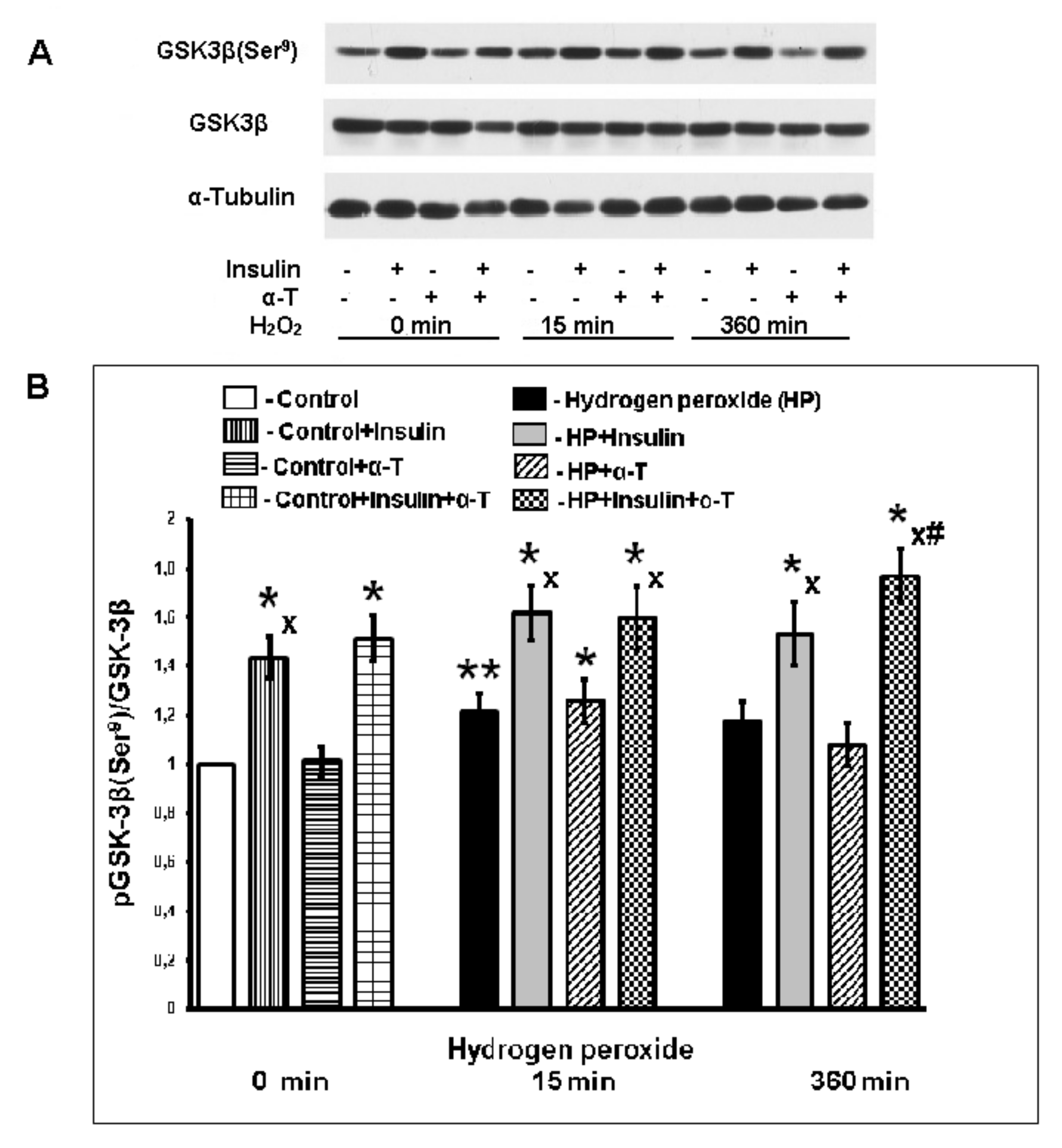
2.5. Effect of Insulin and α-T on pGSK-3beta (Ser9) Level in Control and Exposed to Hydrogen Peroxide Brain Cortical Neurons
2.6. Effect of Insulin and α-T on ERK1/2 Activity (pERK1/2/ERK1/2 Ratio) in Control and Hydrogen Peroxide-Exposed Brain Cortical Neurons
2.7. Effect of Insulin and α-T on Mitochondrial Membrane Potential in Control and Hydrogen Peroxide-Exposed Brain Cortical Neurons
2.8. The Combination of Intranasally Administered Insulin Together with Orally Administered α-T Diminished the Accumulation of Schiff Bases in the Brain Cortex of Rats with Ischemia/Reperfusion to Higher Extent than Monotherapy with These Drugs
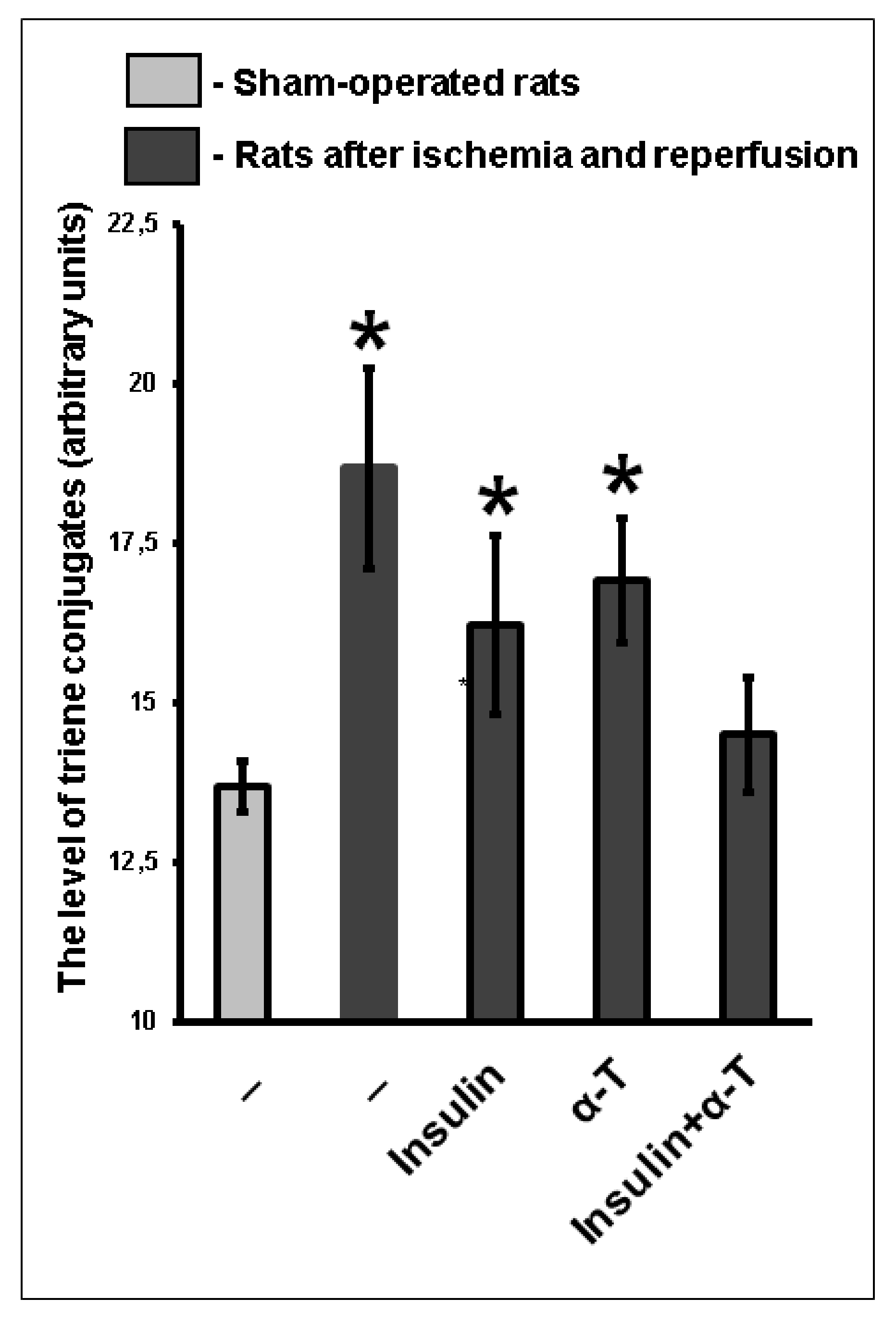
2.9. The Combination of Intranasally Administered Insulin Together with Orally Administered α-T Normalizes the Level of Conjugated Dienes and Trienes in the Brain Cortex of Rats with Ischemia/Reperfusion to a Greater Extent than Monotherapy with These Drugs
2.10. Additive Activating Effect of the Intranasally Administered Insulin and Orally Administered α-T on Na+,K+-ATPase Partially Inactivated in Brain Cortex of Rats with Two-Vessel Ischemia and Subsequent Reperfusion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Brain Cortical Neurons in Culture
4.3. Determination of the Viability of Brain Cortical Neurons by MTT Method and of Activation of Caspoase-3 Measuring the Level of Its 17–19 kDa Fragment
4.4. Determination of ROS Formation in Brain Cortical Neurons
4.5. Evaluation of Insulin, α-T and Hydrogen Peroxide Effects on Akt, GSK-3beta and ERK1/2 Activities and Expression of These Protein Kinases and of Cleaved Caspase-3 Using Western Blot Analysis
4.6. Determination of the Effects of Hydrogen Peroxide Application and of Pre-Incubation with Insulin and α-Tocopherol on Mitochondrial Membrane Potentials in Brain Cortical Neurons in Culture
4.7. Two-Vessel Forebrain Ischemia in Wistar Rats
4.8. Determination of Lipid Peroxidation Products, Na+,K+-ATPase Activity and Na+,K+-ATPase subunits expression in Brain Cortex of Rats after Two-Vessel Ischemia and Reperfusion
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-T | α-Tocopherol |
| Akt | Protein kinase B |
| ERK1/2 | Extracellular signal-regulated protein kinase |
| GSK-3beta | Glycogen synthase kinase-3beta |
| IGF-1 | Insulin-like growth factor-1 |
| LPO | Lipid peroxidation |
| MTT | 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| ROS | Reactive oxygen species |
| TMRM | Tetramethylrhodamine methyl ester |
References
- Fine, J.M.; Stroebel, B.M.; Faltesek, K.A.; Terai, K.; Haase, L.; Knutzen, K.E.; Kosyakovsky, J.; Bowe, T.J.; Fuller, A.K.; Frey, W.H.; et al. Intranasal delivery of low-dose insulin ameliorates motor dysfunction and dopaminergic cell death in a 6-OHDA rat model of Parkinson’s Disease. Neurosci. Lett. 2020, 714, 134567. [Google Scholar] [CrossRef]
- Hendrickx, J.O.; Moudt, S.; Calus, E.; Martinet, W.; Pieter-Jan, D.F.; Guns, P.-J.D.F.; Roth, L.; Peter, P.; De Deyn, P.P.; Van Dam, D.; et al. Serum corticosterone and insulin resistance as early biomarkers in the hAPP23 overexpressing mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 6656. [Google Scholar] [CrossRef]
- Toljan, K.; Homolak, J. Circadian changes in Alzheimer’s disease: Neurobiology, clinical problems, and therapeutic opportunities. Handb. Clin. Neurol. 2021, 179, 285–300. [Google Scholar] [CrossRef]
- Chua, L.-M.; Lim, M.-L.; Chong, P.-R.; Hu, Z.P.; Cheung, N.S.; Wong, B.S. Impaired neuronal insulin signaling precedes Aβ42 accumulation in female AβPPsw/PS1ΔE9 mice. J. Alzheimers Dis. 2012, 29, 783–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhao, Y.; Dai, C.-L.; Liang, Z.; Run, X.; Iqbal, K.; Liu, F.; Gong, C.-X. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Aβ level and microglia activation in the brains of 3xTg-AD mice. Exp. Neurol. 2014, 261, 610–619. [Google Scholar] [CrossRef]
- Rajasekar, N.; Hanif, K.; Shukla, R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)-induced memory impaired rats. Life Sci. 2017, 173, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, J.M.; Calva, C.B.; Reagan, L.P.; Fadel, J.R. Intranasal insulin and orexins to treat age-related cognitive decline. Physiol. Behav. 2021, 234, 113370. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferre, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Derkach, K.V.; Bogush, I.V.; Berstein, L.M.; Shpakov, A.O. The influence of intranasal insulin on hypothalamic-pituitary axis in normal and diabetic rats. Horm. Metab. Res. 2015, 47, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H. Central insulin-mediated regulation of hepatic glucose production. Endocr. J. 2016, 63, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.W.; Carter, K.; Bhatt, A.; Pang, Y. Rapid transport of insulin to the brain following intranasal administration in rats. Neural Regen. Res. 2019, 14, 1046–1051. [Google Scholar] [CrossRef]
- Tashima, T. Shortcut approaches to substance delivery into the brain based on intranasal administration using nanodelivery strategies for insulin. Molecules 2020, 25, 5188. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Mao, Y.F.; Zheng, T.; Zhang, B. Intranasal insulin ameliorates cerebral hypometabolism, neuronal loss, and astrogliosis in streptosotocin-induced Alzheimer’s rat model. Neurotox. Res. 2018, 33, 716–724. [Google Scholar] [CrossRef]
- Lv, H.; Tang, L.; Guo, C.; Jiang, Y.; Gao, C.; Wang, Y.; Jian, C. Intranasal insulin administration may be highly effective in improving cognitive function in mice with cognitive dysfunction by reversing brain insulin resistance. Cogn. Neurodyn. 2020, 14, 323–338. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Hanson, A.J.; Trittschuh, E.H.; Collerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimers Dis. 2015, 44, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Collerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: A pilot clinical trial. J. Alzheimers Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Avgerinos, K.I.; Kalaitzidis, G.; Malli, A.; Kalaitzoglou, D.; Myserlis, P.G.; Lioutas, V.-A. Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment. A systematic review. J. Neurol. 2018, 265, 1497–1510. [Google Scholar] [CrossRef]
- Miziak, B.; Błaszczyk, B.; Czuczwar, S.J. Some candidate drugs for pharmacotherapy of Alzheimer’s disease. Pharmaceuticals 2021, 14, 458. [Google Scholar] [CrossRef]
- Hallschmid, M. Intranasal insulin. J. Neuroendocrinol. 2021, 33, e12934. [Google Scholar] [CrossRef]
- Novak, P.; Maldonado, D.A.P.; Novak, V. Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: A double-blinded placebo-controlled pilot study. PLoS ONE 2019, 14, e0214364. [Google Scholar] [CrossRef] [Green Version]
- Zorina, I.I.; Zakharova, I.O.; Bayunova, L.V.; Avrova, N.F. Insulin administration prevents accumulation of conjugated dienes and trienes and inactivation of Na+,K+-ATPase in the rat cerebral cortex during two-vessel forebrain ischemia and reperfusion. J. Evol. Biochem. Physiol. 2018, 54, 246–249. [Google Scholar] [CrossRef]
- Zorina, I.I.; Fokina, E.A.; Zakharova, I.O.; Bayunova, L.V.; Shpakov, A.O. Characteristics of changes in lipid peroxidation and Na+/K+-ATPase activity in the cortex of old rats in conditions of two-vessel cerebral ischemia/reperfusion. Adv. Geront. 2020, 10, 156–161. [Google Scholar] [CrossRef]
- Russo, V.; Candeloro, P.; Malara, N.; Perozziello, G.; Iannone, M.; Scicchitano, M.; Mollace, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; et al. Key role of cytochrome C for apoptosis detection using Raman microimaging in an animal model of brain ischemia with insulin treatment. Appl. Spectrosc. 2019, 73, 1208–1217. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.S.; Lu, Y.J.; Huang, J.P.; Wu, Y.T.; Day, Y.J.; Hung, L.M.J. The essential role of endothelial nitric oxide synthase activation in insulin-mediated neuroprotection against ischemic stroke in diabetes. Vasc. Surg. 2014, 59, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Rizk, N.N.; Rafols, J.A.; Dunbar, J.C. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: Effects of insulin and C-peptide. Brain Res. 2006, 1096, 204–212. [Google Scholar] [CrossRef]
- Liu, X.F.; Fawcett, J.R.; Thorne, R.G.; DeFor, T.A.; Frey, W.H., 2nd. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 2001, 187, 91–97. [Google Scholar] [CrossRef]
- Lin, S.; Rhodes, P.G.; Cai, Z. Whole body hypothermia broadens the therapeutic window of intranasally administered IGF-1 in a neonatal rat model of cerebral hypoxia-ischemia. Brain Res. 2011, 1385, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Gu, X.; Wei, Z.Z.; Wu, A.; Liu, X. Combinatorial intranasal delivery of bone marrow mesenchymal stem cells and insulin-like growth factor-1 improves neurovascularization and functional outcomes following focal cerebral ischemia in mice. Exp. Neurol. 2021, 337, 113542. [Google Scholar] [CrossRef]
- Lioutas, V.A.; Alfaro-Martinez, F.; Bedoya, F.; Chung, C.C.; Pimentel, D.A.; Novak, V. Intranasal insulin and insulin-like growth factor-1 as neuroprotectants in acute ischemic stroke. Transl. Stroke Res. 2015, 6, 264–275. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D. Effect of vitamin E and memantine on functional decline in Alzheimer’s disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Farina, N.; Llewellyn, D.; Nasr, I.M.G.K.; Tabet, N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2017, 4, CD002854. [Google Scholar] [CrossRef]
- Wei, Z.; Koya, J.; Reznik, S.E. Insulin resistance exacerbates Alzheimer disease via multiple mechanisms (mini-review). Front. Neurosci. 2021. [Google Scholar] [CrossRef]
- Ashley, S.; Bradburn, S.; Murgatroyd, C. A meta-analysis of peripheral tocopherol levels in age-related cognitive decline and Alzheimer’s disease. Nutr. Neurosci. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.; Sicard, F.; Sperber, S.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Krug, A.W. DHEA reduces NGF-mediated cell survival in serum-deprived PC12 cells. Ann. N. Y. Acad. Sci. 2006, 1073, 306–311. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rice-Evans, C.; Williams, R.J.; Spencer, J.P. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J. Neurochem. 2007, 103, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; DeFranco, D.B. Opposing roles for ERK 1/2 in neuronal oxidative toxicity: Distinct mechanisms of ERK 1/2 action at early versus late phases of oxidative stress. J. Biol. Chem. 2006, 281, 16436–16442. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Qin, L.; Huang, F.; Wang, X.; Yang, L.; Shi, H.; Wu, H.; Zhang, B.; Chen, Z.; Wu, X. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol. Appl. Pharmacol. 2017, 319, 80–90. [Google Scholar] [CrossRef]
- Ho, Y.; Samarasinghe, R.; Knoch, M.E.; Lewis, M.E.; Aizenman, E.; DeFranco, D.B. Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellar signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol. Pharmacol. 2008, 74, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, I.O.; Sokolova, T.V.; Vlasova, Y.A.; Bayunova, L.V.; Rychkova, M.P.; Avrova, N.F. α-Tocopherol at nanomolar concentration protects cortical neurons against oxidative stress. Int. J. Mol. Sci. 2017, 18, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease: The Alzheimer’s disease cooperative study. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Page, R.; Morrel, C.; Shea, T.B. Maintenance of cognitive performance and mood for individuals with Alzheimer’s disease following consumption of a nutraceutical formulation: A one-year, open-label study. J. Alzheimers Dis. 2016, 51, 991–995. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Sokolova, T.V.; Bayunova, L.V.; Vlasova, Y.A.; Rychkova, M.P.; Avrova, N.F. α-Tocopherol at nanomolar concentration protects PC12 cells from hydrogen peroxide-induced death and modulates protein kinase activities. Int. J. Mol. Sci. 2012, 13, 11543–11568. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Sokolova, T.V.; Bayunova, L.V.; Zorina, I.I.; Rychkova, M.P.; Shpakov, A.O.; Avrova, N.F. The protective effect of insulin on rat cortical neurons in oxidative Stress and its dependence on the modulation of Akt, GSK-3beta, ERK1/2, and AMPK activities. Int. J. Mol. Sci. 2019, 20, 3702. [Google Scholar] [CrossRef] [Green Version]
- Vélez, D.E.; Mestre-Cordero, V.E.; Hermann, R.; Perego, J.; Harriet, S.; Fernandez-Pazos, M.L.M.; Mourglia, J.; Marina-Prendes, M.G. Rosuvastatin protects isolated hearts against ischemia-reperfusion injury: Role of Akt-GSK-3beta, metabolic environment, and mitochondrial permeability transition pore. J. Physiol. Biochem. 2020, 76, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.; Jiang, Q.; Jiang, Y.; Zhang, Y.; Cao, J.; Lu, L.; Li, C.; Wei, P.; Wang, Q.; et al. Cryptotanshinone Ameliorates Doxorubicin-Induced Cardiotoxicity by Targeting Akt-GSK-3β-mPTP Pathway In Vitro. Molecules 2021, 26, 1460. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; He, W.X.; Li, C.; Chang, M.J. Enteric glial cells exert neuroprotection from hyperglycemia-induced damage via Akt/GSK3β pathway. Neuroreport 2021, 32, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Namura, S.; Iihara, K.; Takami, S.; Nagata, I.; Kikuchi, H.; Matsushita, K.; Moskowitz, M.A.; Bonventre, J.V.; Alessandrini, A. Intravenous administration of MEK inhibitor UO126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc. Natl. Acad. Sci. USA 2001, 98, 11569–11574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.T.; Levinthal, D.J.; Kulich, S.M.; Chalovich, E.M.; DeFranco, D.B. Oxidative neuronal injury. The dark side of ERK1/2. Eur. J. Biochem. 2004, 271, 2060–2066. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Liang, C.L.; Liliang, P.C.; Yang, S.-H.; Cho, C.L.; Weng, H.C.; Tsai, Y.-D.; Wang, K.W.; Chen, H.J. Inhibition of extracellular signal-regulated kinases 1/2 provides neuroprotection in spinal cord ischemia/reperfusion injury in rats: Relationship with the nuclear factor-kB-regulated anti-apoptotic mechanisms. J. Neurochem. 2010, 114, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Xie, L.; Zou, X.S.; Li, N.; Yang, Y.G.; Wu, Z.J.; Tian, X.Y.; Zhao, G.Y.; Chen, M.H. Inhibition of extracellular signal-regulated kinase/calpain-2 pathway reduces neuroinflammation and necroptosis after cerebral ischemia-reperfusion injury in a rat model of cardiac arrest. Int. Immunopharmacol. 2021, 93, 107377. [Google Scholar] [CrossRef]
- Derkach, K.V.; Ivantsov, A.O.; Chistyakova, O.V.; Sukhov, I.B.; Buzanakov, D.M.; Kulikova, A.A.; Shpakov, A.O. Intranasal insulin restores metabolic parameters and insulin sensitivity in rats with metabolic syndrome. Bull. Exp. Biol. Med. 2017, 163, 184–189. [Google Scholar] [CrossRef]
- Beirami, E.; Oryan, S.; Seyedhosseini Tamijani, S.M.; Ahmadiani, A.; Dargahi, L. Intranasal insulin treatment restores cognitive deficits and insulin signaling impairment induced by repeated methamphetamine exposure. J. Cell. Biochem. 2018, 119, 2345–2355. [Google Scholar] [CrossRef]
- Chaudhary, G.; Sinha, K.; Gupta, Y.K. Protective effect of exogenous administration of alpha-tocopherol in middle cerebral artery occlusion model of cerebral ischemia in rats. Fundam. Clin. Pharmacol. 2003, 17, 703–707. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Satish Kumar, C.S.; Rani, M.U.; Srikanth, M.K.; Boobalan, G.; Reddy, A.G. An evaluation of the protective role of alpha-tocopherol on free radical induced hepatotoxicity and nephrotoxicity due to chromium in rats. Indian J. Pharmacol. 2013, 45, 490–495. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Tuz, K. Volume changes in neurons: Hyperexcitability and neuronal death. Contrib. Nephrol. 2006, 152, 221–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, L.R.; Janicot, R.; Stafstrom, C.E. Na+-K+-ATPase functions in the developing hippocampus: Regional differences in CA1 and CA3 neuronal excitability and role in epileptiform network bursting. J. Neurophysiol. 2021, 125, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, A.; Miyake, H.; Ohyashiki, T. Contribution of lipid dynamics on the inhibition of bovine brain synaptosomal Na+-K+-ATPase activity induced by 4-hydroxy-2-nonenal. Biol. Pharm. Bull. 2003, 26, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, H.; Kadoya, A.; Ohyashiki, T. Increase in molecular rigidity of the protein conformation of brain Na+-K+-ATPase by modification with 4-hydroxy-2-nonenal. Biol. Pharm. Bull. 2003, 26, 1652–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Goncalves Nunes, M.A.; Rubin, M.A.; da Cruz, I.B.; et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na+,K+-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef]
- Mironova, E.V.; Evstratova, A.A.; Antonov, S.M. A fluorescence vital assay for the recognition and quantification of excitotoxic cell death by necrosis and apoptosis using confocal microscopy on neurons in culture. J. Neurosci. Methods 2007, 163, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zorina, I.I.; Bayunova, L.V.; Zakharova, I.O.; Avrova, N.F. The dependence of the protective effect of insulin on its concentration and modulation of ERK1/2 activity under the conditions of oxidative stress in cortical neurons. Neurochem. J. 2018, 10, 111–116. [Google Scholar] [CrossRef]
- Vlasova, Y.A.; Zakharova, I.O.; Avrova, N.F. The effect of alpha-tocopherol and H2O2 on the mitochondrial membrane potential and Bax/Bcl-xL ratio in PC12 cells. Neurochem. J. 2016, 10, 318–322. [Google Scholar] [CrossRef]
- Molchanova, S.M.; Moskvin, A.N.; Zakharova, I.Y.; Yurlova, L.A.; Nosova, I.Y.; Avrova, N. Effects of two-vessel forebrain ischemia and of administration of indomethacin and quinacrine on Na+,K+-ATPase activity in various rat brain areas. J. Evol. Biochem. Physiol. 2005, 41, 39–46. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Sarieva, K.V.; Lyanguzov, A.Y.; Galkina, O.V.; Vetrovoy, O.V. The effect of severe hypoxia on HIF1- and Nrf2-mediated mechanisms of antioxidant defense in the rat neocortex. Neurochem. J. 2019, 13, 145–155. [Google Scholar] [CrossRef]
- Ferenczi, S.; Kuti, D.; Cserháti, M.; Krifaton, C.; Szoboszlay, S.; Kukolya, J.; Szőke, Z.; Albert, M.; Kriszt, B.; Kovács, K.J.; et al. Effects of single and repeated oral doses of ochratoxin A on the lipid peroxidation and antioxidant defense systems in mouse kidneys. Toxins 2020, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Avrova, N.F.; Victorov, I.V.; Tyurin, V.A.; Zakharova, I.O.; Sokolova, T.V.; Andreeva, N.A.; Stelmaschuk, E.V.; Tyurina, Y.Y.; Gonchar, V.S. Inhibition of glutamate-induced intensification of free radical reactions by gangliosides: Possible role in their protective effect in rat cerebellar granule cells and brain synaptosomes. Neurochem. Res. 1998, 23, 945–952. [Google Scholar] [CrossRef]





| Sample | Mean ± SEM |
|---|---|
| Control | 71.1 ± 2.88 |
| Control+Insulin | 67.5 ± 0.15 |
| Control+ α-T | 68.3 ± 0.25 |
| Hydrogen peroxide (HP) | 55.9 ± 4.64 a |
| HP+ Insulin | 78.3 ± 0.72 b |
| HP+ α-T | 68.0 ± 0.36 b |
| HP+Insulin+ α-T | 89.0 ± 2.36 b,c |
| Sham-Operated or Ischemic and Reperfused Rats | Administration to Rats | Na+,K+-ATPase Activity (μmol Pi/mg of Protein/h) |
|---|---|---|
| Sham-operated rats | - | 24.6 ± 1.27 |
| Sham-operated rats | 0.25 IU insulin | 22.7 ± 0.48 |
| Sham-operated rats | 50 mg α-T per kg | 24.2 ± 0.51 |
| Sham-operated rats | 0.25 IU insulin and 50 mg α-T per kg | 24.4 ± 0.74 |
| Ischemic and reperfused rats | - | 15.95 ± 0.82 a |
| Ischemic and reperfused rats | 0.25 IU insulin | 18.5 ± 0.82 a,b |
| Ischemic and reperfused rats | 50 mg α-T per kg | 21.3 ± 0.83 c |
| Ischemic and reperfused rats | 0.25 IU insulin and 50 mg α-T per kg | 23.1 ± 0.83 c,d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, I.O.; Bayunova, L.V.; Zorina, I.I.; Sokolova, T.V.; Shpakov, A.O.; Avrova, N.F. Insulin and α-Tocopherol Enhance the Protective Effect of Each Other on Brain Cortical Neurons under Oxidative Stress Conditions and in Rat Two-Vessel Forebrain Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 11768. https://doi.org/10.3390/ijms222111768
Zakharova IO, Bayunova LV, Zorina II, Sokolova TV, Shpakov AO, Avrova NF. Insulin and α-Tocopherol Enhance the Protective Effect of Each Other on Brain Cortical Neurons under Oxidative Stress Conditions and in Rat Two-Vessel Forebrain Ischemia/Reperfusion Injury. International Journal of Molecular Sciences. 2021; 22(21):11768. https://doi.org/10.3390/ijms222111768
Chicago/Turabian StyleZakharova, Irina O., Liubov V. Bayunova, Inna I. Zorina, Tatiana V. Sokolova, Alexander O. Shpakov, and Natalia F. Avrova. 2021. "Insulin and α-Tocopherol Enhance the Protective Effect of Each Other on Brain Cortical Neurons under Oxidative Stress Conditions and in Rat Two-Vessel Forebrain Ischemia/Reperfusion Injury" International Journal of Molecular Sciences 22, no. 21: 11768. https://doi.org/10.3390/ijms222111768
APA StyleZakharova, I. O., Bayunova, L. V., Zorina, I. I., Sokolova, T. V., Shpakov, A. O., & Avrova, N. F. (2021). Insulin and α-Tocopherol Enhance the Protective Effect of Each Other on Brain Cortical Neurons under Oxidative Stress Conditions and in Rat Two-Vessel Forebrain Ischemia/Reperfusion Injury. International Journal of Molecular Sciences, 22(21), 11768. https://doi.org/10.3390/ijms222111768