Molecular Consequences of Depression Treatment: A Potential In Vitro Mechanism for Antidepressants-Induced Reprotoxic Side Effects
Abstract
1. Introduction
2. Results
2.1. Antidepressants Interfere with ATP Production
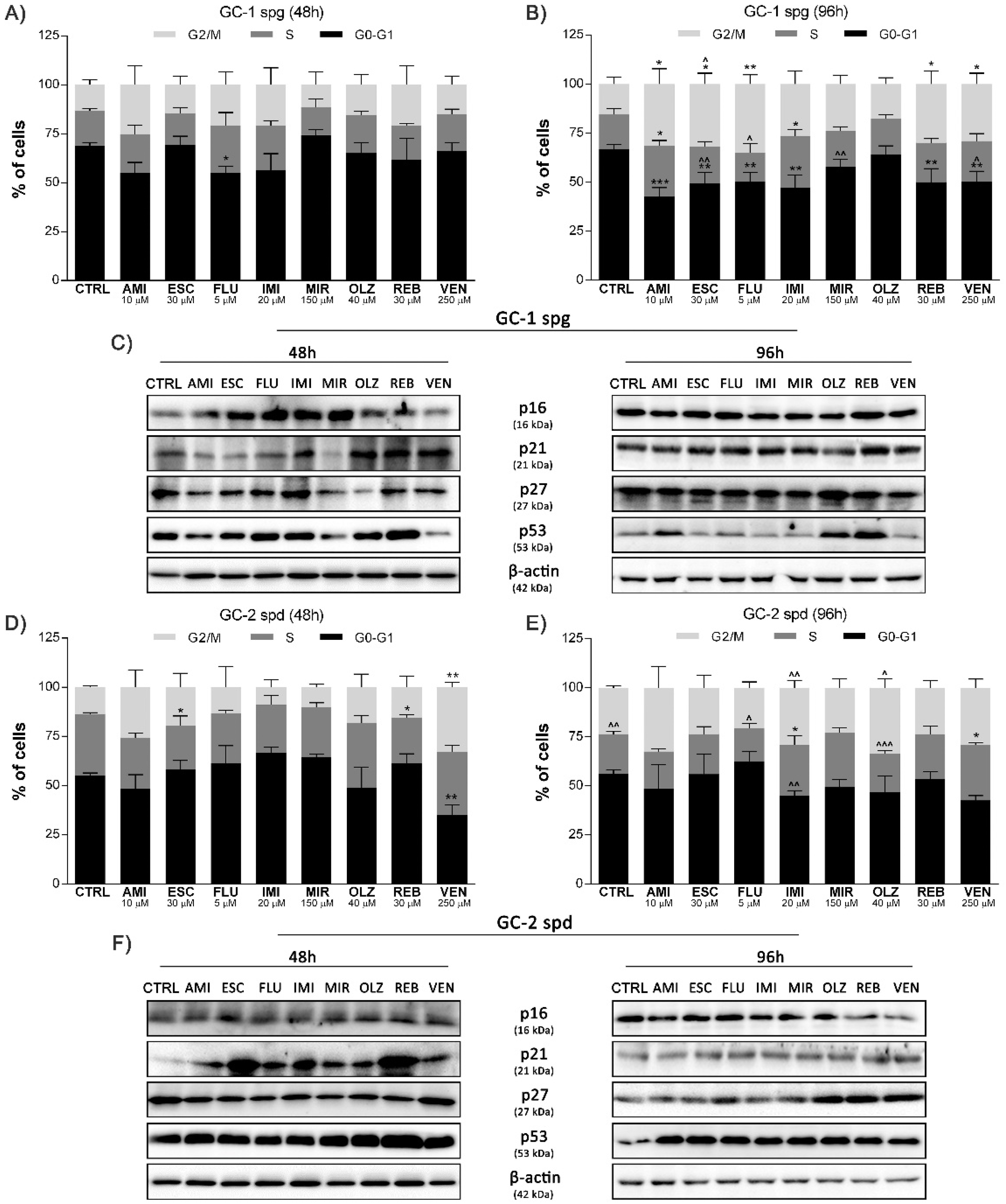
2.2. Antidepressant-Induced Cell Cycle Arrest and Related Protein Network Activation
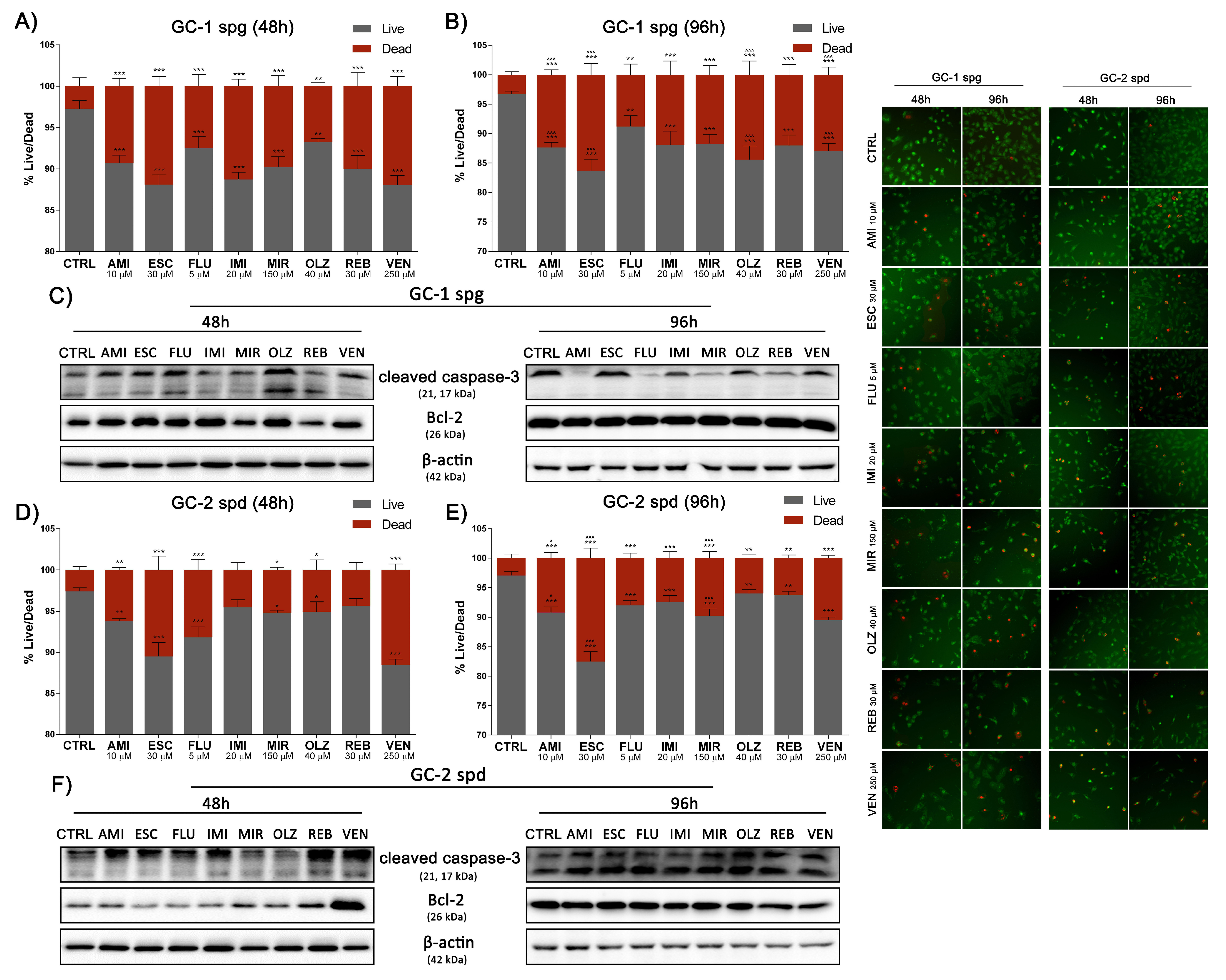
2.3. Antidepressants Initiate the DNA Fragmentation of Spermatogenic Cells
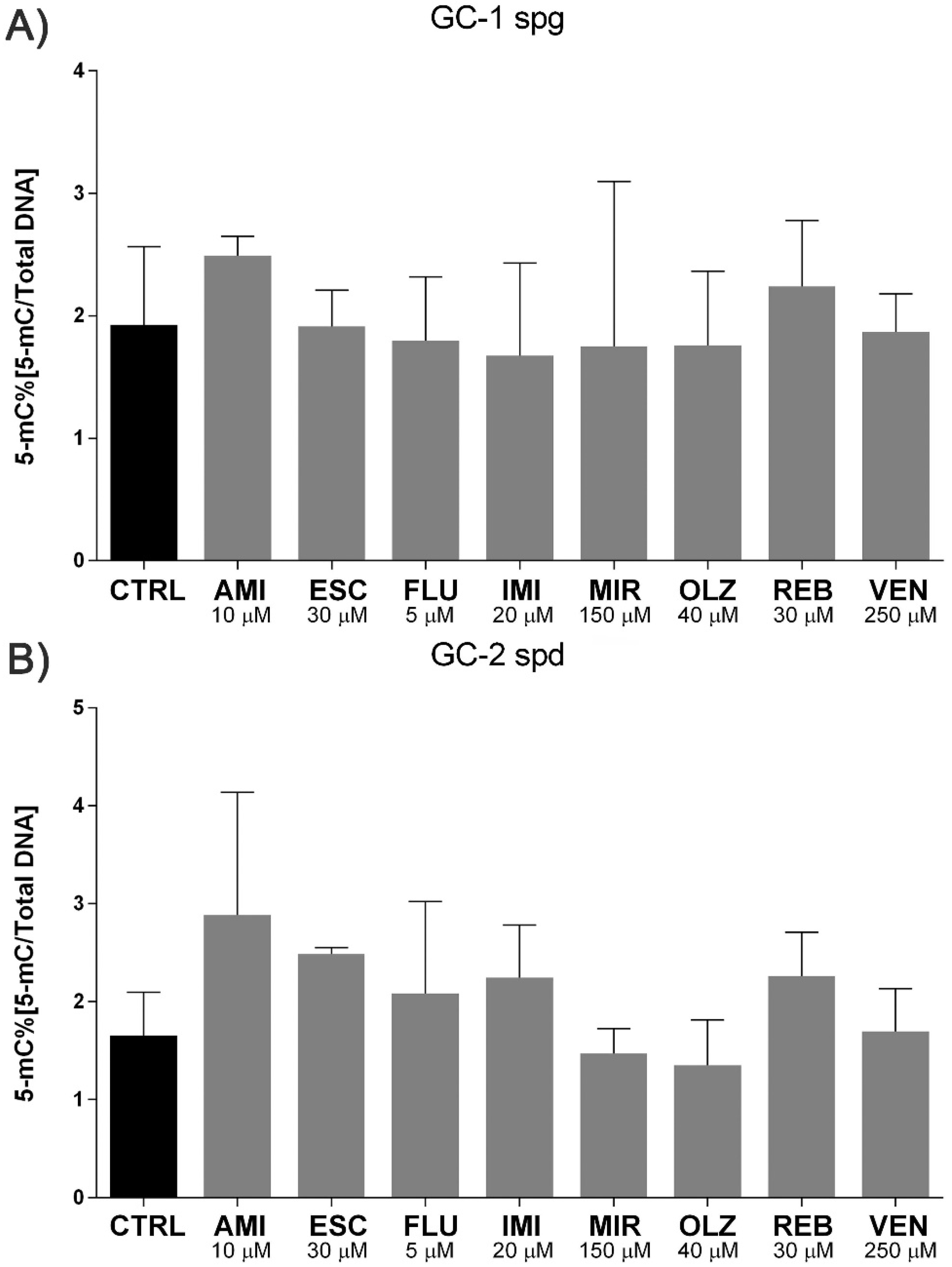
2.4. Antidepressants Induce Micronuclei Formation in Spermatogenic Cells with No Effect on Methylation Status
2.5. Antidepressants Affect NuMa-Tubulin Interactions
2.6. Antidepressants Reduce Spermatogenic Cells Viability
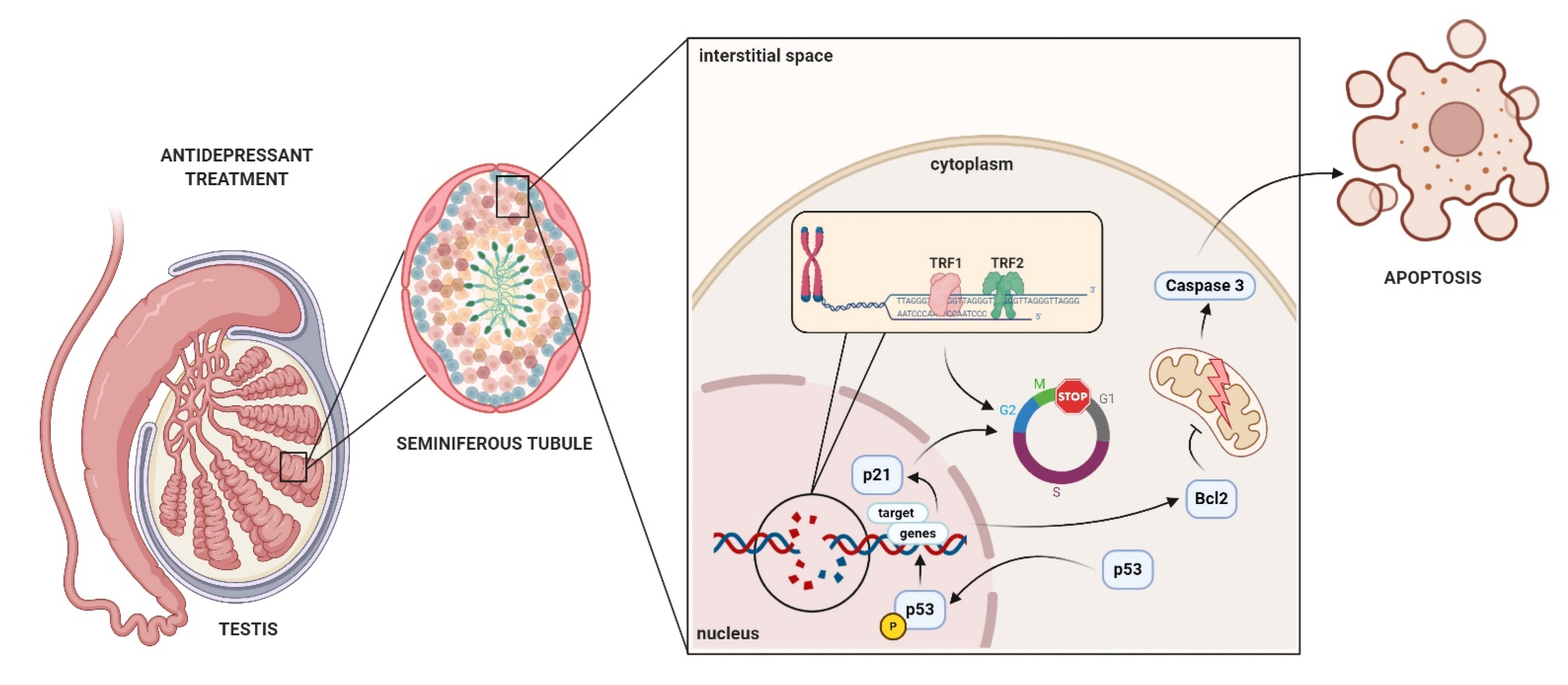
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Drug Application
4.4. ATP Luminescence Assay
4.5. Cell Cycle Profile and Micronuclei Formation
4.6. TUNEL Apoptotic Cell Detection
4.7. Cell Death Assays by Calcein-AM/PI Staining
4.8. Western Blotting
4.9. Methylation Status
4.10. Immunofluorescent Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, J. Antidepressants in the treatment of major depression: A changing landscape for clinical decision making. Clin. Pharm. 2019, 11. [Google Scholar] [CrossRef]
- Sartorius, N.; Baghai, T.C.; Baldwin, D.S.; Barrett, B.; Brand, U.; Fleischhacker, W.; Goodwin, G.; Grunze, H.; Knapp, M.; Leonard, B.E.; et al. Antidepressant medications and other treatments of depressive disorders: A CINP Task Force report based on a review of evidence. Int. J. Neuropsychopharmacol. 2007, 10 (Suppl. 1), S1–S207. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Fricker, A.D.; Devi, L.A.; Gomes, I. Mechanisms of action of antidepressants: From neurotransmitter systems to signaling pathways. Cell. Signal. 2005, 17, 549–557. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.H.; Emslie, G.J.; Mayes, T.L. The use of antidepressants to treat depression in children and adolescents. Can. Med. Assoc. J. 2006, 174, 193–200. [Google Scholar] [CrossRef]
- Uher, R.; Pavlova, B. Long-term effects of depression treatment. Lancet Psychiatry 2016, 3, 95–96. [Google Scholar] [CrossRef]
- Cartwright, C.; Gibson, K.; Read, J.; Cowan, O.; Dehar, T. Long-term antidepressant use: Patient perspectives of benefits and adverse effects. Patient Prefer. Adherence 2016, ume 10, 1401–1407. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.; Salanti, G.; Chaimani, A.; Atkinson, L.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Montejo, A.L.; Prieto, N.; De Alarcón, R.; Casado-Espada, N.; De La Iglesia, J.; Montejo, L. Management Strategies for Antidepressant-Related Sexual Dysfunction: A Clinical Approach. J. Clin. Med. 2019, 8, 1640. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.D.A.; Olivier, B. Antidepressants and Sexual Dysfunctions: A Translational Perspective. Curr. Sex. Health Rep. 2019, 11, 156–166. [Google Scholar] [CrossRef]
- Jing, E.; Straw-Wilson, K. Sexual dysfunction in selective serotonin reuptake inhibitors (SSRIs) and potential solutions: A narrative literature review. Ment. Health Clin. 2016, 6, 191–196. [Google Scholar] [CrossRef]
- Segraves, R.T.; Balon, R. Antidepressant-induced sexual dysfunction in men. Pharmacol. Biochem. Behav. 2014, 121, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.; Nash, M.; Lynch, A.M. Antidepressant-associated sexual dysfunction: Impact, effects, and treatment. Drug Healthc. Patient Saf. 2010, 2, 141–150. [Google Scholar] [CrossRef]
- Hellstrom, W.J.G. Clinical applications of centrally acting agents in male sexual dysfunction. Int. J. Impot. Res. 2008, 20, S17–S23. [Google Scholar] [CrossRef]
- Graf, H.; Malejko, K.; Metzger, C.D.; Walter, M.; Grön, G.; Abler, B. Serotonergic, Dopaminergic, and Noradrenergic Modulation of Erotic Stimulus Processing in the Male Human Brain. J. Clin. Med. 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Solek, P.; Mytych, J.; Tabecka-Lonczynska, A.; Sowa-Kucma, M.; Koziorowski, M. Toxic effect of antidepressants on male reproductive system cells: Evaluation of possible fertility reduction mechanism. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72, 3. [Google Scholar]
- Kumar St, V.S.K.; Sharma, V.L.; Tiwari, P.; Singh, D.; Maikhuri, J.P.; Gupta, G.; Singh, M.M. The spermicidal and antitrichomonas activities of SSRI antidepressants. Bioorg. Med. Chem. Lett. 2006, 16, 2509–2512. [Google Scholar] [CrossRef]
- Charles, E.; Hammadi, M.; Kischel, P.; Delcroix, V.; Demaurex, N.; Castelbout, C.; Vacher, A.-M.; Devin, A.; Ducret, T.; Nunes-Hasler, P.; et al. The antidepressant fluoxetine induces necrosis by energy depletion and mitochondrial calcium overload. Oncotarget 2016, 8, 3181–3196. [Google Scholar] [CrossRef]
- Arimochi, H.; Morita, K. Characterization of cytotoxic actions of tricyclic antidepressants on human HT29 colon carcinoma cells. Eur. J. Pharmacol. 2006, 541, 17–23. [Google Scholar] [CrossRef]
- Mao, X.; Hou, T.; Cao, B.; Wang, W.; Li, Z.; Chen, S.; Fei, M.; Hurren, R.; Gronda, M.; Wu, D.; et al. The Tricyclic Antidepressant Amitriptyline Inhibits d-Cyclin Transactivation and Induces Myeloma Cell Apoptosis by Inhibiting Histone Deacetylases: In Vitro and In Silico Evidence. Mol. Pharmacol. 2011, 79, 672–680. [Google Scholar] [CrossRef]
- Biber, A.; Durusu, I.Z.; Özen, C. In vitro anticancer effect of tricyclic antidepressant nortriptyline on multiple myeloma. Turk. J. Biol. 2018, 42, 414–421. [Google Scholar] [CrossRef]
- Battal, D.; Aktas, A.; Sungur, M.A.; Kadioglu, E.; Eker, E.D.; Sahin, N.O.; Saygi, S. In Vivo Genotoxicity Assessment of Sertraline by Using Alkaline Comet Assay and the Cytokinesis-Block Micronucleus Assay. Basic Clin. Pharmacol. Toxicol. 2013, 113, 339–346. [Google Scholar] [CrossRef]
- Roshdy, H.M. Cytogenetic and Biochemical effects of Antidepression drug (wellbutrin) on Male Mice. N. Y. Sci. 2010, 3, 121–126. [Google Scholar]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef]
- Spiller, C.; Wilhelm, D.; Koopman, P. Cell cycle analysis of fetal germ cells during sex differentiation in mice. Biol. Cell 2009, 101, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Taniguchi, M.; Otoi, T. Studying Spermatogenesis by using In vivo and In vitro Mod-els: Advantages and Disadvantages of these Models for Practical Use. J. Vet. Sci. Technol. 2012, 3, 115. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Takashima, S.; Shinohara, T. Transmission distortion by loss of p21 or p27 cyclin-dependent kinase inhibitors following competitive spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 2010, 107, 6210–6215. [Google Scholar] [CrossRef] [PubMed]
- Stopper, H.; Garcia, S.B.; Waaga-Gasser, A.M.; Kannen, V. Antidepressant fluoxetine and its potential against colon tumors. World J. Gastrointest. Oncol. 2014, 6, 11–21. [Google Scholar] [CrossRef]
- Krishnan, A.; Hariharan, R.; Nair, S.A.; Pillai, M.R. Fluoxetine mediates G0/G1 arrest by inducing functional inhibition of cyclin dependent kinase subunit (CKS)1. Biochem. Pharmacol. 2008, 75, 1924–1934. [Google Scholar] [CrossRef]
- Stepulak, A.; Rzeski, W.; Sifringer, M.; Brocke, K.; Gratopp, A.; Kupisz, K.; Turski, L.; Ikonomidou, C. Fluoxetine inhibits the extracellular signal regulated kinase pathway and suppresses growth of cancer cells. Cancer Biol. Ther. 2008, 7, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Pechnick, R.N.; Zonis, S.; Wawrowsky, K.; Cosgayon, R.; Farrokhi, C.; Lacayo, L.; Chesnokova, V. Antidepressants Stimulate Hippocampal Neurogenesis by Inhibiting p21 Expression in the Subgranular Zone of the Hipppocampus. PLoS ONE 2011, 6, e27290. [Google Scholar] [CrossRef]
- Chen, R.-W.; Chuang, D.-M. Long Term Lithium Treatment Suppresses p53 and Bax Expression but Increases Bcl-2 Expression. J. Biol. Chem. 1999, 274, 6039–6042. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Hassanane, M.S.; Hafiz, N.; Radwan, W.; El-Ghor, A.A. Genotoxic evaluation for the tricyclic antidepressant drug, amitriptyline. Drug Chem. Toxicol. 2011, 35, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Speit, G.; Vasquez, M.; Hartmann, A. The comet assay as an indicator test for germ cell genotoxicity. Mutat. Res. Mutat. Res. 2009, 681, 3–12. [Google Scholar] [CrossRef]
- Alzahrani, H.A.S. Sister chromatid exchanges and sperm abnormalities produced by antidepressant drug fluoxetine in mouse treated in vivo. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2154–2161. [Google Scholar]
- Safarinejad, M.R. Sperm DNA Damage and Semen Quality Impairment after Treatment With Selective Serotonin Reuptake Inhibitors Detected Using Semen Analysis and Sperm Chromatin Structure Assay. J. Urol. 2008, 180, 2124–2128. [Google Scholar] [CrossRef]
- Riggin, L.; Koren, G. Effects of selective serotonin reuptake inhibitors on sperm and male fertility. Can. Fam. Physician 2015, 61, 529–530. [Google Scholar]
- Endo, A.; Moyori, A.; Kobayashi, A.; Wong, R.W. Nuclear mitotic apparatus protein, NuMA, modulates p53-mediated transcription in cancer cells. Cell Death Dis. 2013, 4, e713. [Google Scholar] [CrossRef][Green Version]
- Bhattacharya, S.; Kumar, N.M.; Ganguli, A.; Tantak, M.P.; Kumar, D.; Chakrabarti, G. NMK-TD-100, a Novel Microtubule Modulating Agent, Blocks Mitosis and Induces Apoptosis in HeLa Cells by Binding to Tubulin. PLoS ONE 2013, 8, e76286. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Chang, S.; Li, Y.; Li, Q.; Hu, J.; Liang, J.; Song, L.; Kretzschmar, W.; Gan, X.; Nicod, J.; et al. Molecular Signatures of Major Depression. Curr. Biol. 2015, 25, 1146–1156. [Google Scholar] [CrossRef]
- Picco, V.; Coste, I.; Giraud-Panis, M.-J.; Renno, T.; Gilson, E.; Pagès, G. ERK1/2/MAPK pathway-dependent regulation of the telomeric factor TRF2. Oncotarget 2016, 7, 46615–46627. [Google Scholar] [CrossRef]
- Ridout, K.K.; Ridout, S.J.; Price, L.H.; Sen, S.; Tyrka, A.R. Depression and telomere length: A meta-analysis. J. Affect. Disord. 2016, 191, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Spallarossa, P.; Altieri, P.; Barisione, C.; Passalacqua, M.; Aloi, C.; Fugazza, G.; Frassoni, F.; Podestà, M.; Canepa, M.; Ghigliotti, G.; et al. p38 MAPK and JNK Antagonistically Control Senescence and Cytoplasmic p16INK4A Expression in Doxorubicin-Treated Endothelial Progenitor Cells. PLoS ONE 2010, 5, e15583. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.; Dima, D.; Frangou, S.; Breen, G.D. Telomere Length and Bipolar Disorder. Neuropsychopharmacology 2018, 43, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef]
- Sakr, S.; Mahran, H.; ElDeeb, M. Ameliorative effect of curcumin on fluoxetine-induced reproductive toxicity and oxidative stress in male albino rats. Oxid. Antioxid. Med. Sci. 2013, 2, 29–35. [Google Scholar] [CrossRef]
- Khaksar, M.; Oryan, A.; Sayyari, M.; Rezabakhsh, A.; Rahbarghazi, R. Protective effects of melatonin on long-term administration of fluoxetine in rats. Exp. Toxicol. Pathol. 2017, 69, 564–574. [Google Scholar] [CrossRef]
- Djordjevic, A.; Djordjevic, J.; Elaković, I.; Adzic, M.; Matić, G.; Radojcic, M.B. Fluoxetine affects hippocampal plasticity, apoptosis and depressive-like behavior of chronically isolated rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 92–100. [Google Scholar] [CrossRef]
- Nahon, E.; Israelson, A.; Abu-Hamad, S.; Shoshan-Barmatz, V. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 2005, 579, 5105–5110. [Google Scholar] [CrossRef] [PubMed]
- Kamla, K.S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, E4, 746–754. [Google Scholar] [CrossRef]
- Atli, O.; Baysal, M.; Aydogan-Kilic, G.; Kilic, V.; Ucarcan, S.; Karaduman, B.; Ilgin, S. Sertraline-induced reproductive toxicity in male rats: Evaluation of possible underlying mechanisms. Asian J. Androl. 2017, 19, 672–679. [Google Scholar] [CrossRef]
- Mytych, J.; Wos, I.; Solek, P.; Koziorowski, M. Protective role of klotho protein on epithelial cells upon co-culture with activated or senescent monocytes. Exp. Cell Res. 2017, 350, 358–367. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołek, P.; Mytych, J.; Tabęcka-Łonczyńska, A.; Koziorowski, M. Molecular Consequences of Depression Treatment: A Potential In Vitro Mechanism for Antidepressants-Induced Reprotoxic Side Effects. Int. J. Mol. Sci. 2021, 22, 11855. https://doi.org/10.3390/ijms222111855
Sołek P, Mytych J, Tabęcka-Łonczyńska A, Koziorowski M. Molecular Consequences of Depression Treatment: A Potential In Vitro Mechanism for Antidepressants-Induced Reprotoxic Side Effects. International Journal of Molecular Sciences. 2021; 22(21):11855. https://doi.org/10.3390/ijms222111855
Chicago/Turabian StyleSołek, Przemysław, Jennifer Mytych, Anna Tabęcka-Łonczyńska, and Marek Koziorowski. 2021. "Molecular Consequences of Depression Treatment: A Potential In Vitro Mechanism for Antidepressants-Induced Reprotoxic Side Effects" International Journal of Molecular Sciences 22, no. 21: 11855. https://doi.org/10.3390/ijms222111855
APA StyleSołek, P., Mytych, J., Tabęcka-Łonczyńska, A., & Koziorowski, M. (2021). Molecular Consequences of Depression Treatment: A Potential In Vitro Mechanism for Antidepressants-Induced Reprotoxic Side Effects. International Journal of Molecular Sciences, 22(21), 11855. https://doi.org/10.3390/ijms222111855





