Early Sucrose Degradation and the Dominant Sucrose Cleavage Pattern Influence Lycoris sprengeri Bulblet Regeneration In Vitro
Abstract
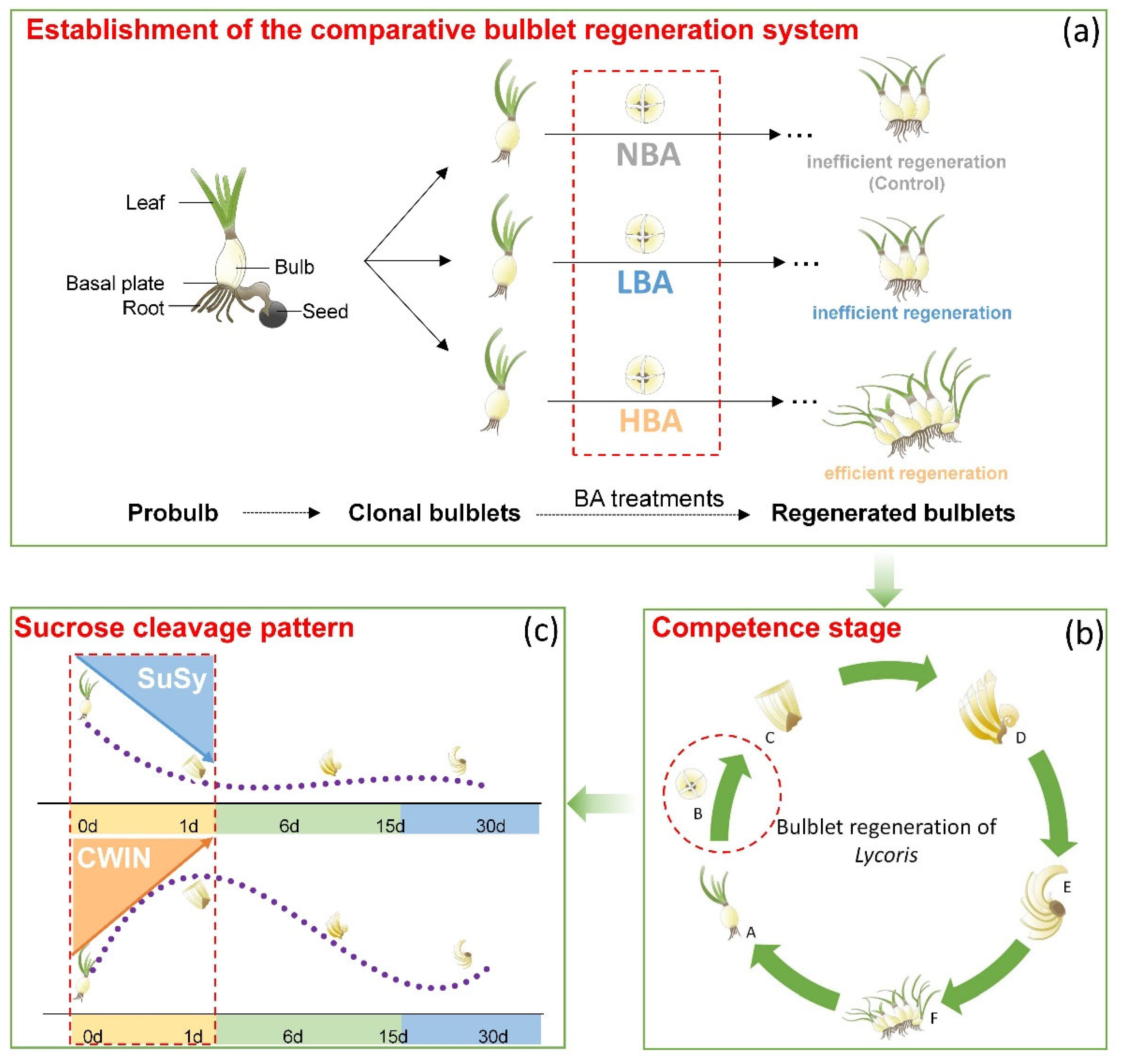
:1. Introduction
2. Results
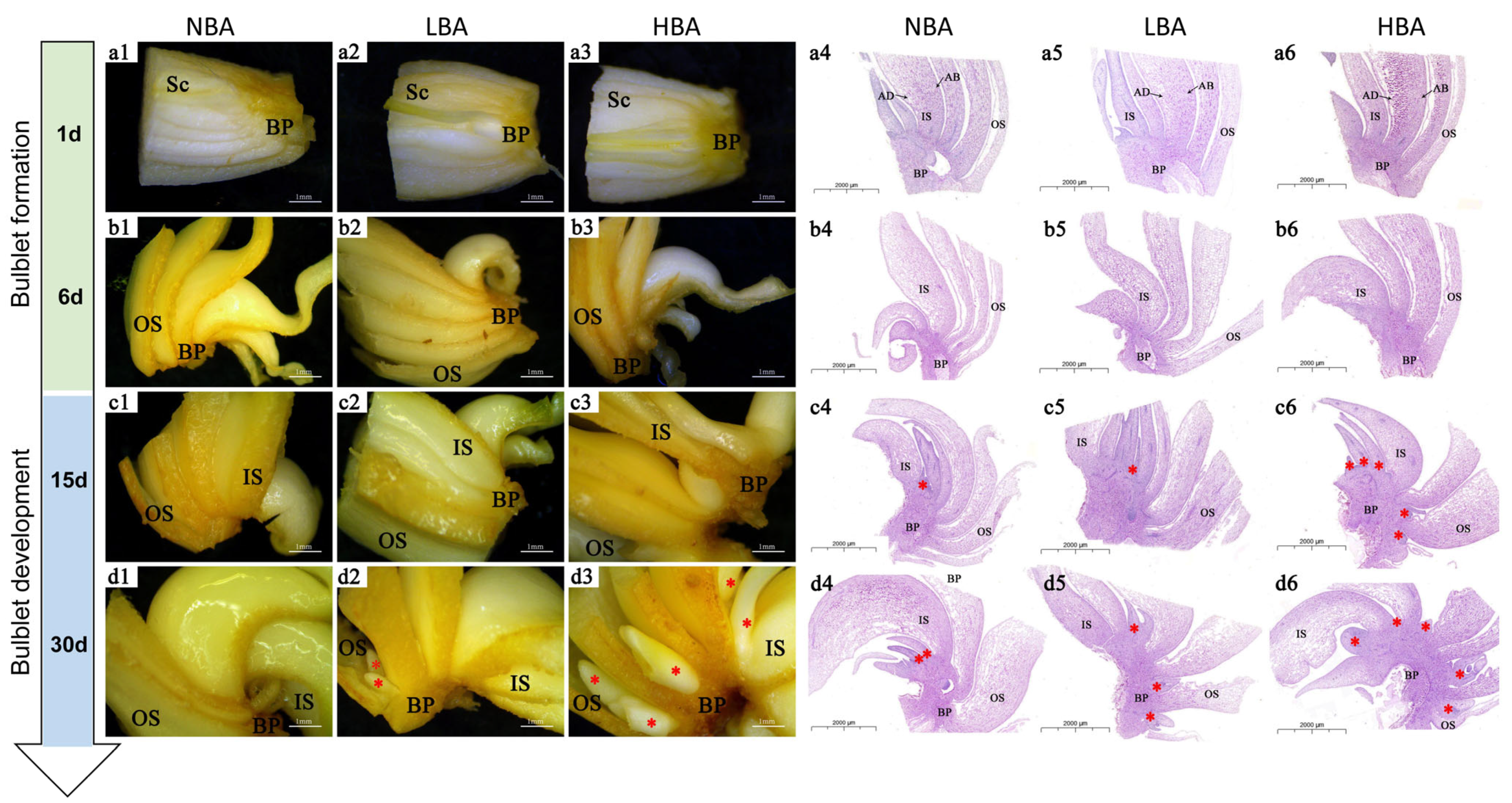
2.1. Histological Structure of Clonal Bulblets Derived In Vitro
2.2. Effects of BA on Bulblet Regeneration In Vitro of Ls
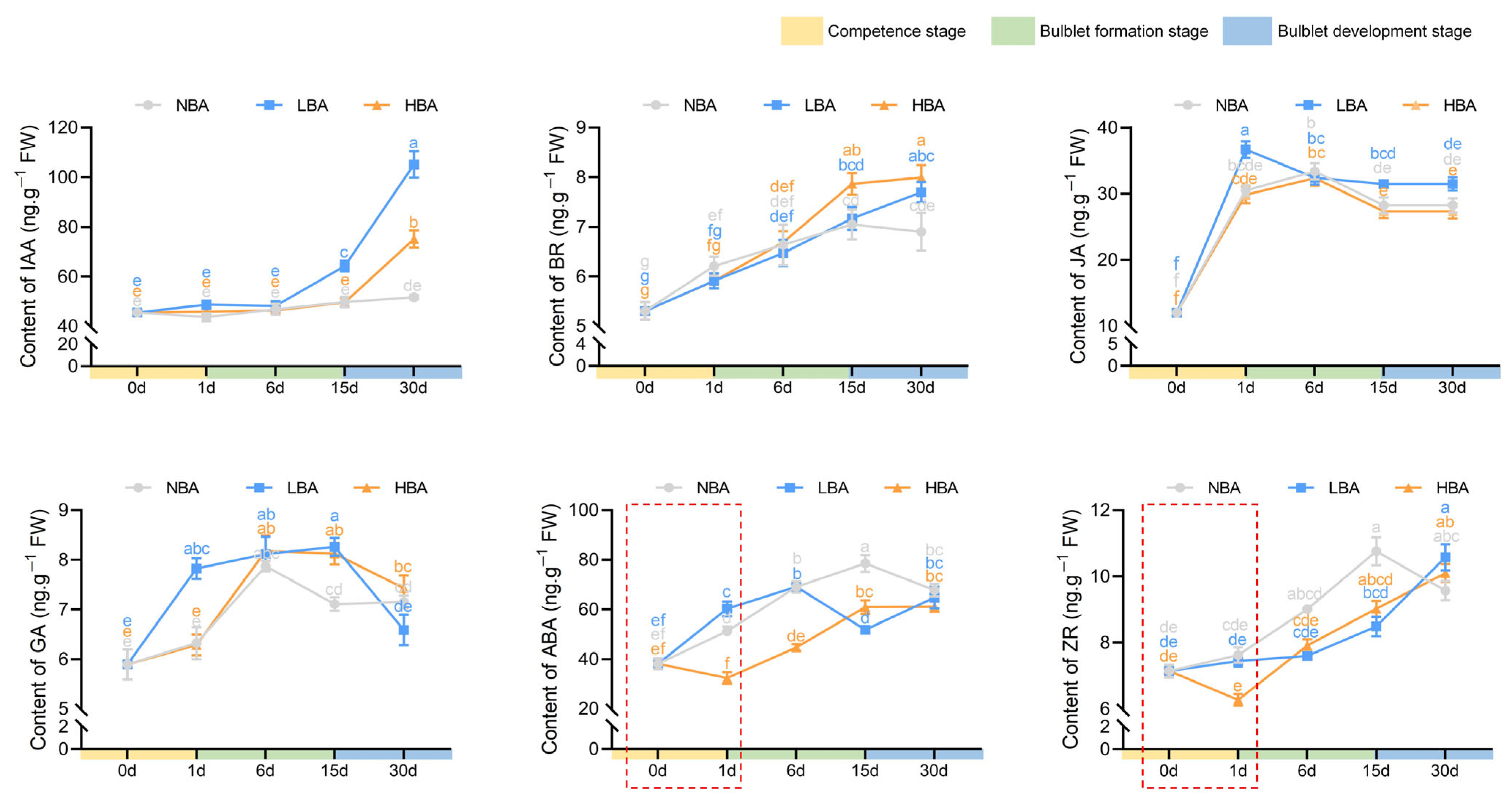
2.3. Effects of BA on Endogenous Hormone Levels during Bulblet Regeneration In Vitro
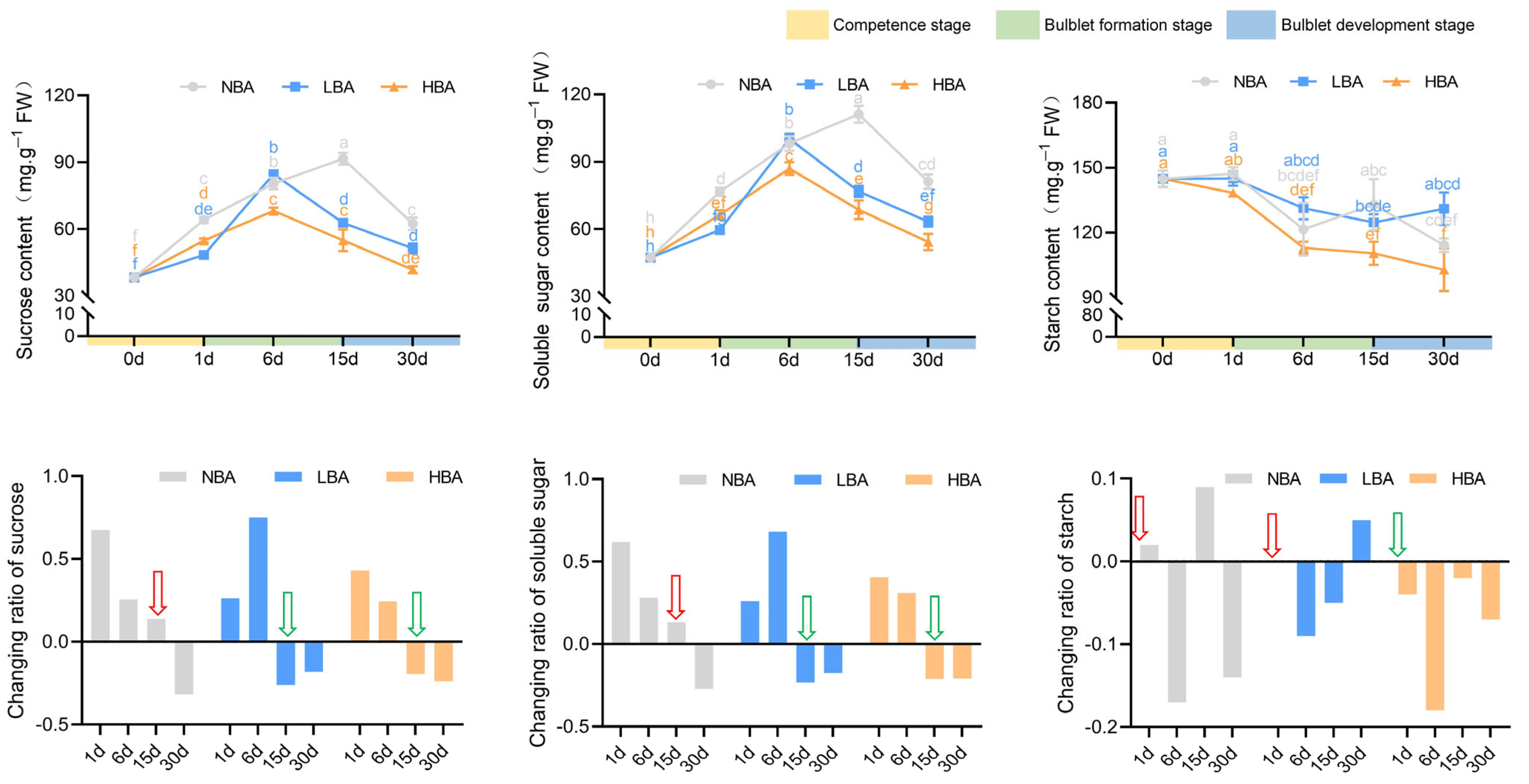
2.4. Effects of BA on Nonstructural Carbohydrate Contents during Bulblet Regeneration In Vitro
2.5. Correlation Analyses
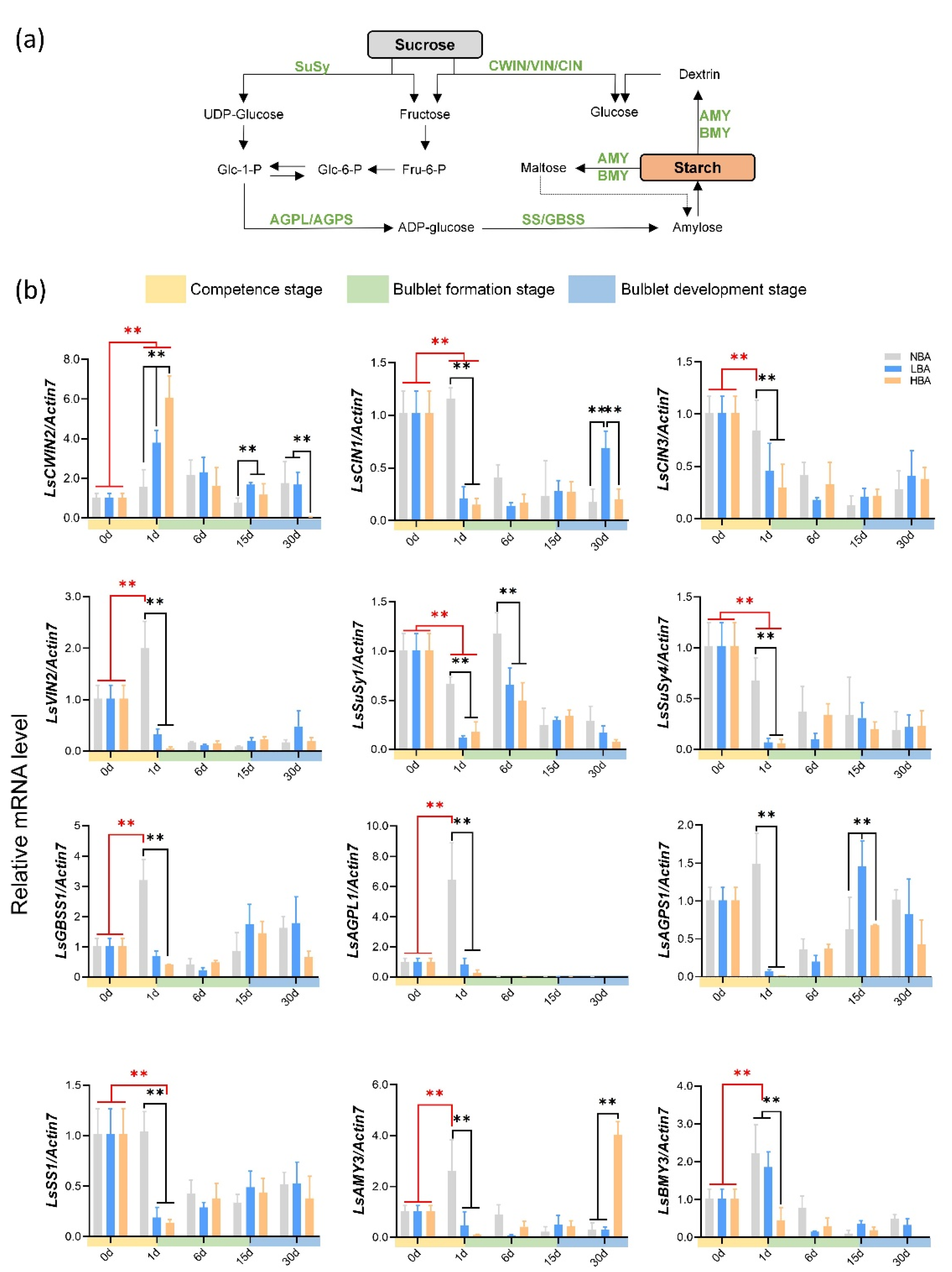
2.6. Expression Patterns of Genes Related to Sucrose and Starch Metabolism during Bulblet Regeneration
3. Discussion
3.1. Establishment of a Comparative In Vitro Bulblet Regeneration System via BA Application
3.2. Exogenous BA Mainly Affected the Incidence of Bulblets by Manipulating Hormone Regulation and Nutrient Utilization in the Competence Stage
3.3. Incidence of Bulblets In Vitro Might Be Associated with the Dominant Sucrose Cleavage Pathway during the Competence Stage
3.4. General Model of the Bulblet Regeneration in Lycoris In Vitro
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Endogenous Hormone Determination
4.3. Nonstructural Carbohydrate Contents Assay
4.4. RNA Extraction and First-Strand cDNA Synthesis
4.5. Real-Time Reverse Transcription PCR Assays
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, S.; Qiu, Y.; Wu, L.; Fu, C. Interspecific relationships of Lycoris (Amaryllidaceae) inferred from inter-simple sequence repeat data. Sci. Hortic. 2006, 110, 285–291. [Google Scholar] [CrossRef]
- He, Q.; Shen, Y.; Wang, M.; Huang, M.; Yang, R.; Zhu, S.; Wang, L.; Xu, Y.; Wu, R. Natural variation in petal color in Lycoris longituba revealed by anthocyanin components. PLoS ONE 2011, 6, e22098. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Xu, S.; Wang, N.; Xia, B.; Jiang, Y.; Wang, R. Transcriptome analysis of secondary metabolism pathway, transcription factors, and transporters in response to methyl jasmonate in Lycoris aurea. Front. Plant Sci. 2016, 7, 1971. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Xia, Y.; Zhang, D.; Li, Y.; Wu, Y. Cytological analysis of the bulblet initiation and development in Lycoris species. Sci. Hortic. 2017, 218, 72–79. [Google Scholar] [CrossRef]
- Jin, Z.; Yao, G. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2019, 36, 1462–1488. [Google Scholar] [CrossRef]
- Tsi, Z.H.; Meerow, A.W. Amaryllidaceae. In Flora of China; Wu, C.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, p. 264. [Google Scholar]
- Ren, Z.; Lin, Y.; Lv, X.; Zhang, J.; Zhang, D.; Gao, C.; Wu, Y.; Xia, Y. Clonal bulblet regeneration and endophytic communities profiling of Lycoris sprengeri, an economically valuable bulbous plant of pharmaceutical and ornamental value. Sci. Hortic. 2021, 279, 109856. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Okubo, H. Ornamental Geophytes: From Basic Science to Sustainable Production; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Wu, Y.; Li, Y.; Ma, Y.D.; Zhang, L.; Ren, Z.M.; Xia, Y.P. Hormone and antioxidant responses of Lilium Oriental hybrids ‘Sorbonne’ bulblets to humic acid treatments in vitro. J. Hortic. Sci. Biotechnol. 2017, 92, 155–167. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Zhou, L.; Fu, X.; Lei, T.; Chen, Q.; Yu, X.; Zhou, Y.; Li, W.; Hu, J.; et al. Sucrose signaling function on the formation and swelling of bulblets of Lilium sargentiae E.H. Wilson. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 135, 143–153. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Cheng, J.; Zhang, J.; da Silva, J.A.; Liu, X.; Duan, X.; Li, T.; Sun, H. Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor. BMC Plant Biol. 2014, 14, 358. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Sun, M.Y.; Zhang, J.P.; Zhang, L.; Ren, Z.M.; Min, R.H.; Wang, X.Y.; Xia, Y.P. Differential effects of paclobutrazol on the bulblet growth of oriental lily cultured in vitro: Growth behavior, carbohydrate metabolism, and antioxidant capacity. J. Plant Growth Regul. 2019, 38, 359–372. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Yang, L.; Li, X.; Wang, Z.; Yang, Z.; Zhang, Y. Changes in starch synthesis and metabolism within developing bulbs of Lycoris radiata during the vegetative growth stage. J. Plant Growth Regul. 2020, 39, 785–794. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Yang, L.; Li, X.; Wang, Z.; Zhang, Y. Changes in carbohydrate metabolism and endogenous hormone regulation during bulblet initiation and development in Lycoris radiata. BMC Plant Biol. 2020, 20, 180. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, H.; Huang, C.; Xu, W. Accumulation and distribution of 14C-photosynthate during bulb development of Lilium oriental hybrid. Nucl. Agric. Sci. 2006, 20, 417–422. (In Chinese) [Google Scholar]
- Wang, X.; Xiong, L.; Wu, X.; Wang, Q.; Chen, M.; Bao, L. Relationship between starch saccharification and propagation of bulblets from scales in oriental hybrid Lily (Lilium L.). Southwest China J. Agric. Sci. 2007, 20, 115–119. (In Chinese) [Google Scholar]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in sucrose cleavage pattern and rapid starch accumulation govern lily shoot-to-bulblet transition in vitro. Front. Plant Sci. 2021, 11, 564713. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dedaldechamp, F.; Perez-Garcia, M.-D.; Oge, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Le Gourrierec, J.; Jiao, F.; Demotes-Mainard, S.; Perez-Garcia, M.D.; Oge, L.; Hamama, L.; Crespel, L.; Bertheloot, J.; Chen, J.; et al. Convergence and divergence of sugar and cytokinin signaling in plant development. Int. J. Mol. Sci. 2021, 22, 1282. [Google Scholar] [CrossRef]
- Sheen, J.; Zhou, L.; Jang, J.C. Sugars as signaling molecules. Curr. Opin. Plant Biol. 1999, 2, 410–418. [Google Scholar] [CrossRef]
- Chiariello, N.R.; Mooney, H.A.; Williams, K. Growth, carbon allocation and cost of plant tissues. In Plant Physiological Ecology; Pearcy, R.W., Ehleringer, J.R., Mooney, H.A., Rundel, P.W., Eds.; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Ruan, Y. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Lemoine, R. Sucrose transporters in plants: Update on function and structure. Biochim. Biophys. Acta 2000, 1465, 246–262. [Google Scholar] [CrossRef] [Green Version]
- Ljung, K.; Nemhauser, J.L.; Perata, P. New mechanistic links between sugar and hormone signalling networks. Curr. Opin. Plant Biol. 2015, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, G.; Wu, M. CLE Peptide signaling and crosstalk with phytohormones and environmental stimuli. Front. Plant Sci. 2015, 6, 1211. [Google Scholar] [CrossRef] [Green Version]
- Raines, T.; Shanks, C.; Cheng, C.Y.; McPherson, D.; Argueso, C.T.; Kim, H.J.; Franco-Zorrilla, J.M.; Lopez-Vidriero, I.; Solano, R.; Vankova, R.; et al. The cytokinin response factors modulate root and shoot growth and promote leaf senescence in Arabidopsis. Plant J. 2016, 85, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Argueso, C.T. Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth-defence trade-off. Ann. Bot. 2017, 119, 725–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenig, M.; Plihalova, L.; Husickova, A.; Nisler, J.; Dolezal, K. Role of Cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef] [Green Version]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [Green Version]
- Bakhshaie, M.; Khosravi, S.; Azadi, P.; Bagheri, H.; van Tuyl, J.M. Biotechnological advances in Lilium. Plant Cell Rep. 2016, 35, 1799–1826. [Google Scholar] [CrossRef] [PubMed]
- Gong, L. Mechanism of Axillary Bud Formation during Ex Vitro Culture of Lycoris chinensis. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2012. (In Chinese). [Google Scholar]
- Xiao, Y. Effects of Plant Growth Regulators on the Development of Bulblets of Lycoris Species. Master’s Thesis, Zhejiang University, Hangzhou, China, 2013. (In Chinese). [Google Scholar]
- Xu, J.; Li, Q.; Li, Y.; Yang, L.; Zhang, Y.; Cai, Y. Effect of exogenous gibberellin, paclobutrazol, abscisic acid, and ethrel application on bulblet development in Lycoris radiata. Front. Plant Sci. 2021, 11, 615547. [Google Scholar] [CrossRef]
- Cabib, E.; Leloir, L.F. Biosynthesis of trehalose phosphate. J. Biol. Chem. 1958, 231, 259–275. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Dung Tien, L.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef] [Green Version]
- Takatsuka, H.; Umeda, M. ABA inhibits root cell elongation through repressing the cytokinin signaling. Plant Signal. Behav. 2019, 14, e1578632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurkman, W.J.; McCue, K.F.; Altenbach, S.B.; Korn, A.; Tanaka, C.K.; Kotharia, K.M.; Johnson, E.L.; Bechtel, D.B.; Wilson, J.D.; Anderson, O.D.; et al. Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci. 2003, 164, 873–881. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol. 2006, 171, 293–303. [Google Scholar] [CrossRef]
- Rubio, S.; Noriega, X.; Perez, F.J. ABA promotes starch synthesis and storage metabolism in dormant grapevine buds. J. Plant Physiol. 2019, 234, 1–8. [Google Scholar] [CrossRef]
- Chang, L.; Xiao, Y.M.; She, L.F.; Xia, Y.P. Analysis of gene expression and enzyme activities related to starch metabolism in Lycoris sprengeri bulbs of different sizes. Sci. Hortic. 2013, 161, 118–124. [Google Scholar] [CrossRef]
- Janssen, B.J.; Thodey, K.; Schaffer, R.J.; Alba, R.; Balakrishnan, L.; Bishop, R.; Bowen, J.H.; Crowhurst, R.N.; Gleave, A.P.; Ledger, S.; et al. Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol. 2008, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Chen, Z.H.; Tecsi, L.I.; Famiani, F.; Lea, P.J.; Leegood, R.C. Phosphoenolpyruvate carboxykinase plays a role in interactions of carbon and nitrogen metabolism during grape seed development. Planta 1999, 210, 9–18. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Loeb, J. Regeneration: From a physico-chemical viewpoint. Nature 1925, 116, 90–91. [Google Scholar]
- Gregory, F.G.; Veale, J.A. A reassessment of the problem of apical dominance. Symp. Soc. Exp. Biol. 1957, 11, 1–20. [Google Scholar]
- Phillips, I.D.J. Apical dominance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1975, 26, 341–367. [Google Scholar] [CrossRef]
- Ayre, B.G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 2011, 4, 377–394. [Google Scholar] [CrossRef] [Green Version]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Wang, L.; Li, J.; Ruan, Y.L. Cell wall invertase is essential for ovule development through sugar signaling rather than provision of carbon nutrients. Plant Physiol. 2020, 183, 1126–1144. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mowry, R.W. The special value of methods that color both acidic and vicinal hydroxyl groups in the histochemical study of mucins. With revised directions for the colloidal iron stain, the use of alcian blue g8x and their combinations with the periodic acid-schiff reaction. Ann. N. Y. Acad. Sci. 1963, 106, 402–423. [Google Scholar]
- Yang, Y.M.; Xu, C.N.; Wang, B.M.; Jia, J.Z. Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regul. 2001, 35, 233–237. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- McCready, R.M.; Guggolz, J.; Silviera, V.; Owens, H.S. Determination of starch and amylose in vegetables-application to peas. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Livak, L.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Xu, Y.; Lvy, X.; Zhang, D.; Gao, C.; Lin, Y.; Liu, Y.; Wu, Y.; Xia, Y. Early Sucrose Degradation and the Dominant Sucrose Cleavage Pattern Influence Lycoris sprengeri Bulblet Regeneration In Vitro. Int. J. Mol. Sci. 2021, 22, 11890. https://doi.org/10.3390/ijms222111890
Ren Z, Xu Y, Lvy X, Zhang D, Gao C, Lin Y, Liu Y, Wu Y, Xia Y. Early Sucrose Degradation and the Dominant Sucrose Cleavage Pattern Influence Lycoris sprengeri Bulblet Regeneration In Vitro. International Journal of Molecular Sciences. 2021; 22(21):11890. https://doi.org/10.3390/ijms222111890
Chicago/Turabian StyleRen, Ziming, Yunchen Xu, Xuesi Lvy, Dong Zhang, Cong Gao, Yefan Lin, Yue Liu, Yun Wu, and Yiping Xia. 2021. "Early Sucrose Degradation and the Dominant Sucrose Cleavage Pattern Influence Lycoris sprengeri Bulblet Regeneration In Vitro" International Journal of Molecular Sciences 22, no. 21: 11890. https://doi.org/10.3390/ijms222111890
APA StyleRen, Z., Xu, Y., Lvy, X., Zhang, D., Gao, C., Lin, Y., Liu, Y., Wu, Y., & Xia, Y. (2021). Early Sucrose Degradation and the Dominant Sucrose Cleavage Pattern Influence Lycoris sprengeri Bulblet Regeneration In Vitro. International Journal of Molecular Sciences, 22(21), 11890. https://doi.org/10.3390/ijms222111890






