New Insights into the Role of PD-1 and Its Ligands in Allergic Disease
Abstract
:1. Introduction
2. Molecular Mechanisms of Allergy
3. PD-1 and Its Ligands PD-L1 and PD-L2
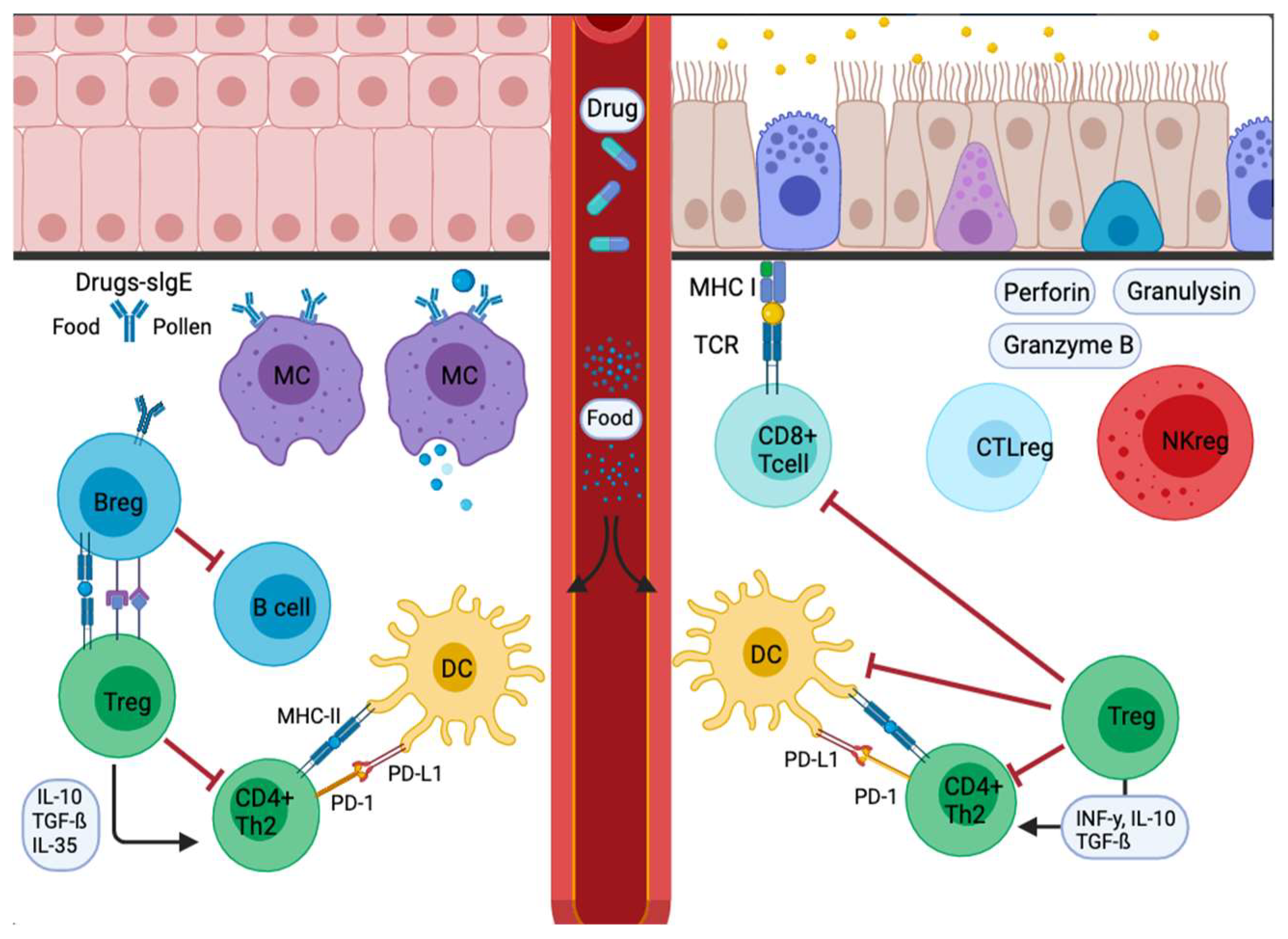
4. PD-1 and Its Ligands in Allergy Diseases
4.1. Allergic Rhinitis and Conjunctivitis
4.2. Asthma
4.3. Food Allergy
4.4. Skin Immune Response
4.5. Anaphylaxis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Krempski, J.W.; Nadeau, K. Advances and novel developments in mechanisms of allergic inflammation. Allergy 2020, 75, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, M.C.; Kim, B.S.; Spergel, J.M.; Artis, D. Basophils and allergic inflammation. J. Allergy Clin. Immunol. 2013, 132, 789–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Veen, W.; Wirz, O.F.; Globinska, A.; Akdis, M. Novel mechanisms in immune tolerance to allergens during natural allergen exposure and allergen-specific immunotherapy. Curr. Opin. Immunol. 2017, 48, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Stock, P.; DeKruyff, R.H.; Umetsu, D.T. Inhibition of the allergic response by regulatory T cells. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 12–16. [Google Scholar] [CrossRef]
- Hansbro, P.M.; Kaiko, G.E.; Foster, P.S. Cytokine/anti-cytokine therapy-novel treatments for asthma? Br. J. Pharmacol. 2011, 163, 81–95. [Google Scholar] [CrossRef]
- Wing, K.; Sakaguchi, S. Regulatory T cells as potential immunotherapy in allergy. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 482–488. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Lv, Z.; Chen, Y.; Li, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Bronchial Allergen Challenge of Patients with Atopic Asthma Triggers an Alarmin (IL-33, TSLP, and IL-25) Response in the Airways Epithelium and Submucosa. J. Immunol. 2018, 201, 2221–2231. [Google Scholar] [CrossRef] [Green Version]
- Gubernatorova, E.O.; Namakanova, O.A.; Tumanov, A.V.; Drutskaya, M.S.; Nedospasov, S.A. Mouse models of severe asthma for evaluation of therapeutic cytokine targeting. Immunol. Lett. 2019, 207, 73–83. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Namakanova, O.A.; Gorshkova, E.A.; Medvedovskaya, A.D.; Nedospasov, S.A.; Drutskaya, M.S. Novel Anti-Cytokine Strategies for Prevention and Treatment of Respiratory Allergic Diseases. Front. Immunol. 2021, 12, 1704. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Agache, I.; Sugita, K.; Morita, H.; Akdis, M.; Akdis, C.A. The Complex Type 2 Endotype in Allergy and Asthma: From Laboratory to Bedside. Curr. Allergy Asthma Rep. 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Roche, P.A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Canavan, M.; Floudas, A.; Veale, D.J.; Fearon, U. The PD-1:PD-L1 axis in Inflammatory Arthritis. BMC Rheumatol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Schwamborn, K. Imaging mass spectrometry in biomarker discovery and validation. J. Proteomics 2012, 75, 4990–4998. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, Y.; Nagashima, H.; Ushio-Fukai, M.; Croft, M.; Ishii, N.; So, T. IQGAP1 restrains T-cell cosignaling mediated by OX40. FASEB J. 2020, 34, 540–554. [Google Scholar] [CrossRef] [Green Version]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Invest. 2019, 129, 1493–1503. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, B.; Mestre, D.; Martínez-Martín, N. The immunological synapse: A cause or consequence of T-cell receptor triggering? Immunology 2011, 133, 420–425. [Google Scholar] [CrossRef]
- Yokosuka, T.; Saito, T. The immunological synapse, TCR microclusters, and T cell activation. Curr. Top. Microbiol. Immunol. 2010, 340, 81–107. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef]
- Stone, S.F.; Phillips, E.J.; Wiese, M.D.; Heddle, R.J.; Brown, S.G.A. Immediate-type hypersensitivity drug reactions. Br. J. Clin. Pharmacol. 2014, 78, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection; Frontiers: Lausanne, Switzerland, 2020; Volume 11, p. 487. [Google Scholar]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubat, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of Programmed Death 1 Ligands by Murine T Cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef] [Green Version]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovas, C.; Casazza, J.P.; Brenchley, J.M.; Price, D.A.; Gostick, E.; Adams, W.C.; Precopio, M.L.; Schacker, T.; Roederer, M.; Douek, D.C.; et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006, 203, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Ballesteros, P. Journal of Neuroscience Research 85:3244–3253 (2007). J. Neurosci. Res. 2007, 3253, 3244–3253. [Google Scholar] [CrossRef]
- Kinter, A.L.; Godbout, E.J.; McNally, J.P.; Sereti, I.; Roby, G.A.; O’Shea, M.A.; Fauci, A.S. The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. J. Immunol. 2008, 181, 6738 LP–6746. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Karwacz, K.; Bricogne, C.; MacDonald, D.; Arce, F.; Bennett, C.L.; Collins, M.; Escors, D. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol. Med. 2011, 3, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Ohashi, P.S. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat. Rev. Immunol. 2015, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, A.; Fernandez-Hinojal, G.; Zuazo-Ibarra, M.; Arasanz, H.; Garcia-Granda, M.; Hernandez, C.; Ibañez, M.; Hernandez-Marin, B.; Martinez-Aguillo, M.; Lecumberri, M.; et al. PD-L1 Expression in Systemic Immune Cell Populations as a Potential Predictive Biomarker of Responses to PD-L1/PD-1 Blockade Therapy in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escors, D.; Gato-Cañas, M.; Zuazo, M.; Arasanz, H.; García-Granda, M.J.; Vera, R.; Kochan, G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct. Target. Ther. 2018, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast Cells Enhance T Cell Activation: Importance of Mast Cell Costimulatory Molecules and Secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef] [Green Version]
- Ghiotto, M.; Gauthier, L.; Serriari, N.; Pastor, S.; Truneh, A.; Nunès, J.A.; Olive, D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int. Immunol. 2010, 22, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Loke, P.; Allison, J.P. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5336–5341. [Google Scholar] [CrossRef] [Green Version]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Gato-Cañas, M.; Zuazo, M.; Arasanz, H.; Ibañez-Vea, M.; Lorenzo, L.; Fernandez-Hinojal, G.; Vera, R.; Smerdou, C.; Martisova, E.; Arozarena, I.; et al. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017, 20, 1818–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, Y.; Li, D.; Wang, L.; Liang, Z.; Chen, Y.; Ma, G.; Wu, H.; Jiao, W.; Niu, H. Glucose metabolism involved in PD-L1-mediated immune escape in the malignant kidney tumour microenvironment. Cell Death Discov. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Eppihimer, M.J.; Gunn, J.; Freeman, G.J.; Greenfield, E.A.; Chernova, T.; Erickson, J.; Leonard, J.P. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 2002, 9, 133–145. [Google Scholar] [CrossRef]
- Palomares, O.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef]
- Canonica, G.W.; Mullol, J.; Pradalier, A.; Didier, A. Patient Perceptions of Allergic Rhinitis and Quality of Life. World Allergy Organ. J. 2008, 1, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Strachan, D.; Sibbald, B.; Weiland, S.; Ait-Khaled, N.; Anabwani, G.; Anderson, H.R.; Asher, M.I.; Beasley, R.; Bjorksten, B.; Burr, M.; et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr. Allergy Immunol. 1997, 8, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Corren, J. Allergic Rhinitis and Conjunctivitis. In Middleton’s Allergy Essentials; O’Hehir, R.E., Holgate, S.T., Sheikh, A.B.T.-M.A.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 205–224. ISBN 9780323392730. [Google Scholar]
- Wang, Z.; Tan, F. The blockade of PD-1/PD-L1 pathway promotes the apoptosis of CD19+CD25+ Bregs and suppresses the secretion of IL-10 in patients with allergic rhinitis. Scand. J. Immunol. 2020, 91, e12836. [Google Scholar] [CrossRef]
- Sin, B.; Togias, A. Pathophysiology of allergic and nonallergic rhinitis. Proc. Am. Thorac. Soc. 2011, 8, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A. Exertion of the suppressive effects of IFN-gamma on experimental immune mediated blepharoconjunctivitis in Brown Norway rats during the induction phase but not the effector phase. Br. J. Ophthalmol. 2002, 86, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, A.; Ozaki, A.; Fukata, K.; Ishida, W.; Ueno, H. Ag-specific recognition, activation, and effector function of T cells in the conjunctiva with experimental immune-mediated blepharoconjunctivitis. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4366–4374. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, A.; Yamaguchi, T.; Azuma, M.; Yagita, H.; Ueno, H. Involvement of programmed death-ligand 2 (PD-L2) in the development of experimental allergic conjunctivitis in mice. Br. J. Ophthalmol. 2006, 90, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Gavrieli, M.; Sedy, J.R.; Yang, J.; Fallarino, F.; Loftin, S.K.; Hurchla, M.A.; Zimmerman, N.; Sim, J.; Zang, X.; et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003, 4, 670–679. [Google Scholar] [CrossRef]
- Ishida, W.; Fukuda, K.; Kajisako, M.; Sumi, T.; Matsuda, H.; Yagita, H.; Fukushima, A. B and T lymphocyte attenuator regulates the development of antigen-induced experimental conjunctivitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Inoue, H.; Nakano, T.; Tsuda, M.; Yoshiura, Y.; Fukuyama, S.; Tsushima, F.; Hoshino, T.; Aizawa, H.; Akiba, H.; et al. B7-DC Regulates Asthmatic Response by an IFN-γ-Dependent Mechanism. J. Immunol. 2004, 172, 2530–2541. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Hupin, C.; Froidure, A.; Detry, B.; Pilette, C. Impaired ICOSL in human myeloid dendritic cells promotes Th2 responses in patients with allergic rhinitis and asthma. Clin. Exp. Allergy 2014, 44, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Kwon, H.J.; Chung, Y.-S.; Lee, B.-J.; Jang, Y.J. Infection Rate and Virus-Induced Cytokine Secretion in Experimental Rhinovirus Infection in Mucosal Organ Culture: Comparison Between Specimens From Patients With Chronic Rhinosinusitis With Nasal Polyps and Those From Normal Subjects. Arch. Otolaryngol. Neck Surg. 2008, 134, 424–427. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.-K.; Zhang, Y.-L.; Wee, J.H.; Han, D.H.; Kim, H.J.; Rhee, C.-S. Human Rhinovirus Infection Enhances the Th2 Environment in Allergic and Non-allergic Patients with Chronic Rhinosinusitis. Clin. Exp. Otorhinolaryngol. 2021, 14, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.L.; Li, F.; Zhao, F.; Zuo, J.J.; Deng, Y.Q.; Zhang, W.; Tao, Z.Z. Programmed cell death protein 1 and its ligands regulate immune balance in allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 55, 384–390. [Google Scholar] [CrossRef]
- Kortekaas Krohn, I.; Bobic, S.; Dooley, J.; Lan, F.; Zhang, N.; Bachert, C.; Steelant, B.; Bullens, D.M.; Liston, A.; Ceuppens, J.L.; et al. Programmed cell death-1 expression correlates with disease severity and IL-5 in chronic rhinosinusitis with nasal polyps. Allergy 2017, 72, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Nasiri Kalmarzi, R.; Fattahi, N.; Kaviani, Z.; Ataee, P.; Mansouri, M.; Moradi, G.; Yousefzade, A.; Abbassi, J.M. Inverse correlation of soluble programmed cell death-1 ligand-1 (sPD-L1) with eosinophil count and clinical severity in allergic rhinitis patients. Allergol. Int. 2017, 66, 326–331. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Brusasco, V.; Crimi, E.; Pellegrino, R. Airway hyperresponsiveness in asthma: Not just a matter of airway inflammation. Thorax 1998, 53, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Lemanske, R.F.J. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef]
- Zhao, S.-T.; Wang, C.-Z. Regulatory T cells and asthma. J. Zhejiang Univ. Sci. B 2018, 19, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Fluhr, J.W.; Ruwwe-Glösenkamp, C.; Stevanovic, K.; Bergmann, K.-C.; Zuberbier, T. Role of IL-17 in atopy—A systematic review. Clin. Transl. Allergy 2021, 11, e12047. [Google Scholar] [CrossRef]
- Li, J.; Sha, J.; Sun, L.; Zhu, D.; Meng, C. Contribution of Regulatory T Cell Methylation Modifications to the Pathogenesis of Allergic Airway Diseases. J. Immunol. Res. 2021, 2021, 5590217. [Google Scholar] [CrossRef]
- Akdis, M.; Verhagen, J.; Taylor, A.; Karamloo, F.; Karagiannidis, C.; Crameri, R.; Thunberg, S.; Deniz, G.; Valenta, R.; Fiebig, H.; et al. Immune Responses in Healthy and Allergic Individuals Are Characterized by a Fine Balance between Allergen-specific T Regulatory 1 and T Helper 2 Cells. J. Exp. Med. 2004, 199, 1567–1575. [Google Scholar] [CrossRef]
- Wei, F.; Zhong, S.; Ma, Z.; Kong, H.; Medvec, A.; Ahmed, R.; Freeman, G.J.; Krogsgaard, M.; Riley, J.L. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc. Natl. Acad. Sci. USA 2013, 110, E2480–E2489. [Google Scholar] [CrossRef] [Green Version]
- Zuazo, M.; Arasanz, H.; Fernández-Hinojal, G.; García-Granda, M.J.; Gato, M.; Bocanegra, A.; Martínez, M.; Hernández, B.; Teijeira, L.; Morilla, I.; et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol. Med. 2019, 11, e10293. [Google Scholar] [CrossRef]
- Nurieva, R.; Thomas, S.; Nguyen, T.; Martin-Orozco, N.; Wang, Y.; Kaja, M.-K.; Yu, X.-Z.; Dong, C. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006, 25, 2623–2633. [Google Scholar] [CrossRef] [Green Version]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Stock, P.; Akbari, O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy 2011, 66, 155–162. [Google Scholar] [CrossRef]
- Akbari, O.; Stock, P.; Singh, A.K.; Lombardi, V.; Lee, W.-L.; Freeman, G.J.; Sharpe, A.H.; Umetsu, D.T.; Dekruyff, R.H. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010, 3, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlees, J.W.; Lajoie, S.; Dienger, K.; Sproles, A.A.; Richgels, P.K.; Yang, Y.; Khodoun, M.; Azuma, M.; Yagita, H.; Fulkerson, P.C.; et al. Differential control of CD4(+) T-cell subsets by the PD-1/PD-L1 axis in a mouse model of allergic asthma. Eur. J. Immunol. 2015, 45, 1019–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oflazoglu, E.; Swart, D.A.; Anders-Bartholo, P.; Jessup, H.K.; Norment, A.M.; Lawrence, W.A.; Brasel, K.; Tocker, J.E.; Horan, T.; Welcher, A.A.; et al. Paradoxical role of programmed death-1 ligand 2 in Th2 immune responses in vitro and in a mouse asthma model in vivo. Eur. J. Immunol. 2004, 34, 3326–3336. [Google Scholar] [CrossRef]
- Bratke, K.; Fritz, L.; Nokodian, F.; Geißler, K.; Garbe, K.; Lommatzsch, M.; Virchow, J.C. Differential regulation of PD-1 and its ligands in allergic asthma. Clin. Exp. allergy J. Br. Soc. Allergy Clin. Immunol. 2017, 47, 1417–1425. [Google Scholar] [CrossRef]
- Kazanova, A.; Rudd, C.E. Programmed cell death 1 ligand (PD-L1) on T cells generates Treg suppression from memory. PLoS Biol. 2021, 19, e3001272. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Sözener, Z.C.; Akdis, M.; Akdis, C.A. The role of Treg cell subsets in allergic disease. Asian Pacific J. Allergy Immunol. 2020, 38, 139–149. [Google Scholar] [CrossRef]
- McGee, H.S.; Yagita, H.; Shao, Z.; Agrawal, D.K. Programmed Death-1 antibody blocks therapeutic effects of T-regulatory cells in cockroach antigen-induced allergic asthma. Am. J. Respir. Cell Mol. Biol. 2010, 43, 432–442. [Google Scholar] [CrossRef] [Green Version]
- Helou, D.G.; Shafiei-Jahani, P.; Lo, R.; Howard, E.; Hurrell, B.P.; Galle-Treger, L.; Painter, J.D.; Lewis, G.; Soroosh, P.; Sharpe, A.H.; et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat. Commun. 2020, 11, 3998. [Google Scholar] [CrossRef]
- Xi, X.; Liu, J.-M.; Guo, J.-Y. Correlation of PD-1/PD-L1 Signaling Pathway with Treg/Th17 Imbalance from Asthmatic Children. Int. Arch. Allergy Immunol. 2018, 176, 255–267. [Google Scholar] [CrossRef]
- Mosayebian, A.; Koohini, Z.; Hossein-Nataj, H.; Abediankenari, S.; Abedi, S.; Asgarian-Omran, H. Elevated Expression of Tim-3 and PD-1 Immune Checkpoint Receptors on T-CD4+ Lymphocytes of Patients with Asthma. Iran. J. Allergy, Asthma Immunol. 2019, 17, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Lewkowich, I.P.; Lajoie, S.; Stoffers, S.L.; Suzuki, Y.; Richgels, P.K.; Dienger, K.; Sproles, A.A.; Yagita, H.; Hamid, Q.; Wills-Karp, M. PD-L2 modulates asthma severity by directly decreasing dendritic cell IL-12 production. Mucosal Immunol. 2013, 6, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, T.B.; Biswas, M.; Terhorst, C.; Daniell, H.; Herzog, R.W.; Piñeros, A.R. Role of orally induced regulatory T cells in immunotherapy and tolerance. Cell. Immunol. 2021, 359, 104251. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Mayer, L.; Berin, M.C. Mucosal immunology of tolerance and allergy in the gastrointestinal tract. Immunol. Res. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef] [Green Version]
- Dubois, B.; Chapat, L.; Goubier, A.; Papiernik, M.; Nicolas, J.-F.; Kaiserlian, D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood 2003, 102, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Takagi, H.; Sato, Y.; Sato, K.; Eizumi, K.; Taya, H.; Shin, T.; Chen, L.; Dong, C.; Azuma, M.; et al. Crucial roles of B7-H1 and B7-DC expressed on mesenteric lymph node dendritic cells in the generation of antigen-specific CD4+Foxp3+ regulatory T cells in the establishment of oral tolerance. Blood 2010, 116, 2266–2276. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Kim, K.S.; Hong, S.-W.; Han, D.; Yi, J.; Jung, J.; Yang, B.-G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Ikebuchi, R.; Chtanova, T.; Kusumoto, Y.; Okuyama, H.; Moriya, T.; Honda, T.; Kabashima, K.; Watanabe, T.; Sakai, Y.; et al. Regulatory T cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunol. 2018, 11, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Hew, M.; Lee, J.; Susanto, N.H.; Prasad, S.; Bardin, P.G.; Barnes, S.; Ruane, L.; Southcott, A.M.; Gillman, A.; Young, A.; et al. The 2016 Melbourne thunderstorm asthma epidemic: Risk factors for severe attacks requiring hospital admission. Allergy 2019, 74, 122–130. [Google Scholar] [CrossRef]
- Manabe, T.; Sato, S.; Yanagida, N.; Hayashi, N.; Nishino, M.; Takahashi, K.; Nagakura, K.-I.; Asaumi, T.; Ogura, K.; Ebisawa, M. Long-term outcomes after sustained unresponsiveness in patients who underwent oral immunotherapy for egg, cow’s milk, or wheat allergy. Allergol. Int. 2019, 68, 527–528. [Google Scholar] [CrossRef]
- Reier-Nilsen, T.; Michelsen, M.M.; Lødrup Carlsen, K.C.; Carlsen, K.-H.; Mowinckel, P.; Nygaard, U.C.; Namork, E.; Borres, M.P.; Håland, G. Feasibility of desensitizing children highly allergic to peanut by high-dose oral immunotherapy. Allergy 2019, 74, 337–348. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef]
- Bonamichi-Santos, R.; Aun, M.V.; Kalil, J.; Castells, M.C.; Giavina-Bianchi, P. PD-L1 Blockade During Allergen Sensitization Inhibits the Synthesis of Specific Antibodies and Decreases Mast Cell Activation in a Murine Model of Active Cutaneous Anaphylaxis. Front. Immunol. 2021, 12, 655958. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016, 28, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor–related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Saito, A.; Nakamura, Y.; Ishitsuka, Y.; Watanabe, R.; Fujisawa, Y.; Mizuno, S.; Takahashi, S.; et al. Differential Involvement of Programmed Cell Death Ligands in Skin Immune Responses. J. Invest. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Immune Checkpoint Inhibitors and Their Side Effects. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html (accessed on 15 July 2021).
- Minkis, K.; Garden, B.C.; Wu, S.; Pulitzer, M.P.; Lacouture, M.E. The risk of rash associated with ipilimumab in patients with cancer: A systematic review of the literature and meta-analysis. J. Am. Acad. Dermatol. 2013, 69, e121–e128. [Google Scholar] [CrossRef]
- Coleman, E.; Ko, C.; Dai, F.; Tomayko, M.M.; Kluger, H.; Leventhal, J.S. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J. Am. Acad. Dermatol. 2019, 80, 990–997. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Schaberg, K.B.; Novoa, R.A.; Wakelee, H.A.; Kim, J.; Cheung, C.; Srinivas, S.; Kwong, B.Y. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J. Cutan. Pathol. 2016, 43, 339–346. [Google Scholar] [CrossRef]
- Naidoo, J.; Schindler, K.; Querfeld, C.; Busam, K.; Cunningham, J.; Page, D.B.; Postow, M.A.; Weinstein, A.; Lucas, A.S.; Ciccolini, K.T.; et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol. Res. 2016, 4, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N.; et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016, 152, 45. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Otsuka, A.; Miyachi, Y.; Kabashima, K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J. Eur. Acad. Dermatol. Venereol. 2016, 30, e89–e91. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Ohtsuka, M.; Kikuchi, N.; Yamamoto, T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm. Venereol. 2016, 96, 259–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivar, K.L.; Deschaine, M.; Messina, J.; Divine, J.M.; Rabionet, A.; Patel, N.; Harrington, M.A.; Seminario-Vidal, L. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J. Cutan. Pathol. 2017, 44, 381–384. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ardusso, L.R.F.; Bilò, M.B.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.M.; Lieberman, P.; Lockey, R.F.; Muraro, A.; Roberts, G.; et al. International consensus on (ICON) anaphylaxis. World Allergy Organ. J. 2014, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Oyoshi, M.K.; Oettgen, H.C.; Chatila, T.A.; Geha, R.S.; Bryce, P.J. Food allergy: Insights into etiology, prevention, and treatment provided by murine models. J. Allergy Clin. Immunol. 2014, 133, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Bartnikas, L.M.; Gurish, M.F.; Burton, O.T.; Leisten, S.; Janssen, E.; Oettgen, H.C.; Beaupré, J.; Lewis, C.N.; Austen, K.F.; Schulte, S.; et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gramberg, J.L.; de Veer, M.J.; O’Hehir, R.E.; Meeusen, E.N.T.; Bischof, R.J. Use of animal models to investigate major allergens associated with food allergy. J. Allergy 2013, 2013, 635695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habicht, A.; Kewalaramani, R.; Vu, M.D.; Demirci, G.; Blazar, B.R.; Sayegh, M.H.; Li, X.C. Striking Dichotomy of PD-L1 and PD-L2 Pathways in Regulating Alloreactive CD4 + and CD8 + T Cells In Vivo. Am. J. Transplant. 2007, 7, 2683–2692. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galván Morales, M.A.; Montero-Vargas, J.M.; Vizuet-de-Rueda, J.C.; Teran, L.M. New Insights into the Role of PD-1 and Its Ligands in Allergic Disease. Int. J. Mol. Sci. 2021, 22, 11898. https://doi.org/10.3390/ijms222111898
Galván Morales MA, Montero-Vargas JM, Vizuet-de-Rueda JC, Teran LM. New Insights into the Role of PD-1 and Its Ligands in Allergic Disease. International Journal of Molecular Sciences. 2021; 22(21):11898. https://doi.org/10.3390/ijms222111898
Chicago/Turabian StyleGalván Morales, Miguel Angel, Josaphat Miguel Montero-Vargas, Juan Carlos Vizuet-de-Rueda, and Luis M Teran. 2021. "New Insights into the Role of PD-1 and Its Ligands in Allergic Disease" International Journal of Molecular Sciences 22, no. 21: 11898. https://doi.org/10.3390/ijms222111898





