Hierarchically Structured Polystyrene-Based Surfaces Amplifying Fluorescence Signals: Cytocompatibility with Human Induced Pluripotent Stem Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Hierarchically Structured Substrates
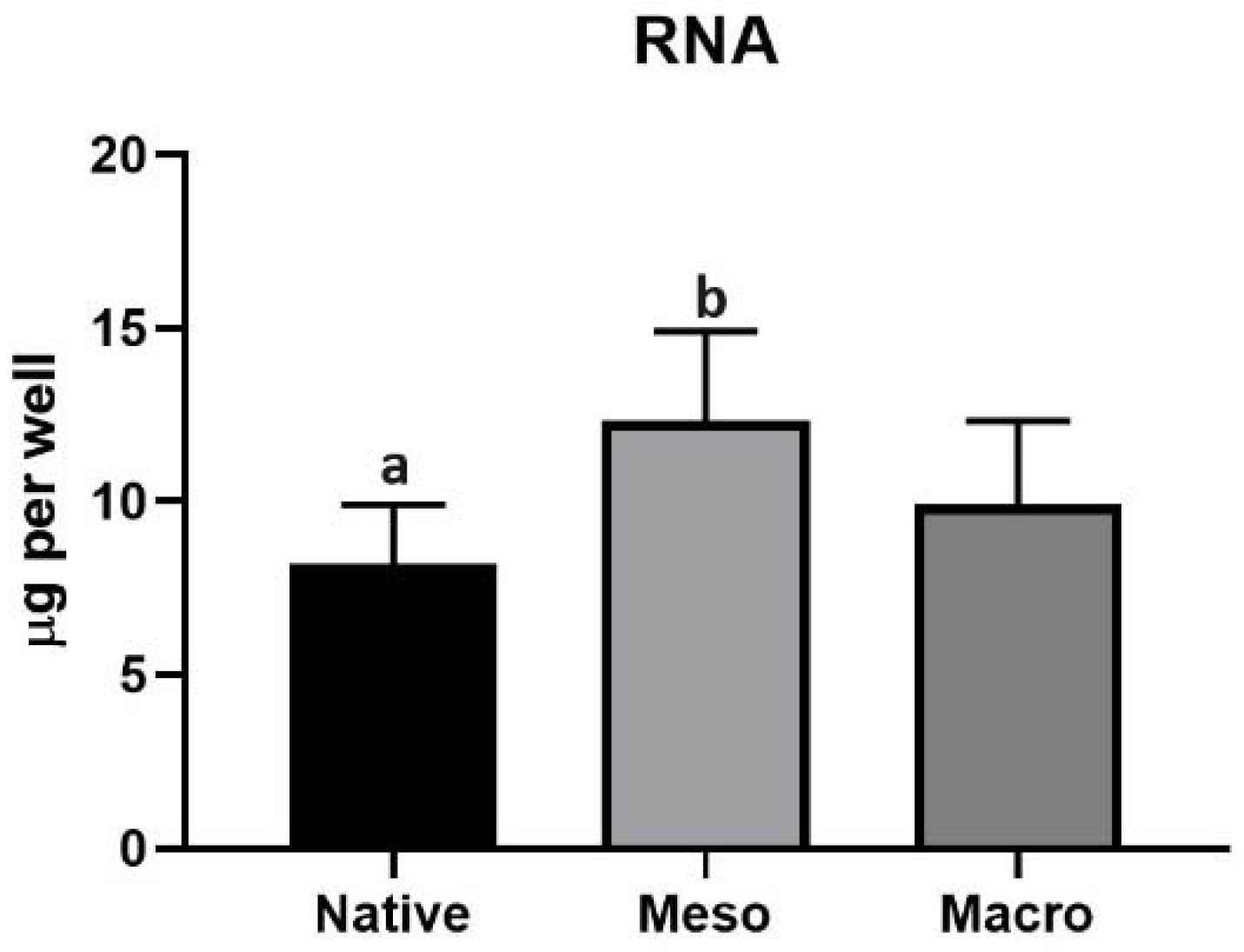
2.2. Cytocompatibility and Cardiomygenesis of hiPSC
2.3. Proliferation of Undifferentiated hiPSC
2.4. Cardiomyocyte Induction and Maturation
2.5. Structured Surface Amplified Fluorescence Signal
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation and Characterization of Hierarchically Structured Substrates
3.3. Cytocompatibility and Cardiomygenesis of hiPSC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| ETH | 2-ethoxyethanol |
| hESC | human embryonic stem cells |
| iPSC | induced pluripotent stem cells |
| iPSC-CM | iPSC-derived cardiomyocytes |
| PDMS | polydimethylsiloxane |
| Ra | roughness |
| SEM | scanning electron microscopy |
| THF | tetrahydrofuran |
References
- Pera, M.F.; Reubinoff, B.; Trounson, A. Human embryonic stem cells. J. Cell Sci. 2000, 113 Pt 1, 5–10. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Wilson, H.K.; Canfield, S.G.; Shusta, E.V.; Palecek, S.P. Concise review: Tissue-specific microvascular endothelial cells derived from human pluripotent stem cells. Stem Cells 2014, 32, 3037–3045. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Kong, J.; Cui, Y.Y.; Liu, P.; Wen, J.Y. Is Human-induced Pluripotent Stem Cell the Best Optimal? Chin. Med. J. 2018, 131, 852–856. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Swingen, C.; Zhang, J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr. Cardiol. Rev. 2013, 9, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwi, L.; Caspi, O.; Arbel, G.; Huber, I.; Gepstein, A.; Park, I.H.; Gepstein, L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 2009, 120, 1513–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csöbönyeiová, M.; Polák, Š.; Danišovič, L. Perspectives of induced pluripotent stem cells for cardiovascular system regeneration. Exp. Biol. Med. 2015, 240, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, T.J.; Martinez-Fernandez, A.; Yamada, S.; Perez-Terzic, C.; Ikeda, Y.; Terzic, A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 2009, 120, 408–416. [Google Scholar] [CrossRef]
- Carson, D.; Hnilova, M.; Yang, X.; Nemeth, C.L.; Tsui, J.H.; Smith, A.S.; Jiao, A.; Regnier, M.; Murry, C.E.; Tamerler, C.; et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 21923–21932. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, L.; Yu, Y.; Yin, F.; Zhang, X.; Jiang, L.; Qin, J. Bioinspired onion epithelium-like structure promotes the maturation of cardiomyocytes derived from human pluripotent stem cells. Biomater. Sci. 2017, 5, 1810–1819. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr. Top. Dev. Biol. 2018, 130, 1–37. [Google Scholar] [CrossRef]
- Lien, C.L.; McAnally, J.; Richardson, J.A.; Olson, E.N. Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev. Biol. 2002, 244, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelb, B.D.; Chin, S.E. Chapter 34-Genetics of Congenital Heart Disease; Muscle Hill, J.A., Olson, E.N., Eds.; Academic Press: Boston/Waltham, MA, USA, 2012; pp. 473–480. [Google Scholar]
- Cullup, T.; Lamont, P.J.; Cirak, S.; Damian, M.S.; Wallefeld, W.; Gooding, R.; Tan, S.V.; Sheehan, J.; Muntoni, F.; Abbs, S.; et al. Mutations in MYH7 cause Multi-minicore Disease (MmD) with variable cardiac involvement. Neuromuscul. Disord. 2012, 22, 1096–1104. [Google Scholar] [CrossRef]
- McNally, E.; Dellefave, L. Sarcomere mutations in cardiogenesis and ventricular noncompaction. Trends Cardiovasc. Med. 2009, 19, 17–21. [Google Scholar] [CrossRef] [PubMed]
- England, J.; Loughna, S. Heavy and light roles: Myosin in the morphogenesis of the heart. Cell Mol. Life Sci. 2013, 70, 1221–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, D.A.; Braam, S.R.; Koutsis, K.; Ng, E.S.; Jenny, R.; Lagerqvist, E.L.; Biben, C.; Hatzistavrou, T.; Hirst, C.E.; Yu, Q.C.; et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat. Methods 2011, 8, 1037–1040. [Google Scholar] [CrossRef]
- Moses, K.A.; DeMayo, F.; Braun, R.M.; Reecy, J.L.; Schwartz, R.J. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis 2001, 31, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Louch, W.E.; Koivumäki, J.T.; Tavi, P. Calcium signalling in developing cardiomyocytes: Implications for model systems and disease. J. Physiol. 2015, 593, 1047–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minařík, M.; Wrzecionko, E.; Minařík, A.; Grulich, O.; Smolka, P.; Musilová, L.; Junkar, I.; Primc, G.; Ptošková, B.; Mozetič, M.; et al. Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination. Coatings 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Wrzecionko, E.; Minařík, A.; Smolka, P.; Minařík, M.; Humpolíček, P.; Rejmontová, P.; Mráček, A.; Minaříková, M.; Gřundělová, L. Variations of Polymer Porous Surface Structures via the Time-Sequenced Dosing of Mixed Solvents. ACS Appl. Mater. Interfaces 2017, 9, 6472–6481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasenská, D.; Kašpárková, V.; Radaszkiewicz, K.A.; Capáková, Z.; Pacherník, J.; Trchová, M.; Minařík, A.; Vajďák, J.; Bárta, T.; Stejskal, J.; et al. Conducting composite films based on chitosan or sodium hyaluronate. Properties and cytocompatibility with human induced pluripotent stem cells. Carbohydr. Polym. 2021, 253, 117244. [Google Scholar] [CrossRef] [PubMed]
- Konopka, R.; Fau, H.M.; Kubala, L.; Fau, K.L.; Pacherník, J. New luminescence-based approach to measurement of luciferase gene expression reporter activity and adenosine triphosphate-based determination of cell viability. Folia Biol. 2010, 56, 66–71. [Google Scholar]
- Radaszkiewicz, K.A.; Sýkorová, D.; Karas, P.; Kudová, J.; Kohút, L.; Binó, L.; Večeřa, J.; Víteček, J.; Kubala, L.; Pacherník, J. Simple non-invasive analysis of embryonic stem cell-derived cardiomyocytes beating in vitro. Rev. Sci. Instrum. 2016, 87, 024301. [Google Scholar] [CrossRef]
- Radaszkiewicz, T.; Nosková, M.; Gömöryová, K.; Vondálová Blanářová, O.; Radaszkiewicz, K.A.; Picková, M.; Víchová, R.; Gybel’, T.; Kaiser, K.; Demková, L.; et al. RNF43 inhibits WNT5A driven signaling and suppresses melanoma invasion. bioRxiv 2021. [Google Scholar] [CrossRef]






| NCBI Reference Sequence Gene | Primer Sequence | Tanealing (°C) | UPL Probe No. |
|---|---|---|---|
| NM_004387.3 NKX2.5 | F cacctcaacagctccctga R ctaggtctccgcaggagtga | 59 60 | #7 |
| NM_021223.2 MYL7 | F gggtggtgaacaaggatgag R gtgtcagggcgaacatctg | 60 60 | #2 |
| NM_000432.3 MYL2 | F gcaggcggagaggttttc R agttgccagtcacgtcagg | 60 60 | #63 |
| NM_000257.3 MYH7 | F catctcccaaggagagacca R ccagcacatcaaaagcgtta | 60 59 | #73 |
| NM_002471.3 MYH6 | F ctcaagctcatggccactct R gcctcctttgcttttaccact | 60 59 | #63 |
| NM_000194.2 HPRT1 | F tgaccttgatttattttgcatacc R cgagcaagacgttcagtcct | 59 60 | #73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopalová, K.; Radaszkiewicz, K.A.; Kadlečková, M.; Pacherník, J.; Minařík, A.; Capáková, Z.; Kašpárková, V.; Mráček, A.; Daďová, E.; Humpolíček, P. Hierarchically Structured Polystyrene-Based Surfaces Amplifying Fluorescence Signals: Cytocompatibility with Human Induced Pluripotent Stem Cell. Int. J. Mol. Sci. 2021, 22, 11943. https://doi.org/10.3390/ijms222111943
Skopalová K, Radaszkiewicz KA, Kadlečková M, Pacherník J, Minařík A, Capáková Z, Kašpárková V, Mráček A, Daďová E, Humpolíček P. Hierarchically Structured Polystyrene-Based Surfaces Amplifying Fluorescence Signals: Cytocompatibility with Human Induced Pluripotent Stem Cell. International Journal of Molecular Sciences. 2021; 22(21):11943. https://doi.org/10.3390/ijms222111943
Chicago/Turabian StyleSkopalová, Kateřina, Katarzyna Anna Radaszkiewicz, Markéta Kadlečková, Jiří Pacherník, Antonín Minařík, Zdenka Capáková, Věra Kašpárková, Aleš Mráček, Eliška Daďová, and Petr Humpolíček. 2021. "Hierarchically Structured Polystyrene-Based Surfaces Amplifying Fluorescence Signals: Cytocompatibility with Human Induced Pluripotent Stem Cell" International Journal of Molecular Sciences 22, no. 21: 11943. https://doi.org/10.3390/ijms222111943
APA StyleSkopalová, K., Radaszkiewicz, K. A., Kadlečková, M., Pacherník, J., Minařík, A., Capáková, Z., Kašpárková, V., Mráček, A., Daďová, E., & Humpolíček, P. (2021). Hierarchically Structured Polystyrene-Based Surfaces Amplifying Fluorescence Signals: Cytocompatibility with Human Induced Pluripotent Stem Cell. International Journal of Molecular Sciences, 22(21), 11943. https://doi.org/10.3390/ijms222111943








