Sprouty3, but Not Sprouty1, Expression Is Beneficial for the Malignant Potential of Osteosarcoma Cells
Abstract
:1. Introduction
2. Results
2.1. Osteosarcoma-Derived Cell Lines Express Substantial Levels of Spry3 Protein
2.2. Irrespective of Mitogen Availability, Spry3 and Spry1 Proteins Are Usually Expressed in Osteosarcoma
2.3. In Osteosarcoma-Derived Cells, Spry3, but Not Spry1, Accelerates Cell Proliferation
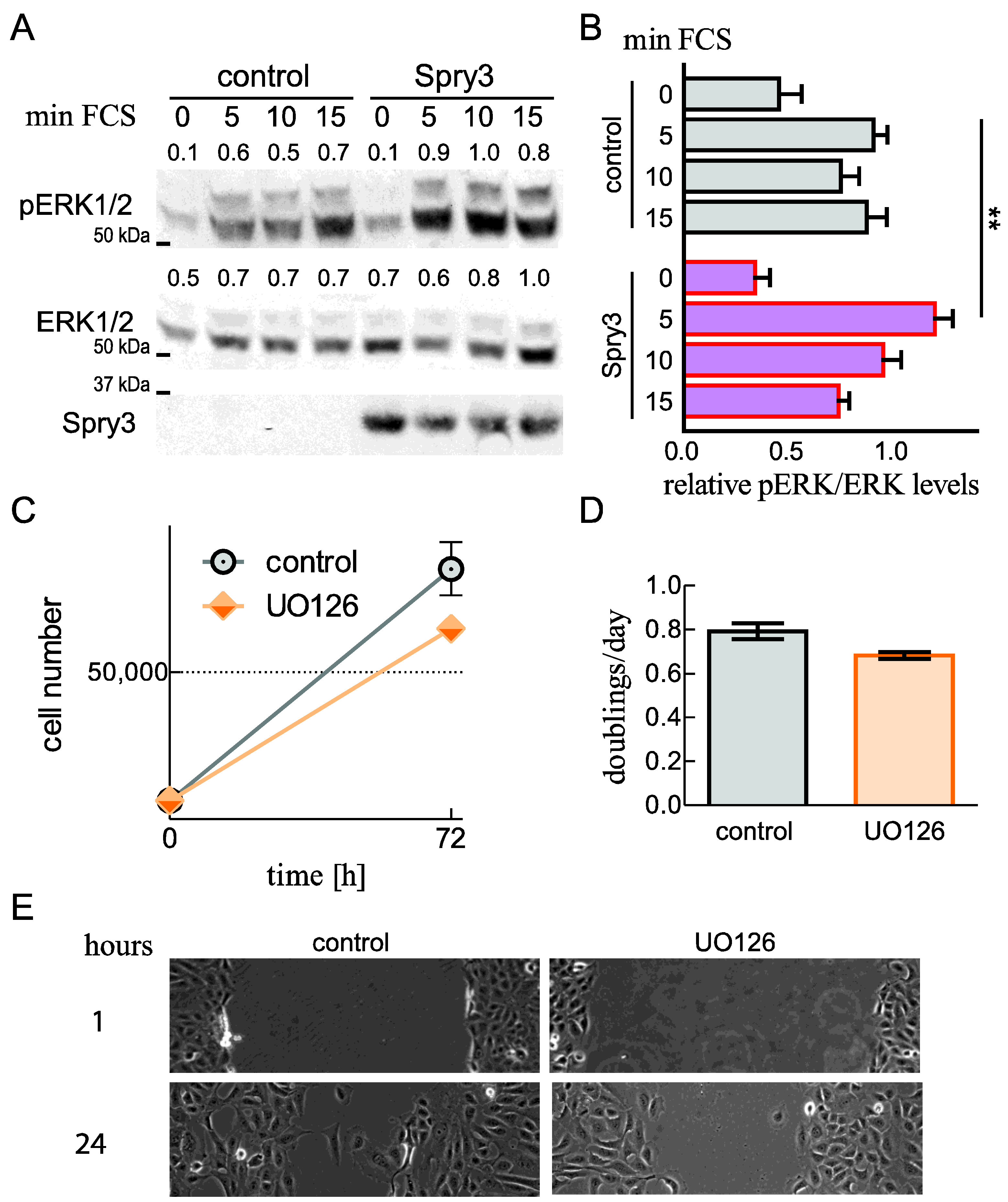
2.4. A Tumor Promoting Role of Spry3 Is Additionally Suggested by Its Beneficial Role on Cell Migration of Osteosarcoma-Derived Cells
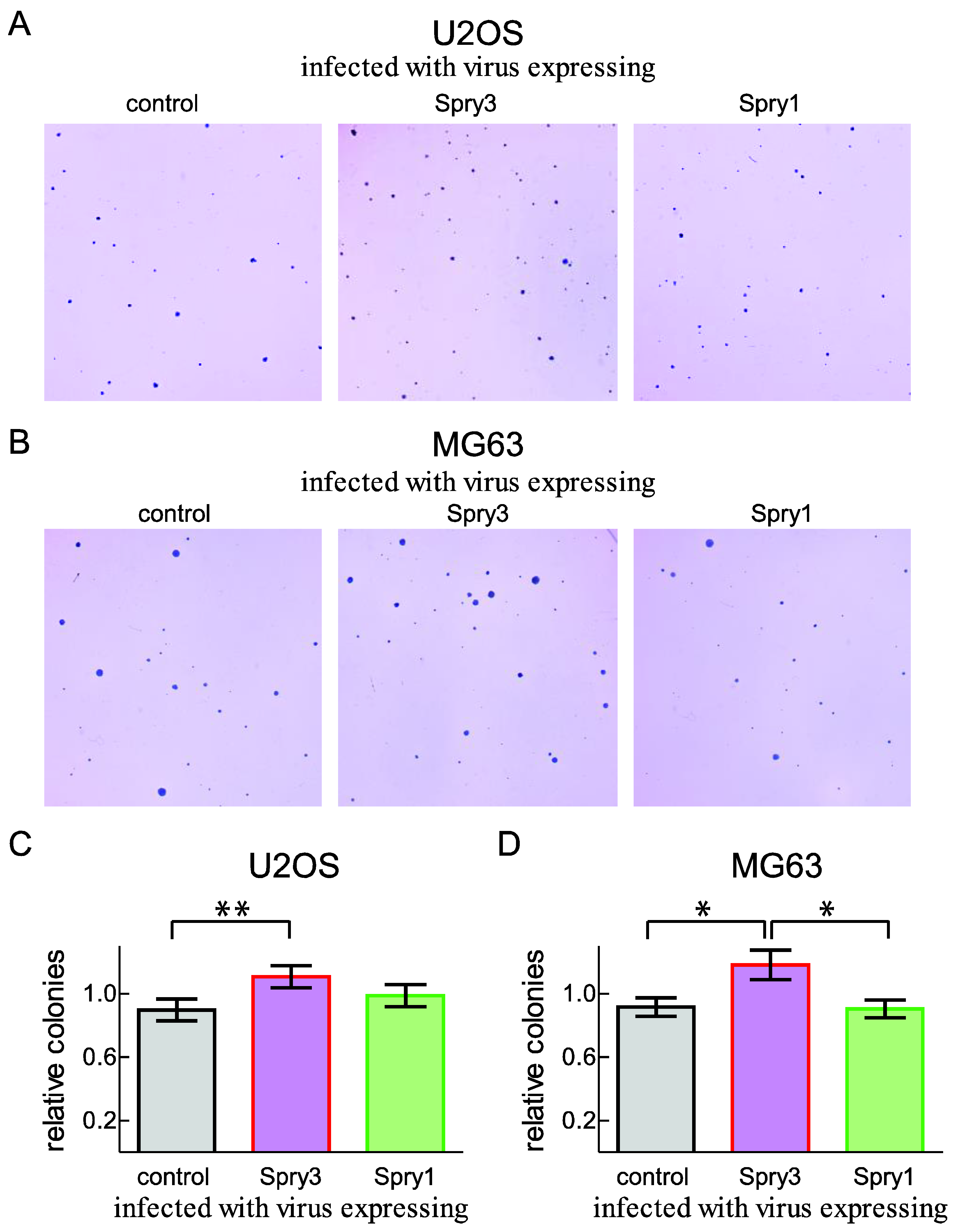
2.5. Anchorage-Independent Growth of the Osteosarcoma-Derived Cell Line U2OS Is Facilitated by Increased Spry3 Levels
2.6. Spry3 Levels Are Beneficial for ERK Phosphorylation
2.7. Mutation of the Tyrosine in the N-Terminal Homologous Box of Spry3 Creates a Dominant-Negative Protein with an Inhibitory Role on Cell Migration
2.8. Spry3 Expression Is Elevated in Tumor-Derived Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Adenoviral Infection of Cells
4.3. Immunoblotting
4.4. Growth Curve
4.5. Scratch Assay
4.6. Soft Agar Colony Formation Assay
4.7. Cell Signaling Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [Green Version]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Bishop, M.W.; Janeway, K.A.; Gorlick, R. Future directions in the treatment of osteosarcoma. Curr. Opin. Pediatr. 2016, 28, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Morrow, J.J.; Khanna, C. Osteosarcoma genetics and epigenetics: Emerging biology and candidate therapies. Crit. Rev. Oncog. 2015, 20, 173–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, S.R.; Miller, W.T. Receptor tyrosine kinases: Mechanisms of activation and signaling. Curr. Opin. Cell Biol. 2007, 19, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M. Feedback control of intercellular signalling in development. Nature 2000, 408, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Minowada, G.; Jarvis, L.A.; Chi, C.L.; Neubuser, A.; Sun, X.; Hacohen, N.; Krasnow, M.A.; Martin, G.R. Vertebrate sprouty genes are induced by fgf signaling and can cause chondrodysplasia when overexpressed. Development 1999, 126, 4465. [Google Scholar] [CrossRef]
- Taniguchi, K.; Ayada, T.; Ichiyama, K.; Kohno, R.; Yonemitsu, Y.; Minami, Y.; Kikuchi, A.; Maehara, Y.; Yoshimura, A. Sprouty2 and sprouty4 are essential for embryonic morphogenesis and regulation of fgf signaling. Biochem. Biophys. Res. Commun. 2007, 352, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.A.; Akbulut, S.; Watson-Johnson, J.; Simon, R.; Carroll, T.J.; Shakya, R.; Gross, I.; Martin, G.R.; Lufkin, T.; McMahon, A.P.; et al. Sprouty1 is a critical regulator of gdnf/ret-mediated kidney induction. Dev. Cell 2005, 8, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basson, M.A.; Watson-Johnson, J.; Shakya, R.; Akbulut, S.; Hyink, D.; Costantini, F.D.; Wilson, P.D.; Mason, I.J.; Licht, J.D. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of gdnf and sprouty1. Dev. Biol. 2006, 299, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Shim, K.; Minowada, G.; Coling, D.E.; Martin, G.R. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing fgf signaling. Dev. Cell 2005, 8, 553–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taketomi, T.; Yoshiga, D.; Taniguchi, K.; Kobayashi, T.; Nonami, A.; Kato, R.; Sasaki, M.; Sasaki, A.; Ishibashi, H.; Moriyama, M.; et al. Loss of mammalian sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat. Neurosci. 2005, 8, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Gross, I.; Morrison, D.J.; Hyink, D.P.; Georgas, K.; English, M.A.; Mericskay, M.; Hosono, S.; Sassoon, D.; Wilson, P.D.; Little, M.; et al. The receptor tyrosine kinase regulator sprouty1 is a target of the tumor suppressor wt1 and important for kidney development. J. Biol. Chem. 2003, 278, 41420–41430. [Google Scholar] [CrossRef] [Green Version]
- Hanafusa, H.; Torii, S.; Yasunaga, T.; Nishida, E. Sprouty1 and sprouty2 provide a control mechanism for the ras/mapk signalling pathway. Nat. Cell Biol. 2002, 4, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Taketomi, T.; Kato, R.; Saeki, K.; Nonami, A.; Sasaki, M.; Kuriyama, M.; Saito, N.; Shibuya, M.; Yoshimura, A. Mammalian sprouty4 suppresses ras-independent erk activation by binding to raf1. Nat. Cell Biol. 2003, 5, 427–432. [Google Scholar] [CrossRef]
- Edwin, F.; Singh, R.; Endersby, R.; Baker, S.J.; Patel, T.B. The tumor suppressor pten is necessary for human sprouty 2-mediated inhibition of cell proliferation. J. Biol. Chem. 2006, 281, 4816–4822. [Google Scholar] [CrossRef]
- Akbulut, S.; Reddi, A.L.; Aggarwal, P.; Ambardekar, C.; Canciani, B.; Kim, M.K.; Hix, L.; Vilimas, T.; Mason, J.; Basson, M.A.; et al. Sprouty proteins inhibit receptor-mediated activation of phosphatidylinositol-specific phospholipase c. Mol. Biol. Cell 2010, 21, 3487–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Bar-Sagi, D. Modulation of signalling by sprouty: A developing story. Nat. Rev. Mol. Cell Biol. 2004, 5, 441–450. [Google Scholar] [CrossRef]
- Wong, E.S.; Lim, J.; Low, B.C.; Chen, Q.; Guy, G.R. Evidence for direct interaction between sprouty and cbl. J. Biol. Chem. 2001, 276, 5866–5875. [Google Scholar] [CrossRef] [Green Version]
- Masoumi-Moghaddam, S.; Amini, A.; Morris, D.L. The developing story of sprouty and cancer. Cancer Metastasis Rev. 2014, 33, 695–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, A.T.; Meissner, A.; Dowdle, J.A.; Crowley, D.; Magendantz, M.; Ouyang, C.; Parisi, T.; Rajagopal, J.; Blank, L.J.; Bronson, R.T.; et al. Sprouty-2 regulates oncogenic k-ras in lung development and tumorigenesis. Genes Dev. 2007, 21, 694–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutterluty, H.; Mayer, C.E.; Setinek, U.; Attems, J.; Ovtcharov, S.; Mikula, M.; Mikulits, W.; Micksche, M.; Berger, W. Down-regulation of sprouty2 in non-small cell lung cancer contributes to tumor malignancy via extracellular signal-regulated kinase pathway-dependent and -independent mechanisms. Mol. Cancer Res. 2007, 5, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, T.L.; Yusoff, P.; Fong, C.W.; Guo, K.; McCaw, B.J.; Phillips, W.A.; Yang, H.; Wong, E.S.; Leong, H.F.; Zeng, Q.; et al. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Res. 2004, 64, 6127–6136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, C.W.; Chua, M.S.; McKie, A.B.; Ling, S.H.; Mason, V.; Li, R.; Yusoff, P.; Lo, T.L.; Leung, H.Y.; So, S.K.; et al. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res. 2006, 66, 2048–2058. [Google Scholar] [CrossRef] [Green Version]
- Holgren, C.; Dougherty, U.; Edwin, F.; Cerasi, D.; Taylor, I.; Fichera, A.; Joseph, L.; Bissonnette, M.; Khare, S. Sprouty-2 controls c-met expression and metastatic potential of colon cancer cells: Sprouty/c-met upregulation in human colonic adenocarcinomas. Oncogene 2010, 29, 5241–5253. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.M.; Kapoor, G.S.; Buonato, J.M.; Mathew, L.K.; Bi, Y.; Davuluri, R.V.; Martinez-Lage, M.; Simon, M.C.; O’Rourke, D.M.; Lazzara, M.J. Sprouty2 drives drug resistance and proliferation in glioblastoma. Mol. Cancer Res. 2015, 13, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Wollmann, G.; Urbiola, C.; Fogli, B.; Florio, T.; Geley, S.; Klimaschewski, L. Sprouty2 enhances the tumorigenic potential of glioblastoma cells. Neuro Oncol. 2018, 20, 1044–1054. [Google Scholar] [CrossRef] [Green Version]
- Tennis, M.A.; Van Scoyk, M.M.; Freeman, S.V.; Vandervest, K.M.; Nemenoff, R.A.; Winn, R.A. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by wnt7a through ppargamma in non-small cell lung cancer. Mol. Cancer Res. 2010, 8, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanas, V.; Muhlbacher, E.; Kral, R.; Sutterluty-Fall, H. Sprouty4 interferes with cell proliferation and migration of breast cancer-derived cell lines. Tumour Biol. 2014, 35, 4447–4456. [Google Scholar] [CrossRef]
- Celik-Selvi, B.E.; Stutz, A.; Mayer, C.E.; Salhi, J.; Siegwart, G.; Sutterluty, H. Sprouty3 and sprouty4, two members of a family known to inhibit fgf-mediated signaling, exert opposing roles on proliferation and migration of glioblastoma-derived cells. Cells 2019, 8, 808. [Google Scholar] [CrossRef] [Green Version]
- Schaaf, G.; Hamdi, M.; Zwijnenburg, D.; Lakeman, A.; Geerts, D.; Versteeg, R.; Kool, M. Silencing of spry1 triggers complete regression of rhabdomyosarcoma tumors carrying a mutated ras gene. Cancer Res. 2010, 70, 762–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwabi-Addo, B.; Wang, J.; Erdem, H.; Vaid, A.; Castro, P.; Ayala, G.; Ittmann, M. The expression of sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res. 2004, 64, 4728–4735. [Google Scholar] [CrossRef] [Green Version]
- Macia, A.; Gallel, P.; Vaquero, M.; Gou-Fabregas, M.; Santacana, M.; Maliszewska, A.; Robledo, M.; Gardiner, J.R.; Basson, M.A.; Matias-Guiu, X.; et al. Sprouty1 is a candidate tumor-suppressor gene in medullary thyroid carcinoma. Oncogene 2012, 31, 3961–3972. [Google Scholar] [CrossRef] [Green Version]
- Rathmanner, N.; Haigl, B.; Vanas, V.; Doriguzzi, A.; Gsur, A.; Sutterluty-Fall, H. Sprouty2 but not sprouty4 is a potent inhibitor of cell proliferation and migration of osteosarcoma cells. FEBS Lett. 2013, 587, 2597–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, S.; Chang, J.T.; Andrechek, E.R.; Matsumura, N.; Baba, T.; Yao, G.; Kim, J.W.; Gatza, M.; Murphy, S.; Nevins, J.R. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene 2009, 28, 2796–2805. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.M.; Morrison, D.J.; Bassit, B.; Dimri, M.; Band, H.; Licht, J.D.; Gross, I. Tyrosine phosphorylation of sprouty proteins regulates their ability to inhibit growth factor signaling: A dual feedback loop. Mol. Biol. Cell 2004, 15, 2176–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; Taketomi, T.; Wakioka, T.; Kato, R.; Yoshimura, A. Identification of a dominant negative mutant of sprouty that potentiates fibroblast growth factor- but not epidermal growth factor-induced erk activation. J. Biol. Chem. 2001, 276, 36804–36808. [Google Scholar] [CrossRef] [Green Version]
- Ning, Z.; McLellan, A.S.; Ball, M.; Wynne, F.; O’Neill, C.; Mills, W.; Quinn, J.P.; Kleinjan, D.A.; Anney, R.J.; Carmody, R.J.; et al. Regulation of spry3 by x chromosome and par2-linked promoters in an autism susceptibility region. Hum. Mol. Genet. 2015, 24, 7450. [Google Scholar] [CrossRef] [Green Version]
- Cidre-Aranaz, F.; Grunewald, T.G.; Surdez, D.; Garcia-Garcia, L.; Carlos Lazaro, J.; Kirchner, T.; Gonzalez-Gonzalez, L.; Sastre, A.; Garcia-Miguel, P.; Lopez-Perez, S.E.; et al. Ews-fli1-mediated suppression of the ras-antagonist sprouty 1 (spry1) confers aggressiveness to ewing sarcoma. Oncogene 2017, 36, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.E.; Haigl, B.; Jantscher, F.; Siegwart, G.; Grusch, M.; Berger, W.; Sutterluty, H. Bimodal expression of sprouty2 during the cell cycle is mediated by phase-specific ras/mapk and c-cbl activities. Cell. Mol. Life Sci. 2010, 67, 3299–3311. [Google Scholar] [CrossRef]
- Jiang, Z.; Price, C.A. Differential actions of fibroblast growth factors on intracellular pathways and target gene expression in bovine ovarian granulosa cells. Reproduction 2012, 144, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, K.; Kadomoto, R.; Asato, K.; Tanimura, S.; Itoh, N.; Kohno, M. Erk pathway positively regulates the expression of sprouty genes. Biochem. Biophys. Res. Commun. 2001, 285, 1084–1088. [Google Scholar] [CrossRef]
- Kral, R.; Doriguzzi, A.; Mayer, C.E.; Krenbek, D.; Setinek, U.; Sutterluty-Fall, H. Differential effects of variations at codon 106 on sprouty2 functions in lung cancer-derived cells. J. Cell Biochem. 2016, 117, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A comprehensive survey of ras mutations in cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekkawy, A.H.; Pourgholami, M.H.; Morris, D.L. Human sprouty1 suppresses growth, migration, and invasion in human breast cancer cells. Tumour Biol. 2014, 35, 5037–5048. [Google Scholar] [CrossRef]
- Barbachano, A.; Ordonez-Moran, P.; Garcia, J.M.; Sanchez, A.; Pereira, F.; Larriba, M.J.; Martinez, N.; Hernandez, J.; Landolfi, S.; Bonilla, F.; et al. Sprouty-2 and e-cadherin regulate reciprocally and dictate colon cancer cell tumourigenicity. Oncogene 2010, 29, 4800–4813. [Google Scholar] [CrossRef] [Green Version]
- Wong, E.S.; Fong, C.W.; Lim, J.; Yusoff, P.; Low, B.C.; Langdon, W.Y.; Guy, G.R. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances ras/erk signalling. EMBO J. 2002, 21, 4796–4808. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, K.; Miyazaki, S.; Tanimura, S.; Kohno, M. Efficient suppression of fgf-2-induced erk activation by the cooperative interaction among mammalian sprouty isoforms. J. Cell Sci. 2005, 118, 5861–5871. [Google Scholar] [CrossRef] [Green Version]
- Vaquero, M.; Cuesta, S.; Anerillas, C.; Altes, G.; Ribera, J.; Basson, M.A.; Licht, J.D.; Egea, J.; Encinas, M. Sprouty1 controls genitourinary development via its n-terminal tyrosine. J. Am. Soc. Nephrol. 2019, 30, 1398–1411. [Google Scholar] [CrossRef]
- Sanui, T.; Tanaka, U.; Fukuda, T.; Toyoda, K.; Taketomi, T.; Atomura, R.; Yamamichi, K.; Nishimura, F. Mutation of spry2 induces proliferation and differentiation of osteoblasts but inhibits proliferation of gingival epithelial cells. J. Cell Biochem. 2015, 116, 628–639. [Google Scholar] [CrossRef]
- Taketomi, T.; Onimura, T.; Yoshiga, D.; Muratsu, D.; Sanui, T.; Fukuda, T.; Kusukawa, J.; Nakamura, S. Sprouty2 is involved in the control of osteoblast proliferation and differentiation through the fgf and bmp signaling pathways. Cell Biol. Int. 2018, 42, 1106–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Webster, J.B.; Kovalenko, D.; Nadeau, R.J.; Zubanova, O.; Chen, P.Y.; Friesel, R. Sprouty genes are expressed in osteoblasts and inhibit fibroblast growth factor-mediated osteoblast responses. Calcif. Tissue Int. 2006, 78, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Vanas, V.; Haigl, B.; Stockhammer, V.; Sutterluty-Fall, H. Microrna-21 increases proliferation and cisplatin sensitivity of osteosarcoma-derived cells. PLoS ONE 2016, 11, e0161023. [Google Scholar] [CrossRef] [Green Version]
- Sutterluty, H.; Chatelain, E.; Marti, A.; Wirbelauer, C.; Senften, M.; Muller, U.; Krek, W. P45skp2 promotes p27kip1 degradation and induces s phase in quiescent cells. Nat. Cell Biol. 1999, 1, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kral, R.M.; Mayer, C.E.; Vanas, V.; Gsur, A.; Sutterluty-Fall, H. In non-small cell lung cancer mitogenic signaling leaves sprouty1 protein levels unaffected. Cell Biochem. Funct. 2014, 32, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Stutz, A.; Kamptner, A.Z.M.; Sutterluty, H. A sprouty4 mutation identified in kallmann syndrome increases the inhibitory potency of the protein towards fgf and connected processes. Int. J. Mol. Sci. 2021, 22, 2145. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamptner, A.Z.M.; Mayer, C.-E.; Sutterlüty, H. Sprouty3, but Not Sprouty1, Expression Is Beneficial for the Malignant Potential of Osteosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 11944. https://doi.org/10.3390/ijms222111944
Kamptner AZM, Mayer C-E, Sutterlüty H. Sprouty3, but Not Sprouty1, Expression Is Beneficial for the Malignant Potential of Osteosarcoma Cells. International Journal of Molecular Sciences. 2021; 22(21):11944. https://doi.org/10.3390/ijms222111944
Chicago/Turabian StyleKamptner, Anna Zita Mehira, Christoph-Erik Mayer, and Hedwig Sutterlüty. 2021. "Sprouty3, but Not Sprouty1, Expression Is Beneficial for the Malignant Potential of Osteosarcoma Cells" International Journal of Molecular Sciences 22, no. 21: 11944. https://doi.org/10.3390/ijms222111944





