Cross-Tolerance and Autoimmunity as Missing Links in Abiotic and Biotic Stress Responses in Plants: A Perspective toward Secondary Metabolic Engineering
Abstract
:1. Introduction
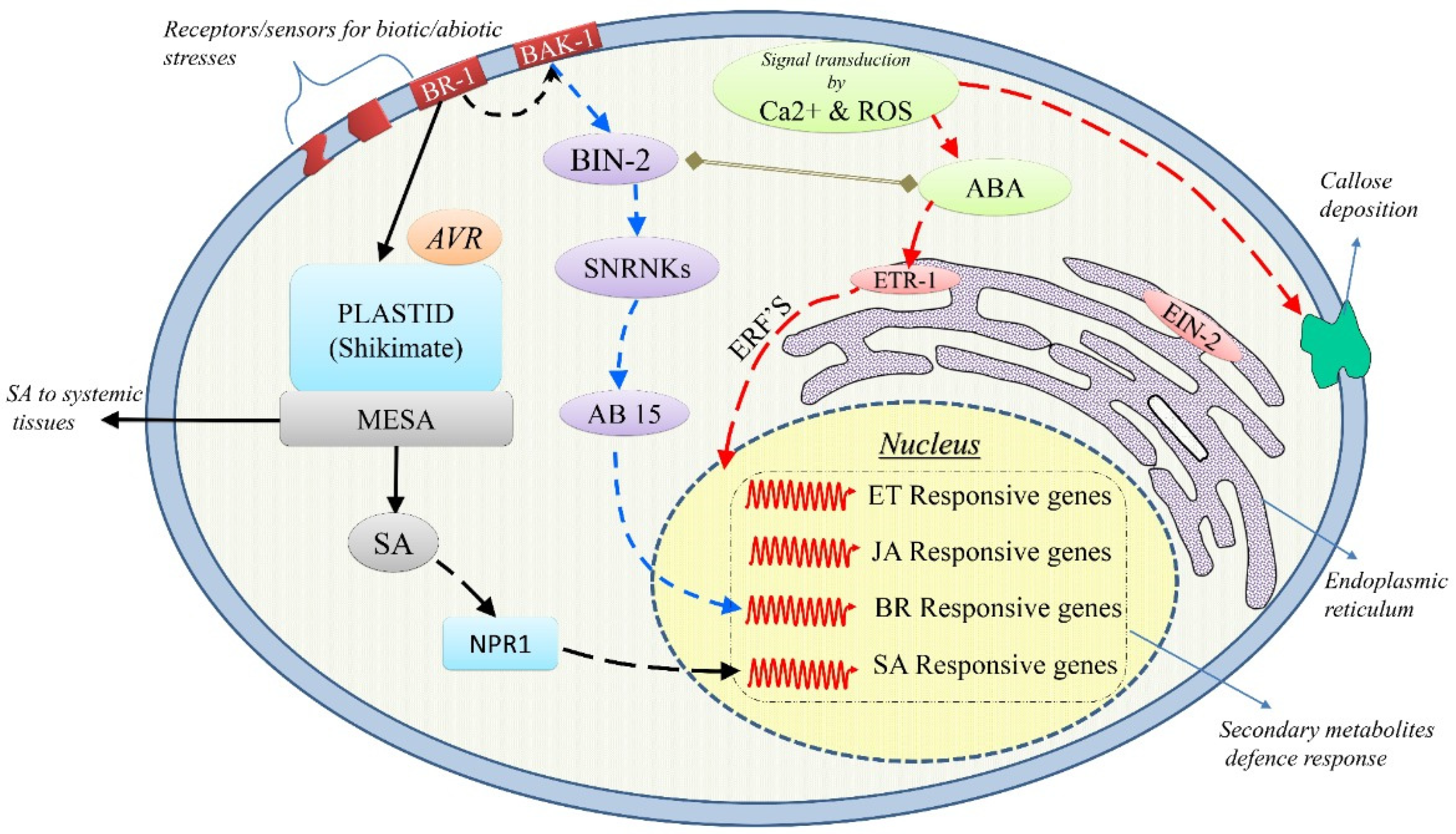
2. Common Abiotic and Biotic Stress Tolerance Mechanisms
2.1. Abiotic Stress Tolerance
2.2. Biotic Stress Tolerance
3. The Concepts of Cross-Talk and Cross-Tolerance
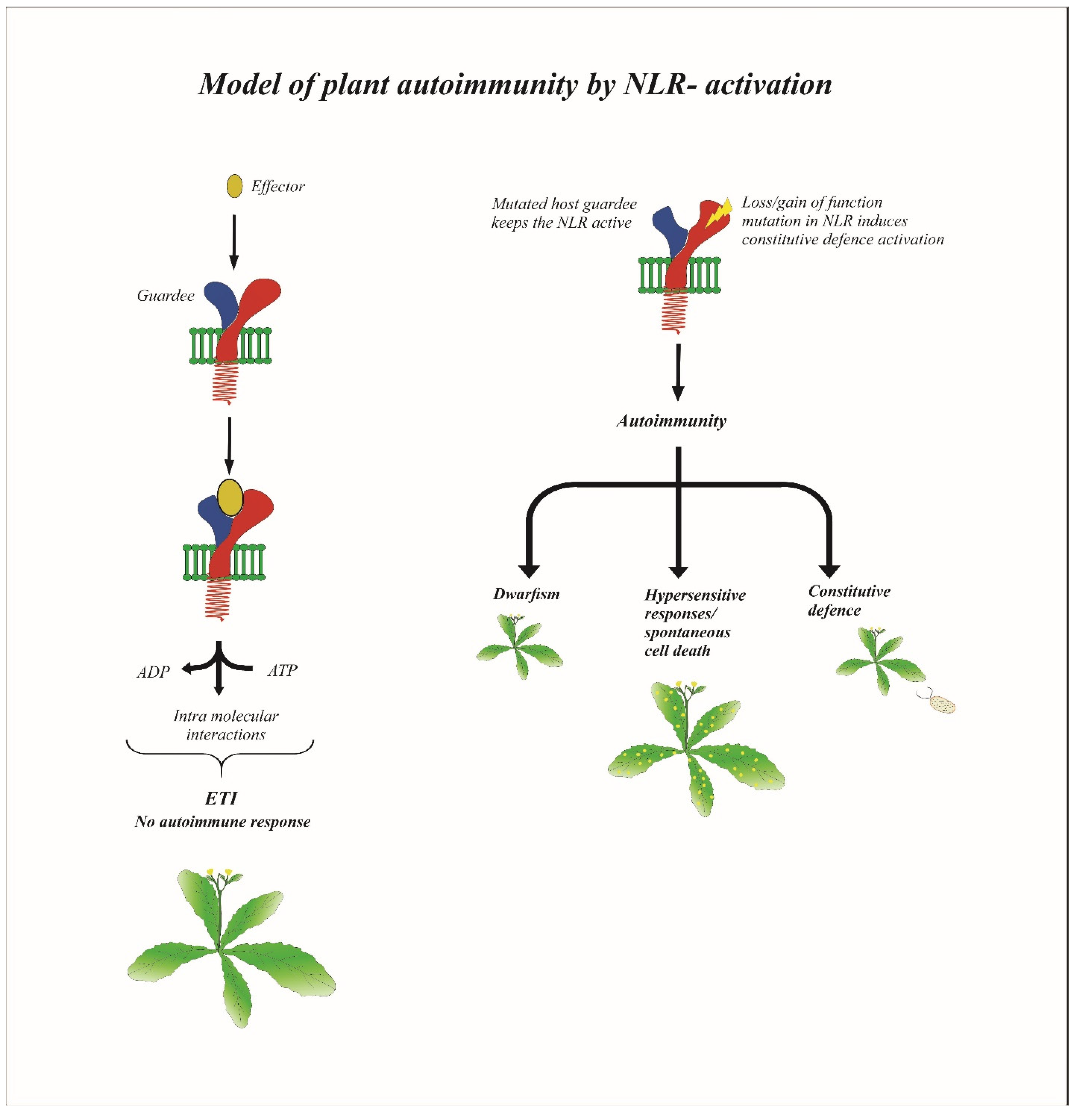
4. Expediency in Plant Autoimmunity: The Propitiously Misdirected Process
5. Targeting Secondary Metabolite Biosynthetic Pathways for Multiple-Stress Resilience and Induced Autoimmunity
6. Managing the Reactive Oxygen Species
| Mutant Type | Encoding Protein | Activity | References |
|---|---|---|---|
| Constitutive expression of potato aspartate proteases in Arabidopsis | Aspartic proteases | Induces expression of genes under JA and SA signaling pathways such as PDF1.2 and PR1. | [71] |
| Transgenic lines expressing pokeweed antiviral protein | Antiviral protein | Constitutive expression of isoforms of PR-I and II genes | [99] |
| At edr1 mutant | Putative MAP kinase | Priming of PR1 and BGL2 genes | [100] |
| Constitutive expression of VSP1 | Vegetative storage protein | Increases anthocyanin accumulation, constitutive expression of defense genes such as VSP, VSP2, Thi2.1, PDF1.2, CHI.B | [101] |
| Overexpression of At JMT | S-Adenosyl L- Methionine Jasmonic acid carboxyl methyl transferase | Constitutive expression of JA-responsive genes such as VSP and PDF1.2 | [102] |
| Constitutive expression of At ERF-1 | Transcription factor | Activates CHI and PDF1.2 | [103] |
| Knockout of At ACD11 gene | Sphingosine transfer protein | PCD and defense-related proteins | [104,105] |
| Overexpression of At WRKY70 | Transcription factor | Constitutive expression of SA-responsive genes | [106] |
| Suppression of At WRKY70 | Transcription factor | Constitutive expression of JA-responsive PR genes | [106] |
| Ectopic expression of OsWRKY11 | Transcription factor | Constitutive expression of pathogen /drought defense responsive genes | [107] |
| Constitutive expression of AtPROPEP1 gene | Elicitor of defense-related genes | Constitutive transcription of PDF1.2 and defense-related proteins | [108] |
| Transgenic combination of wheat Lr34res and Lr34sus alleles | ABCG type transporter | Constitutive expression of defense- and secondary metabolite-related genes | [109] |
| EDS1/PAD4 over-expression | Lipase-like protein | R genes activation | [110] |
| Constitutive expression of Senescence associated gene SAG101 in Arabidopsis | Lipase-like defense regulator | Basal defense activation, R-mediated resistance | [111] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Glossary
| Mutation | A permanent alteration of the nucleotides in the genome sequence of an organism resulting a change in the gene product or affecting the function of gene partially or completely. |
| Apoptosis | Programmed cell death due to biochemical and structural changes in the cell. |
| Autoimmunity | Reduction in the activity of negative regulators or constitutive expression of defense genes that manifest retardation in growth and spontaneous lesion formation. |
| Metabolism | All chemical processes occurring in the plant cell for maintaining the cellular activities and for survival. |
| PR genes | Pathogenesis related genes are involved in the production of proteins that defend the plant in the event of a pathogen infection. |
| Phytoalexin | Antimicrobial compounds produced by plant tissues in response to contact with a pathogen and specifically inhibits its growth. |
| Oxidative stress | Stress generated by the reactive oxygen species when the plant system fails to detoxify them with the antioxidant enzymes. |
| Lignification | Process of strengthening the cell wall in plants by the synthesis and deposition of lignin. |
| TF | Transcription factors are proteins that controls the rate of transcription of DNA to RNA. |
| SA | Salicylic acid is a phenolic phytohormone that have roles in plant growth, photosynthesis and also mediate the defense pathways during pathogen attack. |
| JA | Jasmonic acid is a lipid based plant hormone that functions in photosynthesis, reproduction and biotic stress. |
| ET | Ethylene is a gaseous plant hormone involved in fruit ripening, flower opening and also in leaf abscission. |
| ABA | Abscisic acid is a plant hormone that have functions in stomatal closure, seed and bud dormancy and also in defense against environmental stresses. |
| Phenylpropanoids | Diverse family of secondary metabolites derived from amino acids phenylalanine and tyrosine. |
References
- Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Transgenic Approaches for Abiotic Stress Tolerance in Plants: Retrospect and Prospects. Plant. Cell Rep. 2008, 27, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P.; Farmer, E.E. Jasmonate and Salicylate as Global Signals for Defense Gene Expression. Curr. Opin. Plant Biol. 1998, 1, 404–411. [Google Scholar] [CrossRef]
- Shinozaki, K. Molecular Responses to Dehydration and Low Temperature: Differences and Cross-Talk between Two Stress Signaling Pathways. Curr. Opin. Plant Biol. 2000, 3, 217–223. [Google Scholar] [CrossRef]
- Knight, H.; Knight, M.R. Abiotic Stress Signalling Pathways: Specificity and Cross-Talk. Trends Plant Sci. 2001, 6, 262–267. [Google Scholar] [CrossRef]
- Pastori, G.M.; Foyer, C.H. Common Components, Networks, and Pathways of Cross-Tolerance to Stress. The Central Role of “Redox” and Abscisic Acid-Mediated Controls. Plant Physiol. 2002, 129, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Ghosh, P.; Das, S. Autoimmunity in Plants. Planta 2018, 248, 751–767. [Google Scholar] [CrossRef]
- Erwin, H.B.; Fettig, S.; Knake, C.; Hartig, K.; Bhattarai, T. Specific and Unspecific Responses of Plants to Cold and Drought Stress. J. Biol. Sci. 2007, 32, 501–510. [Google Scholar]
- Rodriguez, E.; El Ghoul, H.; Mundy, J.; Petersen, M. Making Sense of Plant Autoimmunity and “Negative Regulators”. FEBS J. 2016, 283, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Clarke, J.D.; Zhang, Y.; Dong, X. Activation of an EDS1-Mediated R-Gene Pathway in the Snc1 Mutant Leads to Constitutive, NPR1-Independent Pathogen Resistance. Mol. Plant Microbe Interact. 2001, 14, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Habring, A.; Wang, K.; Weigel, D. Atypical Resistance Protein RPW8/HR Triggers Oligomerization of the NLR Immune Receptor RPP7 and Autoimmunity. Cell Host Microbe 2020, 27, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Century, K.; Reuber, T.L.; Ratcliffe, O.J. Regulating the Regulators: The Future Prospects for Transcription-Factor-Based Agricultural Biotechnology Products. Plant Physiol. 2008, 147, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello, J.V.; Lodeyro, A.F.; Zurbriggen, M.D. Novel Perspectives for the Engineering of Abiotic Stress Tolerance in Plants. Curr. Opin. Biotechnol. 2014, 26, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Sopory, S.K.; Kavi Kishor, P.B. Deciphering the Regulatory Mechanisms of Abiotic Stress Tolerance in Plants by Genomic Approaches. Gene 2007, 388, 1–13. [Google Scholar] [CrossRef]
- Seki, M.; Narusaka, M.; Abe, H.; Kasuga, M.; Yamaguchi-Shinozaki, K.; Carninci, P.; Hayashizaki, Y.; Shinozaki, K. Monitoring the Expression Profiles of 7000 Arabidopsis Genes under Drought, Cold and High-Salinity Stresses Using a Full-Length CDNA Microarray. Plant Cell 2002, 31, 279–292. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Varshney, R.K.; Kavi Kishor, P.B.; Weschke, W. Functional Genomics for Tolerance to Abiotic Stress in Cereals; Kluwer Academy Publishers: Dordrecht, The Netherlands, 2005. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, A.; Chellappan, K.P. Accumulation of Proline and Glycine Betaine in Ipomoea Pes-Capre Induced by NaCl. Biol. Plant 1998, 41, 271–276. [Google Scholar] [CrossRef]
- Kishor, P.; Kishor, P.; Sangam, S.; Sangam, S.; Amrutha, R.; Amrutha, R.; Laxmi, P.; Laxmi, P. Uptake and Transport in Higher Plants: Its Implications in Plant Growth and Abiotic Stress. Curr. Sci. 2005, 34, 5–18. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Singh Gill, S.; Khan, N.A.; Naser, B.; Anjum, A.; Tuteja, N. Amelioration of Cadmium Stress in Crop Plants by Nutrient Management: Morphological, Physiological and Biochemical Aspects. Plant Stress 2011, 5, 1–23. [Google Scholar]
- Ozturk, Z.N.; Deyholos, M.; Michalowski, C.B.; Galbraith, D.W.; Gozukirmizi, N.; Tuberosa, R.; Bohnert, H.J. Monitoring Large-Scale Changes in Transcript Abundance in Drought- and Salt-Stressed Barley. Plant Mol. Biol. 2002, 48, 551–573. [Google Scholar] [CrossRef]
- Talamè, V.; Ozturk, N.Z.; Bohnert, H.J.; Tuberosa, R. Barley Transcript Profiles under Dehydration Shock and Drought Stress Treatments: A Comparative Analysis. J. Exp. Bot. 2007, 58, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Takahashi, T.; Michael, A.J.; Kusano, T. A Protective Role for the Polyamine Spermine against Drought Stress in Arabidopsis. Biochem. Biophys. Res. Commun. 2007, 352, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Li, J.; Guo, S.; Kang, Y. Exogenous Spermidine Affects Polyamine Metabolism in Salinity-Stressed Cucumis Sativus Roots and Enhances Short-Term Salinity Tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]
- Alcázar, R.; Cuevas, J.C.; Planas, J.; Zarza, X.; Bortolotti, C.; Carrasco, P.; Salinas, J.; Tiburcio, A.F.; Altabella, T. Integration of Polyamines in the Cold Acclimation Response. Plant Sci. 2011, 180, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genomics 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Legocka, J.; Kluk, A. Effect of Salt and Osmotic Stress on Changes in Polyamine Content and Arginine Decarboxylase Activity in Lupinus Luteus Seedlings. J. Plant Physiol. 2005, 162, 662–668. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [Green Version]
- Mackerness, S.A.-H.; Surplus, S.L.; Blake, P.; John, C.F.; Buchanan-Wollaston, V.; Jordan, B.R.; Thomas, B. Ultraviolet-B-Induced Stress and Changes in Gene Expression in Arabidopsis Thaliana: Role of Signalling Pathways Controlled by Jasmonic Acid, Ethylene and Reactive Oxygen Species. Plant Cell Environ. 1999, 22, 1413–1423. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Wei, X.; Zhao, X.; Wang, B.; Sui, N. Transcription Profiles of Genes Related to Hormonal Regulations Under Salt Stress in Sweet Sorghum. Plant Mol. Biol. Rep. 2017, 35, 586–599. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat. Rev. Genet. 2010, 11, 539. [Google Scholar] [CrossRef]
- Zipfel, C.; Felix, G. Plants and Animals: A Different Taste for Microbes? Curr. Opin. Plant Biol. 2005, 8, 353–360. [Google Scholar] [CrossRef]
- Eulgem, T. Regulation of the Arabidopsis Defense Transcriptome. Trends Plant Sci. 2005, 10, 71–78. [Google Scholar] [CrossRef]
- Jalali, B.L.; Bhargava, S.; Kamble, A. Signal Transduction and Transcriptional Regulation of Plant Defence Responses. J. Phytopathol. 2006, 154, 65–74. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical Diversity and Defence Metabolism: How Plants Cope with Pathogens and Ozone Pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A. Dixon-2001-Natural Products and.Pdf. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Chan, Y.A.; Podevels, A.M.; Kevany, B.M.; Thomas, M.G. Biosynthesis of Polyketide Synthase Extender Units. Nat. Prod. Rep. 2009, 26, 90–114. [Google Scholar] [CrossRef] [Green Version]
- Jonathan, D.G.; Jones, J.; Dang, L. The Plant Immune System. Nat. Rev. 2006, 444, 323–329. [Google Scholar]
- Bostock, R.M. Signal Crosstalk and Induced Resistance: Straddling the Line Between Cost and Benefit. Annu. Rev. Phytopathol. 2005, 43, 545–580. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Ton, J.; van Loon, L.C. Cross-Talk between Plant Defence Signalling Pathways: Boost or Burden? AgBiotechNet 2001, 3, 1–8. [Google Scholar]
- Clarke, J.D.; Volko, S.M.; Ledford, H.; Ausubel, F.M.; Dong, X. Roles of Salicylic Acid, Jasmonic Acid, and Ethylene in Cpr-Induced Resistance in Arabidopsis. Plant Cell 2000, 12, 2175–2190. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, K.; Katagiri, F. Comparing Signaling Mechanisms Engaged in Pattern-Triggered and Effector-Triggered Immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.E. The Ethylene Signal Transduction Pathway in Plants. Science 1995, 268, 667–675. [Google Scholar]
- Creelman, R.A.; Mullet, J.E. Oligosaccharins, Brassinolides, and Jasmonates: Nontraditional Regulators of Plant Growth, Development, and Gene Expression. Plant Cell 1997, 9, 1211–1223. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber 1[W]. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [Green Version]
- Planas-Riverola, A.; Gupta, A.; Betegoń-Putze, I.; Bosch, N.; Ibanḛs, M.; Cano-Delgado, A.I. Brassinosteroid Signaling in Plant Development and Adaptation to Stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [Green Version]
- Checker, V.G.; Kushwaha, H.R.; Kumari, P.; Yadav, S. Role of phytohormones in plant defense: Signaling and cross talk. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Singapore, 2018; pp. 159–184. [Google Scholar] [CrossRef]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 Phosphorylates the Central Regulator EIN2 to Control Ethylene Hormone Signaling from the ER Membrane to the Nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, X.; Zhang, C.; Ji, Y.; Zhao, Q.; He, W.; An, F.; Jiang, L.; Guo, H. Activation of Ethylene Signaling Is Mediated by Nuclear Translocation of the Cleaved EIN2 Carboxyl Terminus. Cell Res. 2012, 22, 1613–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, Y.; Yamoto, N.; Suzuki, Y.; Chiba, Y.; Yamazaki, K.I.; Sato, T.; Yamaguchi, J. The Arabidopsis Transcriptional Repressor ERF9 Participates in Resistance against Necrotrophic Fungi. Plant Sci. 2013, 213, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Chico, J.M.; Sánchez-Serrano, J.J.; Solano, R. Jasmonate-Insensitive1 Encodes a MYC Transcription Factor Essential to Discriminate between Different Jasmonate-Regulated Defense Responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Memelink, J. Jasmonate-Responsive Transcription Factors Regulating Plant Secondary Metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 Are Receptors for the Immune Signal Salicylic Acid in Plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Rockett, K.S.; Kørner, C.J.; Pajerowska-Mukhtar, K.M. Salicylic acid signalling: New insights and prospects at a quarter-century milestone. Essays Biochem. 2015, 58, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between Abiotic and Biotic Stress Responses: A Current View from the Points of Convergence in the Stress Signaling Networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef]
- Narusaka, Y.; Narusaka, M.; Seki, M.; Umezawa, T.; Ishida, J.; Nakajima, M.; Enju, A.; Shinozaki, K. Crosstalk in the Responses to Abiotic and Biotic Stresses in Arabidopsis: Analysis of Gene Expression in Cytochrome P450 Gene Superfamily by CDNA Microarray. Plant Mol. Biol. 2004, 55, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Xia, L.Q.; Chen, M.; Cheng, X.G.; Zhang, R.Y.; Li, L.C.; Zhao, Y.X.; Lu, Y.; Ni, Z.Y.; Liu, L.; et al. Isolation and Molecular Characterization of the Triticum Aestivum, L. Ethylene-Responsive Factor 1 (TaERF1) That Increases Multiple Stress Tolerance. Plant Mol. Biol. 2007, 65, 719–732. [Google Scholar] [CrossRef]
- Nakashima, K.; Tran, L.S.P.; van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of a NAC-Type Transcription Factor OsNAC6 Involved in Abiotic and Biotic Stress-Responsive Gene Expression in Rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of Cis-Acting Regulatory Elements in Osmotic- and Cold-Stress-Responsive Promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY Transcription Factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Chevalier, J.; El Ghoul, H.; Voldum-Clausen, K.; Mundy, J.; Petersen, M. DNA Damage as a Consequence of NLR Activation. PLoS Genet. 2018, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.T.N.; Chung, E.H.; Habring-Müller, A.; Demar, M.; Schwab, R.; Dangl, J.L.; Weigel, D.; Chae, E. Activation of a Plant NLR Complex through Heteromeric Association with an Autoimmune Risk Variant of Another NLR. Curr. Biol. 2017, 27, 1148–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riehs-Kearnan, N.; Gloggnitzer, J.; Dekrout, B.; Jonak, C.; Riha, K. Aberrant Growth and Lethality of Arabidopsis Deficient in Nonsense-Mediated RNA Decay Factors Is Caused by Autoimmune-like Response. Nucleic Acids Res. 2012, 40, 5615–5624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Goritschnig, S.; Dong, X.; Li, X. A Gain-of-Function Mutation in a Plant Disease Resistance Gene Leads to Constitutive Activation of Downstream Signal. Plant Cell 2003, 15, 2636–2646. [Google Scholar] [CrossRef] [Green Version]
- Van Wersch, R.; Li, X.; Zhang, Y. Mighty Dwarfs: Arabidopsis Autoimmune Mutants and Their Usages in Genetic Dissection of Plant Immunity. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, G.M.; Mazzucotelli, E.; Marone, D.; Crosatti, C.; Michelotti, V.; Valè, G.; Mastrangelo, A.M. Regulation and Evolution of NLR Genes: A Close Interconnection for Plant Immunity. Int. J. Mol. Sci. 2018, 19, 1662. [Google Scholar] [CrossRef] [Green Version]
- Broglie, K.; Chet, I.; Holliday, M.; Cressman, R.; Biddle, P.; Knowlton, S.; Mauvais, C.J.; Broglie, R. Transgenic Plants with Enhanced Resistance to the Fungal Pathogen Rhizoctonia Solani. Science 1991, 254, 1194–1197. [Google Scholar] [CrossRef]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Vivek, P.J.; Tuteja, N.; Soniya, E.V. CDPK1 from Ginger Promotes Salinity and Drought Stress Tolerance without Yield Penalty by Improving Growth and Photosynthesis in Nicotiana Tabacum. PLoS ONE 2013, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, M.E.; D’Ippolito, S.; Pepe, A.; Daleo, G.R.; Guevara, M.G. Transgenic Expression of Plant-Specific Insert of Potato Aspartic Proteases (StAP-PSI) Confers Enhanced Resistance to Botrytis Cinerea in Arabidopsis Thaliana. Phytochemistry 2018, 149, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.T.; Qu, N.; Zhao, Q.; Bi, D.; Li, X. Negative Regulation of Defense Responses in Arabidopsis by Two NPR1 Paralogs. Plant J. 2006, 48, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Koukol, K.; Conn, E.E. The Metabolism of Aromatic Compounds in Higher Plants. J. Biol. Chem. 1961, 236, 2692–2698. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Strid, Å.; Chow, W.S.; Anderson, J.M. UV-B Damage and Protection at the Molecular Level in Plants. Photosynth. Res. 1994, 39, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, X.; Gao, J.; Guo, Y.; Huang, Z.; Du, Y. The Tomato Hoffman’s Anthocyaninless Gene Encodes a BHLH Transcription Factor Involved in Anthocyanin Biosynthesis That Is Developmentally Regulated and Induced by Low Temperatures. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signaling Molecules in Plant-Microbe Symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Peck, M.C.; Fisher, R.F.; Long, S.R. Diverse Flavonoids Stimulate NodD1 Binding to Nod Gene Promoters in Sinorhizobium Meliloti. J. Bacteriol. 2006, 188, 5417–5427. [Google Scholar] [CrossRef] [Green Version]
- Kamboj, D.V.; Bhatia, R.; Pathak, D.V.; Sharma, P.K. Role of NodD Gene Product and Flavonoid Interactions in Induction of Nodulation Genes in Mesorhizobium Ciceri. Physiol. Mol. Biol. Plants 2010, 16, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Forkmann, G.; Martens, S. Metabolic Engineering and Applications of Flavonoids. Curr. Opin. Biotechnol. 2001, 12, 155–160. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Plant Polyketide Synthases: A Fascinating Group of Enzymes. Plant Physiol. Biochem. 2009, 47, 167–174. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ogata, H.; Goto, S. Type III Polyketide Synthases: Functional Classification and Phylogenomics. ChemBioChem 2017, 18, 50–65. [Google Scholar] [CrossRef]
- Morita, H.; Wong, C.P.; Abe, I. How Structural Subtleties Lead to Molecular Diversity for the Type III Polyketide Synthases. J. Biol. Chem. 2019, 294, 15121–15136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/Function Analysis of a Type III Polyketide Synthase in the Brown Alga Ectocarpus Siliculosus Reveals a Biochemical Pathway in Phlorotannin Monomer Biosynthesis. Plant Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef] [Green Version]
- Meyer, P.; Heidmann, I.; Forkmannr, G. A New Petunia Flower Colour Generated. Nature 1987, 330, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Dixon Richard, A. Isoflavonoids: Biochemistry, Molecular Bilolgy, and Biological Functions. Compr. Nat. Prod. Chem. 1999, 1, 773–823. [Google Scholar]
- Resmi, M.S.; Vivek, P.J.; Soniya, E.V. Over-Expression of Bael Quinolone Synthase in Tobacco Improves Plant Vigor under Favorable Conditions, Drought, or Salt Stress. FEBS Lett. 2015, 589, 332–341. [Google Scholar] [CrossRef]
- Aiswarya, G.; Mallika, V.; Mur, L.A.J.; Soniya, E.V. Ectopic Expression and Functional Characterization of Type III Polyketide Synthase Mutants from Emblica Officinalis Gaertn. Plant Cell Rep. 2016, 35, 2077–2090. [Google Scholar] [CrossRef]
- Mehrtens, F.; Kranz, H.; Bednarek, P.; Weisshaar, B. The Arabidopsis Transcription Factor MYB12 Is a Flavonol-Specific Regulator of Phenylpropanoid Biosynthesis. Plant Physiol. 2005, 138, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Jirschitzka, J.; Mattern, D.J.; Gershenzon, J.; D’Auria, J.C. Learning from Nature: New Approaches to the Metabolic Engineering of Plant Defense Pathways. Curr. Opin. Biotechnol. 2013, 24, 320–328. [Google Scholar] [CrossRef]
- Allen, A.; Islamovic, E.; Kaur, J.; Gold, S.; Shah, D.; Smith, T.J. Transgenic Maize Plants Expressing the Totivirus Antifungal Protein, KP4, Are Highly Resistant to Corn Smut. Plant Biotechnol. J. 2011, 9, 857–864. [Google Scholar] [CrossRef]
- Omari Alzahrani, F. Metabolic Engineering of Osmoprotectants to Elucidate the Mechanism(s) of Salt Stress Tolerance in Crop Plants. Planta 2021, 253, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Montero, M.; Company, N.; Badosa, E.; Messeguer, J.; Montesinos, L.; Montesinos, E.; Pla, M. Constitutive Expression of Transgenes Encoding Derivatives of the Synthetic Antimicrobial Peptide BP100: Impact on Rice Host Plant Fitness. BMC Plant Biol. 2012, 12, 159–180. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Kong, W.; Wong, G.; Fu, L.; Peng, R.; Li, Z.; Yao, Q. AtMYB12 Regulates Flavonoids Accumulation and Abiotic Stress Tolerance in Transgenic Arabidopsis Thaliana. Mol. Genet. Genomics 2016, 291, 1545–1559. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.W.; Loake, G.J.; Spoel, S.H. Transcription Dynamics in Plant Immunity. Plant Cell 2011, 23, 2809–2820. [Google Scholar] [CrossRef] [Green Version]
- Zoubenko, O.; Uckun, F.; Hur, Y.; Chet, I.; Tumer, N. Plant Resistance to Fungal Infection Induced by Nontoxic Pokeweed Antiviral Protein Mutants. Nat. Biotechnol. 1997, 15, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Innes, R.W. An Arabidopsis Mutant with Enhanced Resistance to Powdery Mildew. Plant Cell 1998, 10, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Ellis, C.; Turner, J.G. The Arabidopsis Mutant Cev1 Has Constitutively Active Jasmonate and Ethylene Signal Pathways and Enhanced Resistance to Pathogens. Plant Cell 2001, 13, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.S.; Song, J.T.; Cheong, J.-J.; Lee, Y.-H.; Lee, Y.-W.; Hwang, I.; Lee, J.S.; Choi, Y.D. Jasmonic Acid Carboxyl Methyltransferase: A Key Enzyme for Jasmonate-Regulated Plant Responses. Proc. Natl. Acad. Sci. USA 2001, 98, 4788–4793. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, O.; Berrocal-Lobo, M.; Molina, A.; Sanchez-Serrano, J.J.; Solano, R. Role of Ethylene-Response-Factor1 in the Response to Pathogens in Arabidopsis, 2002 ed.; Vendrell, M., Klee, H., Pech, J.C., Romojaro, F., Eds.; IOS Press: Amsterdam, The Netherlands, 2002; Volume 349. [Google Scholar]
- Brodersen, P.; Petersen, M.; Pike, H.M.; Olszak, B.; Skov, S.; Ødum, N.; Jørgensen, L.B.; Brown, R.E.; Mundy, J. Knockout of Arabidopsis Accelerated-Cell-Death11 Encoding a Sphingosine Transfer Protein Causes Activation of Programmed Cell Death and Defense. Genes Dev. 2002, 16, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simanshu, D.K.; Zhai, X.; Munch, D.; Hofius, D.; Markham, J.E.; Bielawski, J.; Bielawska, A.; Malinina, L.; Molotkovsky, J.G.; Mundy, J.W.; et al. Arabidopsis Accelerated Cell Death 11, ACD11, Is a Ceramide-1-Phosphate Transfer Protein and Intermediary Regulator of Phytoceramide Levels. Cell Rep. 2014, 6, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Brader, G.; Kariola, T.; Tapio Palva, E. WRKY70 Modulates the Selection of Signaling Pathways in Plant Defense. Plant J. 2006, 46, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cha, J.; Choi, C.; Choi, N.; Ji, H.-S.; Park, S.R.; Lee, S.; Hwang, D.-J. Rice WRKY11 Plays a Role in Pathogen Defense and Drought Tolerance. Rice 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Huffaker, A.; Pearce, G.; Ryan, C.A. An Endogenous Peptide Signal in Arabidopsis Activates Components of the Innate Immune Response. Proc. Natl. Acad. Sci. USA 2006, 103, 10098–10103. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, H.; Boni, R.; Bucher, R.; Kuhn, B.; Buchmann, G.; Sucher, J.; Selter, L.L.; Hensel, G.; Kumlehn, J.; Bigler, L.; et al. The Wheat Resistance Gene Lr34 Results in the Constitutive Induction of Multiple Defense Pathways in Transgenic Barley. Plant J. 2015, 84, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A Core Function of EDS1 with PAD4 Is to Protect the Salicylic Acid Defense Sector in Arabidopsis Immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Song, N.; Tang, Z.; Wang, X.; Kang, Z.; Dai, L.; Wang, B. Constitutive Expression of Arabidopsis Senescence Associated Gene 101 in Brachypodium Distachyon Enhances Resistance to Puccinia Brachypodii and Magnaporthe Oryzae. Plants 2020, 9, 1316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perincherry, L.; Stępień, Ł.; Vasudevan, S.E. Cross-Tolerance and Autoimmunity as Missing Links in Abiotic and Biotic Stress Responses in Plants: A Perspective toward Secondary Metabolic Engineering. Int. J. Mol. Sci. 2021, 22, 11945. https://doi.org/10.3390/ijms222111945
Perincherry L, Stępień Ł, Vasudevan SE. Cross-Tolerance and Autoimmunity as Missing Links in Abiotic and Biotic Stress Responses in Plants: A Perspective toward Secondary Metabolic Engineering. International Journal of Molecular Sciences. 2021; 22(21):11945. https://doi.org/10.3390/ijms222111945
Chicago/Turabian StylePerincherry, Lakshmipriya, Łukasz Stępień, and Soniya Eppurathu Vasudevan. 2021. "Cross-Tolerance and Autoimmunity as Missing Links in Abiotic and Biotic Stress Responses in Plants: A Perspective toward Secondary Metabolic Engineering" International Journal of Molecular Sciences 22, no. 21: 11945. https://doi.org/10.3390/ijms222111945
APA StylePerincherry, L., Stępień, Ł., & Vasudevan, S. E. (2021). Cross-Tolerance and Autoimmunity as Missing Links in Abiotic and Biotic Stress Responses in Plants: A Perspective toward Secondary Metabolic Engineering. International Journal of Molecular Sciences, 22(21), 11945. https://doi.org/10.3390/ijms222111945







