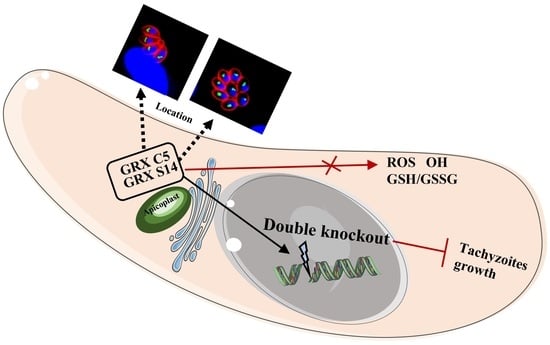
Identification and Function of Apicoplast Glutaredoxins in Neospora caninum
Abstract
:1. Introduction
2. Results
2.1. GRX S14 and GRX C5 Localize to the Apicoplast
2.2. GRX S14 and GRX C5 Together Affect the Growth of Parasites, and Their Function Depends on the CXXC/CXXS Motif
2.3. Redox Homeostasis of Δgrx C5Δgrx S14 Parasites Was Not Affected
2.4. GRX S14 and GRX C5 Are not Involved in the Control of Protein Trafficking to the Apicoplast
2.5. Double-Gene Depletion Affects the Expression of Several Proteins
3. Discussion
4. Materials and Methods
4.1. Parasites and Cell Culture
4.2. Construction of Transgenic Parasite Lines
4.3. Immunoblotting and Immunofluorescence Assays
4.4. Phenotypic Assays
4.4.1. Plaque Assays
4.4.2. Invasion Assay and Intracellular Replication Assay
4.4.3. Egress Assay
4.4.4. N. caninum Mouse Infection
4.4.5. Detection of Reactive Oxygen Species
4.4.6. Detection of Hydroxyl Radicals
4.4.7. GSH and GSSG Determination
4.4.8. Comparative Proteomics Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyon, C. Update on the Diagnosis and Management of Neospora caninum Infections in Dogs. Top. Companion Anim. Med. 2010, 25, 170–175. [Google Scholar] [CrossRef]
- Hall, C.; Reichel, M.; Ellis, J. Neospora abortions in dairy cattle: Diagnosis, mode of transmission and control. Vet. Parasitol. 2005, 128, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J. Recent advances in Neospora and neosporosis. Vet. Parasitol. 1999, 84, 349–367. [Google Scholar] [CrossRef]
- Mohring, F.; Jortzik, E.; Becker, K. Comparison of methods probing the intracellular redox milieu in Plasmodium falciparum. Mol. Biochem. Parasitol. 2016, 206, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.M.; Mieyal, J.J. Protein-Thiol Oxidation and Cell Death: Regulatory Role of Glutaredoxins. Antioxid. Redox Signal. 2012, 17, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Yogavel, M.; Tripathi, T.; Gupta, A.; Banday, M.M.; Rahlfs, S.; Becker, K.; Belrhali, H.; Sharma, A. Atomic resolution crystal structure of glutaredoxin 1 from Plasmodium falciparum and comparison with other glutaredoxins. Acta. Crystallogr. D Biol. Crystallogr. 2014, 70, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begas, P.; Liedgens, L.; Moseler, A.; Meyer, A.J.; DePonte, M. Glutaredoxin catalysis requires two distinct glutathione interaction sites. Nat. Commun. 2017, 8, 14835. [Google Scholar] [CrossRef]
- Manta, B.; Pavan, C.; Sturlese, M.; Medeiros, A.; Crispo, M.; Berndt, C.; Krauth-Siegel, R.L.; Bellanda, M.; Comini, M.A. Iron-Sulfur Cluster Binding by Mitochondrial Monothiol Glutaredoxin-1 of Trypanosoma brucei: Molecular Basis of Iron-Sulfur Cluster Coordination and Relevance for Parasite Infectivity. Antioxid. Redox Signal. 2013, 19, 665–682. [Google Scholar] [CrossRef] [Green Version]
- Ebersoll, S.; Musunda, B.; Schmenger, T.; Dirdjaja, N.; Bonilla, M.; Manta, B.; Ulrich, K.; Comini, M.; Krauth-Siegel, R.L. A glutaredoxin in the mitochondrial intermembrane space has stage-specific functions in the thermo-tolerance and proliferation of African trypanosomes. Redox Biol. 2018, 15, 532–547. [Google Scholar] [CrossRef]
- Musunda, B.; Benítez, D.; Dirdjaja, N.; Comini, M.; Krauth-Siegel, R.L. Glutaredoxin-deficiency confers bloodstream Trypanosoma brucei with improved thermotolerance. Mol. Biochem. Parasitol. 2015, 204, 93–105. [Google Scholar] [CrossRef]
- Marquez, V.E.; Arias, D.G.; Chiribao, M.L.; Faral-Tello, P.; Robello, C.; Iglesias, A.A.; Guerrero, S.A. Redox metabolism in Trypanosoma cruzi. Biochemical characterization of dithiol glutaredoxin dependent cellular pathways. Biochimie 2014, 106, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kehr, S.; Sturm, N.; Rahlfs, S.; Przyborski, J.M.; Becker, K. Compartmentation of Redox Metabolism in Malaria Parasites. PLoS Pathog. 2010, 6, e1001242. [Google Scholar] [CrossRef] [PubMed]
- Jortzik, E.; Becker, K. Thioredoxin and glutathione systems in Plasmodium falciparum. Int. J. Med. Microbiol. 2012, 302, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Mohring, F.; Rahbari, M.; Zechmann, B.; Rahlfs, S.; Przyborski, J.M.; Meyer, A.J.; Becker, K. Determination of glutathione redox potential and pH value in subcellular compartments of malaria parasites. Free Radic. Biol. Med. 2017, 104, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, X.; Xue, Y.; Yang, C.; Wu, K.; Liu, J.; Liu, Q. Glutaredoxin 1 Deficiency Leads to Microneme Protein-Mediated Growth Defects in Neospora caninum. Front. Microbiol. 2020, 11, 536044. [Google Scholar] [CrossRef]
- Biddau, M.; Bouchut, A.; Major, J.; Saveria, T.; Tottey, J.; Oka, O.; Van Lith, M.; Jennings, K.E.; Ovciarikova, J.; DeRocher, A.; et al. Two essential Thioredoxins mediate apicoplast biogenesis, protein import, and gene expression in Toxoplasma gondii. PLoS Pathog. 2018, 14, e1006836. [Google Scholar] [CrossRef] [Green Version]
- McFadden, G.I.; Reith, M.E.; Munholland, J.; Lang-Unnasch, N. Plastid in human parasites. Nat. Cell Biol. 1996, 381, 482. [Google Scholar] [CrossRef]
- McFadden, G.I.; Yeh, E. The apicoplast: Now you see it, now you don’t. Int. J. Parasitol. 2017, 47, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Riondet, C.; Desouris, J.P.; Montoya, J.G.; Chartier, Y.; Meyer, Y.; Reichheld, J.-P. A dicotyledon-specific glutaredoxin GRXC1 family with dimer-dependent redox regulation is functionally redundant with GRXC2. Plant Cell Environ. 2012, 35, 360–373. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Feng, Y.; Liu, J.-Z.; Chen, Y.; Pham, K.; Deng, H.; Hirschi, K.D.; Wang, X.; Cheng, N. Structural insights into the N-terminal GIY-YIG endonuclease activity of Arabidopsis glutaredoxin AtGRXS16 in chloroplasts. Proc. Natl. Acad. Sci. USA 2013, 110, 9565–9570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Huang, C.; Xie, Y.; Song, F.; Zhou, X. A tomato glutaredoxin gene SlGRX1 regulates plant responses to oxidative, drought and salt stresses. Planta 2010, 232, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-H.; Liu, J.; Liu, X.; Wu, Q.; Thompson, S.; Lin, J.; Chang, J.; Whitham, S.A.; Park, S.; Cohen, J.; et al. Arabidopsis Monothiol Glutaredoxin, AtGRXS17, Is Critical for Temperature-dependent Postembryonic Growth and Development via Modulating Auxin Response. J. Biol. Chem. 2011, 286, 20398–20406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuesting, J.; Riondet, C.; Maria, C.; Kruse, I.; Becuwe, N.; König, N.; Berndt, C.; Tourrette, S.; Montoya, J.G.; Herrero, E.; et al. Arabidopsis glutaredoxin S17 and its partner NF-YC11/NC2α contribute to maintenance of the shoot apical meristem under long-day photoperiod. Plant Physiol. 2015, 167, 1643–1658. [Google Scholar] [CrossRef] [Green Version]
- Rey, P.; Becuwe, N.; Tourrette, S.; Rouhier, N. Involvement of Arabidopsis glutaredoxin S14 in the maintenance of chlorophyll content. Plant Cell Environ. 2017, 40, 2319–2332. [Google Scholar] [CrossRef]
- Couturier, J.; Ströher, E.; Albetel, A.N.; Roret, T.; Muthuramalingam, M.; Tarrago, L.; Seidel, T.; Tsan, P.; Jacquot, J.-P.; Johnson, M.K.; et al. Arabidopsis chloroplastic glutaredoxin C5 as a model to explore molecular deter-minants for iron-sulfur cluster binding into glutaredoxins. J. Biol. Chem. 2011, 286, 27515–27527. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.-H.; Hirschi, K.D. Cloning and Characterization of CXIP1, a Novel PICOT Domain-containing Arabidopsis Protein That Associates with CAX1. J. Biol. Chem. 2003, 278, 6503–6509. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.-H.; Liu, J.; Brock, A.; Nelson, R.S.; Hirschi, K.D. AtGRXcp, an Arabidopsis Chloroplastic Glutaredoxin, Is Critical for Protection against Protein Oxidative Damage. J. Biol. Chem. 2006, 281, 26280–26288. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ouyang, B.; Li, Y.; Feng, Y.; Jacquot, J.-P.; Rouhier, N.; Xia, B. Glutathione regulates the transfer of iron-sulfur cluster from monothiol and dithiol glutaredoxins to apo ferredoxin. Protein. Cell 2012, 3, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ren, X.; Li, Y.; Rouhier, N.; Jacquot, J.-P.; Jin, C.; Xia, B. 1H, 13C, and 15N resonance assignments of reduced GrxS14 from Populus tremula × tremuloides. Biomol. NMR Assign. 2010, 5, 121–124. [Google Scholar] [CrossRef]
- Sheiner, L.; Vaidya, A.B.; McFadden, G.I. The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp. Curr. Opin. Microbiol. 2013, 16, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Liu, J.; Ma, L.; Zhang, X.; Zhang, X.; Zhou, B.; Zhu, X.; Liu, Q. NcGRA17 is an important regulator of parasitophorous vacuole morphology and pathogenicity of Neospora caninum. Vet. Parasitol. 2018, 264, 26–34. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Yang, X.; Ying, Z.; Zhang, H.; Liu, J.; Liu, Q. Identification and Function of Apicoplast Glutaredoxins in Neospora caninum. Int. J. Mol. Sci. 2021, 22, 11946. https://doi.org/10.3390/ijms222111946
Song X, Yang X, Ying Z, Zhang H, Liu J, Liu Q. Identification and Function of Apicoplast Glutaredoxins in Neospora caninum. International Journal of Molecular Sciences. 2021; 22(21):11946. https://doi.org/10.3390/ijms222111946
Chicago/Turabian StyleSong, Xingju, Xu Yang, Zhu Ying, Heng Zhang, Jing Liu, and Qun Liu. 2021. "Identification and Function of Apicoplast Glutaredoxins in Neospora caninum" International Journal of Molecular Sciences 22, no. 21: 11946. https://doi.org/10.3390/ijms222111946
APA StyleSong, X., Yang, X., Ying, Z., Zhang, H., Liu, J., & Liu, Q. (2021). Identification and Function of Apicoplast Glutaredoxins in Neospora caninum. International Journal of Molecular Sciences, 22(21), 11946. https://doi.org/10.3390/ijms222111946