3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages
Abstract
1. Introduction
- (i)
- Interstitial ECM (stromal) contains biomolecules that can be organized into two main classes (proteins, glycoproteins) and proteoglycans (polysaccharides) [37]. It consists mainly of several protein molecules such as collagen I and III, self-arranged polysaccharides in fiber networks of glycosaminoglycans (GAG) such as hyaluronic acid (HA), proteoglycan (PG) and fibronectin [33,34,38,39,40];
- (ii)
2. 2D versus 3D Cell Culture
3. Extracellular Matrix Composition
4. Three-Dimensional Cell Culture Scales
4.1. 3D Scaffolding Structures
4.1.1. Hydrogels
- Protein-based EMC
- Natural hydrogels
- Synthetic hydrogels
4.1.2. Synthetic Strategies
- Porous material
- Hydrogel technology
- Collagen Hydrogel by Freeze-Drying (Lyophilization)
- Electrospinning Hydrogel
- 3D-Printing Scaffolding for 3D cell Culture via Stereolithography
- Micro Fluid
4.2. Scaffold-Free Spheroids
4.2.1. Technical Methods of Spheroid Formation
- Pellet Culture
- Hanging drop
- Cultivation of Molded Lozenges and Liquid Overlay (Static Suspension)
- Spinner Culture Technique
4.2.2. Technical Methods of Tumor Spheroid Formation
- Tumorospheres (floating sphere): Tumors are formed from a single cell capable of giving rise to a sphere by clonal expansion (5–7 days up to 1–2 months) under conditions of low adhesion (plastic with low adhesion) and with a stem cell medium (depending on the type of cancer, growth factors may be preferentially added) [414,415,416,417,418].
- Tissue-derived tumor spheres (endoscopic biopsy): Tumor spheres derived from cut (scalpel blade) and minced partially dissociated cancerous tissues are generated by partial dissociation of tumor tissue and compaction/remodeling (2–5 days up to 12–18 days) in conventional FBS-supplemented medium [416,419,420].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fischbach, C.; Kong, H.J.; Hsiong, S.X.; Evangelista, M.B.; Yuen, W.; Mooney, D.J. Cancer Cell Angiogenic Capability Is Regulated by 3D Culture and Integrin Engagement. Proc. Natl. Acad. Sci. USA 2009, 106, 399–404. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Philp, D.; Hoffman, M.P. Role of the Extracellular Matrix in Morphogenesis. Curr. Opin. Biotechnol. 2003, 14, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In Vitro Cell Migration and Invasion Assays. Mutat. Res./Rev. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.L.; Blanchard, D.K. Characterization of Primary Breast Carcinomas Grown in Three-Dimensional Cultures. J. Surg. Res. 2007, 142, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.P.; Gaspar, V.M.; Mano, J.F. Design of Spherically Structured 3D in Vitro Tumor Models-Advances and Prospects. Acta Biomater. 2018, 75, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Ricci, C.; Moroni, L.; Danti, S. Cancer Tissue Engineering—New Perspectives in Understanding the Biology of Solid Tumours—A Critical Review. OA Tissue Eng. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- LaBarbera, D.V.; Reid, B.G.; Yoo, B.H. The Multicellular Tumor Spheroid Model for High-Throughput Cancer Drug Discovery. Expert Opin. Drug Discov. 2012, 7, 819–830. [Google Scholar] [CrossRef]
- Szot, C.S.; Buchanan, C.F.; Freeman, J.W.; Rylander, M.N. 3D in Vitro Bioengineered Tumors Based on Collagen I Hydrogels. Biomaterials 2011, 32, 7905–7912. [Google Scholar] [CrossRef]
- Hutchinson, L.; Kirk, R. High Drug Attrition Rates--Where Are We Going Wrong? Nat. Rev. Clin. Oncol. 2011, 8, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Burg, K.J.L. Three-Dimensional Polymeric Systems for Cancer Cell Studies. Cytotechnology 2007, 54, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Amer, L.D.; Holtzinger, A.; Keller, G.; Mahoney, M.J.; Bryant, S.J. Enzymatically Degradable Poly(Ethylene Glycol) Hydrogels for the 3D Culture and Release of Human Embryonic Stem Cell Derived Pancreatic Precursor Cell Aggregates. Acta Biomater. 2015, 22, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-Parametric Hydrogels Support 3D in Vitro Bioengineered Microenvironment Models of Tumour Angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef]
- Debnath, J.; Brugge, J.S. Modelling Glandular Epithelial Cancers in Three-Dimensional Cultures. Nat. Rev. Cancer 2005, 5, 675–688. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Xu, X.; Wu, H.; Xie, H.; Chen, L.; Lu, T.; Yang, L.; Guo, X.; Sun, G.; et al. Potential Effect of Matrix Stiffness on the Enrichment of Tumor Initiating Cells under Three-Dimensional Culture Conditions. Exp. Cell Res. 2015, 330, 123–134. [Google Scholar] [CrossRef]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Wang, K.; Wu, J.D.; Silber, J.R.; Ellenbogen, R.G.; Lee, J.S.H.; Zhang, M. Proliferation and Enrichment of CD133+ Glioblastoma Cancer Stem Cells on 3D Chitosan-Alginate Scaffolds. Biomaterials 2014, 35, 9137–9143. [Google Scholar] [CrossRef]
- Feng, S.; Duan, X.; Lo, P.-K.; Liu, S.; Liu, X.; Chen, H.; Wang, Q. Expansion of Breast Cancer Stem Cells with Fibrous Scaffolds. Integr. Biol. 2013, 5, 768–777. [Google Scholar] [CrossRef]
- Moysidou, C.-M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 1566. [Google Scholar] [CrossRef]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef] [PubMed]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Redmond, J.; Mccarthy, H.; Buchanan, P.; Levingstone, T.J.; Dunne, N.J. Advances in Biofabrication Techniques for Collagen-Based 3D in Vitro Culture Models for Breast Cancer Research. Mater. Sci. Eng. C 2021, 122, 111944. [Google Scholar] [CrossRef] [PubMed]
- Horning, J.L.; Sahoo, S.K.; Vijayaraghavalu, S.; Dimitrijevic, S.; Vasir, J.K.; Jain, T.K.; Panda, A.K.; Labhasetwar, V. 3-D Tumor Model for in Vitro Evaluation of Anticancer Drugs. Mol. Pharm. 2008, 5, 849–862. [Google Scholar] [CrossRef]
- Kim, J.B. Three-Dimensional Tissue Culture Models in Cancer Biology. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2005; Volume 15, pp. 365–377. [Google Scholar] [CrossRef]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D Tumour Models: Novel in Vitro Approaches to Cancer Studies. J. Cell Commun. Signal. 2011, 5, 239–248. [Google Scholar] [CrossRef]
- Debnath, J.; Muthuswamy, S.K.; Brugge, J.S. Morphogenesis and Oncogenesis of MCF-10A Mammary Epithelial Acini Grown in Three-Dimensional Basement Membrane Cultures. Methods 2003, 30, 256–268. [Google Scholar] [CrossRef]
- Debnath, J.; Mills, K.R.; Collins, N.L.; Reginato, M.J.; Muthuswamy, S.K.; Brugge, J.S. The Role of Apoptosis in Creating and Maintaining Luminal Space within Normal and Oncogene-Expressing Mammary Acini. Cell 2002, 111, 29–40. [Google Scholar] [CrossRef]
- Muthuswamy, S.K.; Li, D.; Lelievre, S.; Bissell, M.J.; Brugge, J.S. ErbB2, but Not ErbB1, Reinitiates Proliferation and Induces Luminal Repopulation in Epithelial Acini. Nat. Cell Biol. 2001, 3, 785–792. [Google Scholar] [CrossRef]
- Kumar, S.; Weaver, V.M. Mechanics, Malignancy, and Metastasis: The Force Journey of a Tumor Cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef]
- Geiger, B.; Yamada, K.M. Molecular Architecture and Function of Matrix Adhesions. Cold Spring Harbor Perspect. Biol. 2011, 3, a005033. [Google Scholar] [CrossRef]
- Sawicki, L.A.; Choe, L.H.; Wiley, K.L.; Lee, K.H.; Kloxin, A.M. Isolation and Identification of Proteins Secreted by Cells Cultured within Synthetic Hydrogel-Based Matrices. ACS Biomater. Sci. Eng. 2018, 4, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and Biochemical Remodeling of Stromal Extracellular Matrix in Cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Tekle Abegaz, S. We Are IntechOpen, the World ’s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%; University of Gondar Institutional Repository: Gondar, Ethiopia, 2021. [Google Scholar]
- Brown, N.H. Extracellular Matrix in Development: Insights from Mechanisms Conserved between Invertebrates and Vertebrates. Cold Spring Harbor Perspect. Biol. 2011, 3, a005082. [Google Scholar] [CrossRef] [PubMed]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, X.R.; Font, A.R.; Ibrahim, A.; Maximilien, N.; Lumbroso, D.; Hurford, A.; Winperry, J.; Wade, S.; Sataloff, R.T.; Johns, M.M.; et al. “We are IntechOpen, the world’s leading publisher of Open Access books Built by scientists, for scientists TOP 1 %,” Intech, vol. 32, no. July, pp. 137–144, 2013. Available online: http://www.intechopen.com/books/trends-in-telecommunications-technologies/gps-total-electron-content-tec-prediction-at-ionosphere-layer-over-the-equatorial-region%0AInTec%0Ahttp://www.asociatiamhc.ro/wp-content/uploads/2013/11/Guide-to-Hydropower.pdf (accessed on 27 October 2021).
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Insua-rodríguez, J.; Oskarsson, T. The Extracellular Matrix in Breast Cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harbor Perspect. Biol. 2011, 3, 1–24. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The Extracellular Matrix in Development and Morphogenesis: A Dynamic View. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Soares, C.P.; Midlej, V.; de Oliveira, M.E.W.; Benchimol, M.; Costa, M.L.; Mermelstein, C. 2D and 3D-Organized Cardiac Cells Shows Differences in Cellular Morphology, Adhesion Junctions, Presence of Myofibrils and Protein Expression. PLoS ONE 2012, 7, e38147. [Google Scholar] [CrossRef]
- Lei, Y.; Schaffer, D.V. A Fully Defined and Scalable 3D Culture System for Human Pluripotent Stem Cell Expansion and Differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, E5039–E5048. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Dinh, T.V.; Hannigan, E.V. Three-Dimensional Endothelial-Tumor Epithelial Cell Interactions in Human Cervical Cancers. In Vitro Cell. Dev. Biol.-Anim. 1997, 33, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery (Review). Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chow, A.B.; Mattingly, R.R. Three-Dimensional Overlay Culture Models of Human Breast Cancer Reveal a Critical Sensitivity to Mitogen-Activated Protein Kinase Kinase Inhibitors. J. Pharmacol. Exp. Ther. 2010, 332, 821–828. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-Dimensional Culture Models of Normal and Malignant Breast Epithelial Cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef]
- Torisawa, Y.-S.; Shiku, H.; Yasukawa, T.; Nishizawa, M.; Matsue, T. Multi-Channel 3-D Cell Culture Device Integrated on a Silicon Chip for Anticancer Drug Sensitivity Test. Biomaterials 2005, 26, 2165–2172. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Li, M.; Wang, L.; Elson, E.L.; Lu, T.J.; Genin, G.M.; Xu, F. An Approach to Quantifying 3D Responses of Cells to Extreme Strain. Sci. Rep. 2016, 6, 19550. [Google Scholar] [CrossRef]
- Do Amaral, R.J.F.C.; Zayed, N.M.A.; Pascu, E.I.; Murphy, C.M.; Sridharan, R.; González-vázquez, A.; Sullivan, B.O. Functionalising Collagen-Based Scaffolds with Platelet-Rich Plasma for Enhanced Skin Wound Healing Potential. Front. Bioeng. Biotechnol. 2019, 7, 371. [Google Scholar] [CrossRef]
- Rosso, F.; Giordano, A.; Barbarisi, M.; Barbarisi, A. From Cell-ECM Interactions to Tissue Engineering. J. Cell. Physiol. 2004, 199, 174–180. [Google Scholar] [CrossRef]
- Yip, D.; Cho, C.H. A Multicellular 3D Heterospheroid Model of Liver Tumor and Stromal Cells in Collagen Gel for Anti-Cancer Drug Testing. Biochem. Biophys. Res. Commun. 2013, 433, 327–332. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Culture of HepG2 Liver Cells on Three Dimensional Polystyrene Scaffolds Enhances Cell Structure and Function during Toxicological Challenge. J. Anat. 2007, 211, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Weaver, V.M.; Petersen, O.W.; Larabell, C.A.; Dedhar, S.; Briand, P.; Lupu, R.; Bissell, M.J. Reciprocal Interactions between Beta1-Integrin and Epidermal Growth Factor Receptor in Three-Dimensional Basement Membrane Breast Cultures: A Different Perspective in Epithelial Biology. Proc. Natl. Acad. Sci. USA 1998, 95, 14821–14826. [Google Scholar] [CrossRef]
- Sokol, E.S.; Miller, D.H.; Breggia, A.; Spencer, K.C.; Arendt, L.M.; Gupta, P.B. Growth of Human Breast Tissues from Patient Cells in 3D Hydrogel Scaffolds. Breast Cancer Res. 2016, 18, 19. [Google Scholar] [CrossRef]
- Weaver, V.M.; Fischer, A.H.; Peterson, O.W.; Bissell, M.J. The Importance of the Microenvironment in Breast Cancer Progression: Recapitulation of Mammary Tumorigenesis Using a Unique Human Mammary Epithelial Cell Model and a Three-Dimensional Culture Assay. Biochem. Cell Biol. 1996, 74, 833–851. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The Morphologies of Breast Cancer Cell Lines in Three-Dimensional Assays Correlate with Their Profiles of Gene Expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular Matrix Molecules: Potential Targets in Pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z.; Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harbor Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Hashmi, S.; Marinkovich, M.P. Molecular Organization of the Basement Membrane Zone. Clin. Dermatol. 2011, 29, 398–411. [Google Scholar] [CrossRef]
- Hohenester, E.; Yurchenco, P.D. Laminins in Basement Membrane Assembly. Cell Adhes. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the Matrisome-An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harbor Perspect. Biol. 2012, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Schaefer, L.; Schaefer, R.M. Proteoglycans: From Structural Compounds to Signaling Molecules. Cell Tissue Res. 2010, 339, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Walter, P. Molecular Biology of the Cell, 6th ed.; Wilson, J., Hunt, T., Eds.; W.W. Norton & Company: New York, NY, USA, 2015; ISBN 0815340834. [Google Scholar] [CrossRef]
- Barsky, S.H.; Karlin, N.J. Myoepithelial Cells: Autocrine and Paracrine Suppressors of Breast Cancer Progression. J. Mammary Gland Biol. Neoplasia 2005, 10, 249–260. [Google Scholar] [CrossRef]
- Tsang, K.Y.; Cheung, M.C.H.; Chan, D.; Cheah, K.S.E. The Developmental Roles of the Extracellular Matrix: Beyond Structure to Regulation. Cell Tissue Res. 2010, 339, 93–110. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E.H.J. Adhesion Signaling—Crosstalk between Integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Varelas, X. The Hippo Pathway Effectors TAZ and YAP in Development, Homeostasis and Disease. Development 2014, 141, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Radisky, D.C.; Rizki, A.; Weaver, V.M.; Petersen, O.W. The Organizing Principle: Microenvironmental Influences in the Normal and Malignant Breast. Differ. Res. Biol. Divers. 2002, 70, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J.; Kivirikko, K.I. Collagens, Modifying Enzymes and Their Mutations in Humans, Flies and Worms. TIG 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Ruggiero, F. The Collagen Superfamily: From the Extracellular Matrix to the Cell Membrane. Pathologie-Biologie 2005, 53, 430–442. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Spicer, A.P.; Brehm-Gibson, T.; Biesterfeldt, J.; Augustine, M.L.; Calabro, A.J.; Kubalak, S.; Klewer, S.E.; McDonald, J.A. Disruption of Hyaluronan Synthase-2 Abrogates Normal Cardiac Morphogenesis and Hyaluronan-Mediated Transformation of Epithelium to Mesenchyme. J. Clin. Investig. 2000, 106, 349–360. [Google Scholar] [CrossRef]
- Chen, X.; Thibeault, S.L. Response of Fibroblasts to Transforming Growth Factor-Β1 on Two-Dimensional and in Three-Dimensional Hyaluronan Hydrogels. Tissue Eng. Part A 2012, 18, 2528–2538. [Google Scholar] [CrossRef]
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal Myofibroblasts Are Drivers of Invasive Cancer Growth. Int. J. Cancer 2008, 123, 2229–2238. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellu. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Schaefer, L.; Iozzo, R.V. Biological Functions of the Small Leucine-Rich Proteoglycans: From Genetics to Signal Transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef]
- Josefsson, A.; Adamo, H.; Hammarsten, P.; Granfors, T.; Stattin, P.; Egevad, L.; Laurent, A.E.; Wikström, P.; Bergh, A. Prostate Cancer Increases Hyaluronan in Surrounding Nonmalignant Stroma, and This Response Is Associated with Tumor Growth and an Unfavorable Outcome. Am. J. Pathol. 2011, 179, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- McAtee, C.O.; Barycki, J.J.; Simpson, M.A. Emerging Roles for Hyaluronidase in Cancer Metastasis and Therapy. Adv. Cancer Res. 2014, 123, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of Fibronectin Extracellular Matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Sivakumar, P.; Jones, C.J.P.; Chen, Q.; Peters, D.M.; Mosher, D.F.; Humphries, M.J.; Kielty, C.M. Fibronectin Regulates Latent Transforming Growth Factor-Beta (TGF Beta) by Controlling Matrix Assembly of Latent TGF Beta-Binding Protein-1. J. Biol. Chem. 2005, 280, 18871–18880. [Google Scholar] [CrossRef] [PubMed]
- Sottile, J.; Hocking, D.C. Fibronectin Polymerization Regulates the Composition and Stability of Extracellular Matrix Fibrils and Cell-Matrix Adhesions. Mol. Biol. Cell 2002, 13, 3546–3559. [Google Scholar] [CrossRef]
- Dzamba, B.J.; Wu, H.; Jaenisch, R.; Peters, D.M. Fibronectin Binding Site in Type I Collagen Regulates Fibronectin Fibril Formation. J. Cell Biol. 1993, 121, 1165–1172. [Google Scholar] [CrossRef]
- Colombi, M.; Zoppi, N.; De Petro, G.; Marchina, E.; Gardella, R.; Tavian, D.; Ferraboli, S.; Barlati, S. Matrix Assembly Induction and Cell Migration and Invasion Inhibition by a 13-Amino Acid Fibronectin Peptide. J. Biol. Chem. 2003, 278, 14346–14355. [Google Scholar] [CrossRef]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional Diversity of Laminins. Annu. Rev. Cell Dev. Biol. 2012, 28, 523–553. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Banerji, S.; Wright, A.J.; Noble, M.; Mahoney, D.J.; Campbell, I.D.; Day, A.J.; Jackson, D.G. Structures of the Cd44-Hyaluronan Complex Provide Insight into a Fundamental Carbohydrate-Protein Interaction. Nat. Struct. Mol. Biol. 2007, 14, 234–239. [Google Scholar] [CrossRef]
- Nelson, J.; McFerran, N.V.; Pivato, G.; Chambers, E.; Doherty, C.; Steele, D.; Timson, D.J. The 67 KDa Laminin Receptor: Structure, Function and Role in Disease. Biosci. Rep. 2008, 28, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin Signalling at a Glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Nutter, F.; Wilkinson, J.M.; Evans, C.A.; Avgoustou, P.; Ottewell, P.D. Human Breast Cancer Bone Metastasis in Vitro and in Vivo: A Novel 3D Model System for Studies of Tumour Cell-Bone Cell Interactions. Clin. Exp. Metastasis 2015, 32, 689–702. [Google Scholar] [CrossRef]
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as Organs: Complex Tissues That Interface with the Entire Organism. Dev. Cell 2010, 18, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, M.; Gayraud, B. Structure and Biological Activity of the Extracellular Matrix. J. Mol. Med. 1998, 76, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking Cell-Matrix Adhesions to the Third Dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Sethi, T.; Rintoul, R.C.; Moore, S.M.; MacKinnon, A.C.; Salter, D.; Choo, C.; Chilvers, E.R.; Dransfield, I.; Donnelly, S.C.; Strieter, R.; et al. Extracellular Matrix Proteins Protect Small Cell Lung Cancer Cells against Apoptosis: A Mechanism for Small Cell Lung Cancer Growth and Drug Resistance in Vivo. Nat. Med. 1999, 5, 662–668. [Google Scholar] [CrossRef]
- Pal, A.; Kleer, C.G. Three Dimensional Cultures: A Tool to Study Normal Acinar Architecture vs. Malignant Transformation of Breast Cells. JoVE 2014, 86, 51311. [Google Scholar] [CrossRef]
- Espinoza-Sánchez, N.A.; Chimal-Ramírez, G.K.; Fuentes-Pananá, E.M. Analyzing the Communication Between Monocytes and Primary Breast Cancer Cells in an Extracellular Matrix Extract (ECME)-Based Three-Dimensional System. JoVE 2018, 131, 56589. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication Strategies for 3D in Vitro Models and Regenerative Medicine. Nat. Rev. Mater. 2021, 3, 21–37. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From Discovery and ECM Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, H.; Hashemi, S.M.; Ashtiani, S.K.; Farrokhi, A. Differentiation of Human Embryonic Stem Cells into Hepatocytes in 2D and 3D Culture Systems in Vitro. Int. J. Dev. Biol. 2006, 50, 645–652. [Google Scholar] [CrossRef]
- Rajakylä, K.; Krishnan, R.; Tojkander, S. Analysis of Contractility and Invasion Potential of Two Canine Mammary Tumor Cell Lines. Front. Vet. Sci. 2017, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Mulfaul, K.; Giacalone, J.C.; Voigt, A.P.; Riker, M.J.; Ochoa, D.; Han, I.C.; Stone, E.M.; Mullins, R.F.; Tucker, B.A. Stepwise Differentiation and Functional Characterization of Human Induced Pluripotent Stem Cell-Derived Choroidal Endothelial Cells. Stem Cell Res. Ther. 2020, 11, 409. [Google Scholar] [CrossRef]
- Chen, D.; Qu, Y.; Hua, X.; Zhang, L.; Liu, Z.; Pflugfelder, S.C.; Li, D.-Q. A Hyaluronan Hydrogel Scaffold-Based Xeno-Free Culture System for Ex Vivo Expansion of Human Corneal Epithelial Stem Cells. Eye 2017, 31, 962–971. [Google Scholar] [CrossRef]
- Devarasetty, M.; Wang, E.; Soker, S.; Skardal, A. Mesenchymal Stem Cells Support Growth and Organization of Host-Liver Colorectal-Tumor Organoids and Possibly Resistance to Chemotherapy. Biofabrication 2017, 9, 21002. [Google Scholar] [CrossRef]
- Mannino, R.G.; Santiago-Miranda, A.N.; Pradhan, P.; Qiu, Y.; Mejias, J.C.; Neelapu, S.S.; Roy, K.; Lam, W.A. 3D Microvascular Model Recapitulates the Diffuse Large B-Cell Lymphoma Tumor Microenvironment in Vitro. Lab. Chip. 2017, 17, 407–414. [Google Scholar] [CrossRef]
- Chen, X.; Thibeault, S.L. Cell-Cell Interaction between Vocal Fold Fibroblasts and Bone Marrow Mesenchymal Stromal Cells in Three-Dimensional Hyaluronan Hydrogel. J. Tissue Eng. Regen. Med. 2016, 10, 437–446. [Google Scholar] [CrossRef][Green Version]
- Engel, B.J.; Constantinou, P.E.; Sablatura, L.K.; Doty, N.J.; Carson, D.D.; Farach-Carson, M.C.; Harrington, D.A.; Zarembinski, T.I. Multilayered, Hyaluronic Acid-Based Hydrogel Formulations Suitable for Automated 3D High Throughput Drug Screening of Cancer-Stromal Cell Cocultures. Adv. Healthc. Mater. 2015, 4, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Milleret, V.; Thompson-Steckel, G.; Huang, N.-P.; Vörös, J.; Simona, B.R.; Ehrbar, M. Soft Hydrogels Featuring In-Depth Surface Density Gradients for the Simple Establishment of 3D Tissue Models for Screening Applications. SLAS Discov. Adv. Life Sci. R&D 2017, 22, 635–644. [Google Scholar] [CrossRef]
- Saha, K.; Pollock, J.F.; Schaffer, D.V.; Healy, K.E. Designing synthetic materials to control stem cell phenotype. Curr. Opin. Chem. Biol. 2008, 11, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.; Otero, T.C.; Panitch, A. Polymeric Biomaterials for Tissue and Organ Regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Haycock, J.W. 3D Cell Culture: A Review of Current Approaches and Techniques. Methods Mol. Biol. 2011, 695, 1–15. [Google Scholar] [CrossRef]
- Sun, T.; Norton, D.; McKean, R.J.; Haycock, J.W.; Ryan, A.J.; MacNeil, S. Development of a 3D Cell Culture System for Investigating Cell Interactions with Electrospun Fibers. Biotechnol. Bioeng. 2007, 97, 1318–1328. [Google Scholar] [CrossRef]
- Aydin, H.M.; El Haj, A.J.; Pişkin, E.; Yang, Y. Improving Pore Interconnectivity in Polymeric Scaffolds for Tissue Engineering. J. Tissue Eng. Regen. Med. 2009, 3, 470–476. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Przyborski, A.; Cameron, N.R. Emulsion-Templated Porous Polymers as Scaffolds for Three Dimensional Cell Culture: Effect of Synthesis Parameters on Scaffold Formation and Homogeneity. J. Mater. Chem. 2007, 17, 4088–4094. [Google Scholar] [CrossRef]
- Salerno, A.; Oliviero, M.; Di Maio, E.; Iannace, S.; Netti, P.A. Design of Porous Polymeric Scaffolds by Gas Foaming of Heterogeneous Blends. J. Mater. Sci. Mater. Med. 2009, 20, 2043–2051. [Google Scholar] [CrossRef]
- Bai, C.; Yang, M.; Fan, Z.; Li, S.; Gao, T.; Fang, Z. Associations of Chemo- and Radio-Resistant Phenotypes with the Gap Junction, Adhesion and Extracellular Matrix in a Three-Dimensional Culture Model of Soft Sarcoma. J. Exp. Clin. Cancer Res. CR 2015, 34, 58. [Google Scholar] [CrossRef]
- Zhang, M.; Rose, B.; Lee, C.S.; Hong, A.M. In Vitro 3-Dimensional Tumor Model for Radiosensitivity of HPV Positive OSCC Cell Lines. Cancer Biol. Ther. 2015, 16, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Elisseeff, J. Hydrogels: Structure Starts to Gel. Nat. Mater. 2008, 7, 271–273. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, C.; Lebourg, M. Three-Dimensional Constructs Using Hyaluronan Cell Carrier as a Tool for the Study of Cancer Stem Cells. J. Biomed. Mater. Research. Part B Appl. Biomater. 2015, 103, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, D.H.; Barbieri, S.; Chevalier, F.; Groetz, J.-E.; Legendre, F.; Demoor, M.; Galera, P.; Lefaix, J.-L.; Saintigny, Y. In Vitro Engineering of Human 3D Chondrosarcoma: A Preclinical Model Relevant for Investigations of Radiation Quality Impact. BMC Cancer 2015, 15, 579. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering Tumors with 3D Scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef]
- Worthington, P.; Pochan, D.J.; Langhans, S.A. Peptide Hydrogels—Versatile Matrices for 3D Cell Culture in Cancer Medicine. Front. Oncol. 2015, 5, 92. [Google Scholar] [CrossRef]
- Dhaliwal, A. 3D Cell Culture: A Review. Mater. Methods 2012, 2, 162. [Google Scholar] [CrossRef]
- Le, V.-M.; Lang, M.-D.; Shi, W.-B.; Liu, J.-W. A Collagen-Based Multicellular Tumor Spheroid Model for Evaluation of the Efficiency of Nanoparticle Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 540–544. [Google Scholar] [CrossRef]
- Haisler, W.L.; Timm, D.M.; Gage, J.A.; Tseng, H.; Killian, T.C.; Souza, G.R. Three-Dimensional Cell Culturing by Magnetic Levitation. Nat. Protoc. 2013, 8, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Domb, A.; Khan, W. Biodegradable Polymers as Drug Carrier Systems. In Encyclopedia of PHARMACEUTICAL TECHNOLOGY, 3rd ed.; Marcel Dekker: New York, NY, USA, 2013; Volume 1, pp. 135–176. ISBN 0824725387. [Google Scholar]
- Zhang, J.; Yun, S.; Du, Y.; Zannettino, A.C.W.; Zhang, H. Fabrication of a Cartilage Patch by Fusing Hydrogel-Derived Cell Aggregates onto Electrospun Film. Tissue Eng. Part A 2020, 26, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Marks, R.; Wolfson, D.; Alsberg, E. Dual-Crosslinked Hydrogel Microwell System for Formation and Culture of Multicellular Human Adipose Tissue-Derived Stem Cell Spheroids. J. Mater. Chem. B 2016, 4, 3526–3533. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Clary, J.M.; Seliktar, D.; Lipke, E.A. A Three-Dimensional Spheroidal Cancer Model Based on PEG-Fibrinogen Hydrogel Microspheres. Biomaterials 2017, 115, 141–154. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Guan, Y.; Zhang, Y. Galactosylated Reversible Hydrogels as Scaffold for HepG2 Spheroid Generation. Acta Biomater. 2014, 10, 1965–1974. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Wong, C.-W.; Hsieh, F.-Y.; Hsu, S. Biomaterial Substrate-Mediated Multicellular Spheroid Formation and Their Applications in Tissue Engineering. Biotechnol. J. 2017, 12, 1700064. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, M.H.; Lee, J.H.; Seliktar, D.; Cho, N.-J.; Tan, L.P. Modulation of Huh7.5 Spheroid Formation and Functionality Using Modified PEG-Based Hydrogels of Different Stiffness. PLoS ONE 2015, 10, e0118123. [Google Scholar] [CrossRef]
- Carvalho, M.P.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Tumor Spheroid Assembly on Hyaluronic Acid-Based Structures: A Review. Carbohydr. Polym. 2016, 150, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yoshida, C.; Hamada, K.; Kikkawa, Y.; Nomizu, M. Development of Three-Dimensional Cell Culture Scaffolds Using Laminin Peptide-Conjugated Agarose Microgels. Biomacromolecules 2020, 21, 3765–3771. [Google Scholar] [CrossRef]
- Barros, A.S.; Costa, E.C.; Nunes, A.S.; de Melo-Diogo, D.; Correia, I.J. Comparative Study of the Therapeutic Effect of Doxorubicin and Resveratrol Combination on 2D and 3D (Spheroids) Cell Culture Models. Int. J. Pharm. 2018, 551, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Härmä, V.; Virtanen, J.; Mäkelä, R.; Happonen, A.; Mpindi, J.-P.; Knuuttila, M.; Kohonen, P.; Lötjönen, J.; Kallioniemi, O.; Nees, M. A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PLoS ONE 2010, 5, e10431. [Google Scholar] [CrossRef]
- Poincloux, R.; Collin, O.; Lizárraga, F.; Romao, M.; Debray, M.; Piel, M.; Chavrier, P. Contractility of the Cell Rear Drives Invasion of Breast Tumor Cells in 3D Matrigel. Proc. Natl. Acad. Sci. USA 2011, 108, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Dolega, M.E.; Abeille, F.; Picollet-D’hahan, N.; Gidrol, X. Controlled 3D Culture in Matrigel Microbeads to Analyze Clonal Acinar Development. Biomaterials 2015, 52, 347–357. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, Z.; Meng, Y.; Zhao, Y.; Han, J.; Su, G.; Chen, B.; Dai, J. Biomaterials The Enhancement of Cancer Stem Cell Properties of MCF-7 Cells in 3D Collagen Scaffolds for Modeling of Cancer and Anti-Cancer Drugs. Biomaterials 2012, 33, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Husmann, A.; Hume, R.D.; Watson, C.J.; Cameron, R.E. Biomaterials Development of Three-Dimensional Collagen Scaffolds with Controlled Architecture for Cell Migration Studies Using Breast Cancer Cell Lines. Biomaterials 2017, 114, 34–43. [Google Scholar] [CrossRef]
- Liverani, C.; De Vita, A.; Minardi, S.; Kang, Y.; Mercatali, L.; Bongiovanni, A.; La Manna, F.; Ibrahim, T.; Tasciotti, E. Open a Biomimetic 3D Model of Hypoxia-Driven Cancer Progression. Sci. Rep. 2019, 9, 12263. [Google Scholar] [CrossRef]
- Liverani, C.; Mercatali, L.; Cristofolini, L.; Giordano, E.; Minardi, S.; Della Porta, G.; De Vita, A.; Miserocchi, G.; Spadazzi, C.; Tasciotti, E.; et al. Investigating the Mechanobiology of Cancer Cell–ECM Interaction Through Collagen-Based 3D Scaffolds. Cell. Mol. Bioeng. 2017, 10, 223–234. [Google Scholar] [CrossRef]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic Interplay between the Collagen Scaffold and Tumor Evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.M.; Andreadis, J.D.; Shaffer, K.M.; Ma, W.; Pancrazio, J.J.; Stenger, D.A. Immobilization of Neural Cells in Three-Dimensional Matrices for Biosensor Applications. Biosens. Bioelectron. 2000, 14, 871–881. [Google Scholar] [CrossRef]
- Cross, V.L.; Zheng, Y.; Won Choi, N.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense Type I Collagen Matrices That Support Cellular Remodeling and Microfabrication for Studies of Tumor Angiogenesis and Vasculogenesis in Vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef] [PubMed]
- Suggs, L.J.; Mikos, A.G. Development of Poly(Propylene Fumarate-Co-Ethylene Glycol) as an Injectable Carrier for Endothelial Cells. Cell Transplant. 1999, 8, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Model, D.; Cancer, B.; Subia, B.; Dey, T.; Sharma, S.; Kundu, S.C. Target Specific Delivery of Anticancer Drug in Silk Fibroin Based 3D Target Specific Delivery of Anticancer Drug in Silk Fibroin Based 3D Distribution Model of Bone−Breast Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 2269–2279. [Google Scholar] [CrossRef]
- Talukdar, S.; Kundu, S.C. A Non-Mulberry Silk Fibroin Protein Based 3D In Vitro Tumor Model for Evaluation of Anticancer Drug Activity. Adv. Funct. Mater. 2012, 22, 4778–4788. [Google Scholar] [CrossRef]
- Talukdar, S.; Mandal, M.; Hutmacher, D.W.; Russell, P.J.; Soekmadji, C.; Kundu, S.C. Biomaterials Engineered Silk Fi Broin Protein 3D Matrices for in vitro Tumor Model. Biomaterials 2011, 32, 2149–2159. [Google Scholar] [CrossRef]
- Gurski, L.A.; Jha, A.K.; Zhang, C.; Jia, X.; Farach-Carson, M.C. Hyaluronic Acid-Based Hydrogels as 3D Matrices for in Vitro Evaluation of Chemotherapeutic Drugs Using Poorly Adherent Prostate Cancer Cells. Biomaterials 2009, 30, 6076–6085. [Google Scholar] [CrossRef]
- Wang, Y.; Mirza, S.; Wu, S.; Zeng, J.; Shi, W. 3D Hydrogel Breast Cancer Models for Studying the Effects of Hypoxia on Epithelial to Mesenchymal Transition. Oncotarget 2018, 9, 32191–32203. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Zhang, Y.; Suo, A.; Cui, N.; Wang, J.; Yao, Y.; Wang, H. Acta Biomaterialia A Double-Network Poly (Ne-Acryloyl L-Lysine)/Hyaluronic Acid Hydrogel as a Mimic of the Breast Tumor Microenvironment. Acta Biomater. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Fisher, S.A.; Anandakumaran, P.N.; Owen, S.C.; Shoichet, M.S. Tuning the Microenvironment: Click-Crosslinked Hyaluronic Acid-Based Hydrogels Provide a Platform for Studying Breast Cancer Cell Invasion. Adv. Funct. Mater. 2015, 25, 7163–7172. [Google Scholar] [CrossRef]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A New Cell-Laden 3D Alginate-Matrigel Hydrogel Resembles Human Breast Cancer Cell Malignant Morphology, Spread and Invasion Capability Observed “ in vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef]
- Qiao, S.; Zhao, Y.; Li, C.; Yin, Y.; Meng, Q.; Lin, F.; Liu, Y.; Hou, X.; Guo, K.; Chen, X.; et al. Acta Biomaterialia An Alginate-Based Platform for Cancer Stem Cell Research. Acta Biomater. 2016, 37, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-lopez, J.G.; Flores-torres, S.; Grant, J.; De Leon-rodriguez, A.; Kinsella, J.M. Directing the Self-Assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef] [PubMed]
- Charmsaz, S.; Hughes, É.; Bane, F.T.; Tibbitts, P.; Mcilroy, M.; Byrne, C.; Cocchiglia, S.; Mcbryan, J.; Hennessy, B.T.; Dwyer, R.M.; et al. S100 β as a Serum Marker in Endocrine Resistant Breast Cancer. BMC Med. 2017, 15, 79. [Google Scholar] [CrossRef]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple Uses of Basement Membrane-like Matrix (BME/Matrigel) In Vitro and In Vivo with Cancer Cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Bissell, M.J. Extracellular Matrix Control of Mammary Gland Morphogenesis and Tumorigenesis: Insights from Imaging. Histochem. Cell Biol. 2008, 130, 1105–1118. [Google Scholar] [CrossRef]
- Raub, C.B.; Unruh, J.; Suresh, V.; Krasieva, T.; Lindmo, T.; Gratton, E.; Tromberg, B.J.; George, S.C. Image Correlation Spectroscopy of Multiphoton Images Correlates with Collagen Mechanical Properties. Biophys. J. 2008, 94, 2361–2373. [Google Scholar] [CrossRef]
- Fridman, R.; Giaccone, G.; Kanemoto, T.; Martin, G.R.; Gazdar, A.F.; Mulshine, J.L. Reconstituted Basement Membrane (Matrigel) and Laminin Can Enhance the Tumorigenicity and the Drug Resistance of Small Cell Lung Cancer Cell Lines. Proc. Natl. Acad. Sci. 1990, 87, 6698–6702. [Google Scholar] [CrossRef]
- Xu, S.; Jia, Z.-F.; Kang, C.; Huang, Q.; Wang, G.; Liu, X.; Zhou, X.; Xu, P.; Pu, P. Upregulation of SEPT7 Gene Inhibits Invasion of Human Glioma Cells. Cancer Investig. 2010, 28, 248–258. [Google Scholar] [CrossRef]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The Endothelial Cell Tube Formation Assay on Basement Membrane Turns 20: State of the Science and the Art. Angiogenesis 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Manuscript, A. Modular Extracellular Matrices: Solutions for the Puzzle. Methods 2009, 45, 93–98. [Google Scholar]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric Scaffolds in Tissue Engineering: A Literature Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Jiang, H.L.; Hoshiba, T.; Akaike, T.; Cho, C.S. Application of Recombinant Fusion Proteins for Tissue Engineering. Ann. Biomed. Eng. 2010, 38, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Kundu, S.C. Acta Biomaterialia Silk Protein-Based Hydrogels: Promising Advanced Materials for Biomedical Applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Conklin, M.W.; Keely, P.J. Why the stroma matters in breast cancer. Cell Adhes. Migr. 2012, 6, 249–260. [Google Scholar] [CrossRef]
- Acerbi Cassereau, I.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integrative Biology 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Han, S.S. 3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering. Polymers 2016, 8, 19. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a Double-Edged Sword in Tumor Progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens-Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2009, 339, 247. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Ramtani, S.; Helary, C. Mechanical Behavior under Unconfined Compression Loading of Dense Fibrillar Collagen Matrices Mimetic Living Tissues Compression Loadings of Dense. J. Mech. Med. Biol. 2010, 10, 35–55. [Google Scholar] [CrossRef]
- Tierney, C.M.; Haugh, M.G.; Liedl, J.; Mulcahy, F.; Hayes, B.; O’Brien, F.J. The Effects of Collagen Concentration and Crosslink Density on the Biological, Structural and Mechanical Properties of Collagen-GAG Scaffolds for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2009, 2, 202–209. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Martin, R.; Farjanel, J.; Eichenberger, D.; Colige, A.; Kessler, E.; Hulmes, D.J.S.; Giraud-Guille, M.M. Liquid Crystalline Ordering of Procollagen as a Determinant of Three-Dimensional Extracellular Matrix Architecture. J. Mol. Biol. 2000, 301, 11–17. [Google Scholar] [CrossRef]
- Achilli, M.; Mantovani, D. Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of PH, Temperature and Ionic Strength on Gelation. Polymers 2010, 2, 664–680. [Google Scholar] [CrossRef]
- Hyllested, J.L.; Veje, K.; Ostergaard, K. Histochemical Studies of the Extracellular Matrix of Human Articular Cartilage—A Review. Osteoarthr. Cartil. 2002, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.F.; Jenkinson, A.; Pohl, K.; O’Brien, F.J.; Morgan, M.P. Osteomimicry of Mammary Adenocarcinoma Cells In Vitro; Increased Expression of Bone Matrix Proteins and Proliferation within a 3D Collagen Environment. PLoS ONE 2012, 7, e41679. [Google Scholar] [CrossRef] [PubMed]
- James-Bhasin, M.; Siegel, P.M.; Nazhat, S.N. A Three-Dimensional Dense Collagen Hydrogel to Model Cancer Cell/Osteoblast Interactions. J. Funct. Biomater. 2018, 9, 72. [Google Scholar] [CrossRef]
- Tom, E.; Jonathan, B. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Folkman, J.; Haudenschild, C. Angiogenesis in Vitro. Nature 1980, 288, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Maffini, M.V.; Soto, A.M.; Sonnenschein, C. The Microenvironment Determines the Breast Cancer Cells’ Phenotype: Organization of MCF7 Cells in 3D Cultures. BMC Cancer 2010, 10, 263. [Google Scholar] [CrossRef]
- Millerot-Serrurot, E.; Guilbert, M.; Fourré, N.; Witkowski, W.; Said, G.; Van Gulick, L.; Terryn, C.; Zahm, J.-M.; Garnotel, R.; Jeannesson, P. 3D Collagen Type I Matrix Inhibits the Antimigratory Effect of Doxorubicin. Cancer Cell Int. 2010, 10, 26. [Google Scholar] [CrossRef]
- Patterson, D.M.; Nazarova, L.A.; Prescher, J.A. Finding the Right (Bioorthogonal) Chemistry. ACS Chem. Biol. 2014, 9, 592–605. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Cerwinka, W.H.; Isoyama, T.; Lokeshwar, B.L. HYAL1 Hyaluronidase in Prostate Cancer: A Tumor Promoter and Suppressor. Cancer Res. 2005, 65, 7782–7789. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in Vitro Culture of Neural Lineage Cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of Osteoblasts in Injectable RGD-Modified PEG Hydrogels for Bone Tissue Engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Burdick, J.A.; Mason, M.N.; Hinman, A.D.; Thorne, K.; Anseth, K.S. Delivery of Osteoinductive Growth Factors from Degradable PEG Hydrogels Influences Osteoblast Differentiation and Mineralization. J. Control. Release 2002, 83, 53–63. [Google Scholar] [CrossRef]
- Bryant, S.J.; Durand, K.L.; Anseth, K.S. Manipulations in Hydrogel Chemistry Control Photoencapsulated Chondrocyte Behavior and Their Extracellular Matrix Production. J. Biomed. Mater. Res. Part A 2003, 67, 1430–1436. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. Hydrogel Properties Influence ECM Production by Chondrocytes Photoencapsulated in Poly(Ethylene Glycol) Hydrogels. J. Biomed. Mater. Res. 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Adelöw, C.; Segura, T.; Hubbell, J.A.; Frey, P. The Effect of Enzymatically Degradable Poly(Ethylene Glycol) Hydrogels on Smooth Muscle Cell Phenotype. Biomaterials 2008, 29, 314–326. [Google Scholar] [CrossRef]
- Strutz, F.; Zeisberg, M.; Renziehausen, A.; Raschke, B.; Becker, V.; van Kooten, C.; Müller, G. TGF-Beta 1 Induces Proliferation in Human Renal Fibroblasts via Induction of Basic Fibroblast Growth Factor (FGF-2). Kidney Int. 2001, 59, 579–592. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Hayward, A.S.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Acrylic-Acid-Functionalized PolyHIPE Scaffolds for Use in 3D Cell Culture. Macromol. Rapid Commun. 2013, 34, 1844–1849. [Google Scholar] [CrossRef]
- Brown, T.E.; Anseth, K.S. Spatiotemporal Hydrogel Biomaterials for Regenerative Medicine. Chem. Soc. Rev. 2017, 46, 6532–6552. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Corbett, S.A.; Lowry, S.F.; Graham, A.M.; Fässler, R.; Li, S. Integrins Are Required for the Differentiation of Visceral Endoderm. J. Cell Sci. 2009, 122, 233–242. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Smyth, H.D.C. Poly(Ethylene Glycol)-Carboxymethyl Chitosan-Based PH-Responsive Hydrogels: Photo-Induced Synthesis, Characterization, Swelling, and in Vitro Evaluation as Potential Drug Carriers. Carbohydr. Res. 2010, 345, 2004–2012. [Google Scholar] [CrossRef]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Acta Biomaterialia Collagen–Hyaluronic Acid Scaffolds for Adipose Tissue Engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Pluen, A.; Boucher, Y.; Ramanujan, S.; McKee, T.D.; Gohongi, T.; di Tomaso, E.; Brown, E.B.; Izumi, Y.; Campbell, R.B.; Berk, D.A.; et al. Role of Tumor-Host Interactions in Interstitial Diffusion of Macromolecules: Cranial vs. Subcutaneous Tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 4628–4633. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan Promotes the Malignant Phenotype. Glycobiology 2002, 12, 37R–42R. [Google Scholar] [CrossRef]
- Gallagher, J.T. Heparan Sulfate: Growth Control with a Restricted Sequence Menu. J. Clin. Investig. 2001, 108, 357–361. [Google Scholar] [CrossRef]
- Guimond, S.; Maccarana, M.; Olwin, B.B.; Lindahl, U.; Rapraeger, A.C. Activating and Inhibitory Heparin Sequences for FGF-2 (Basic FGF). Distinct Requirements for FGF-1, FGF-2, and FGF-4. J. Biol. Chem. 1993, 268, 23906–23914. [Google Scholar] [CrossRef]
- Nikitovic, D.; Assouti, M.; Sifaki, M.; Katonis, P.; Krasagakis, K.; Karamanos, N.K.; Tzanakakis, G.N. Chondroitin Sulfate and Heparan Sulfate-Containing Proteoglycans Are Both Partners and Targets of Basic Fibroblast Growth Factor-Mediated Proliferation in Human Metastatic Melanoma Cell Lines. Int. J. Biochem. Cell Biol. 2008, 40, 72–83. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Venning, F.A.; Wullkopf, L.; Erler, J.T. Targeting ECM Disrupts Cancer Progression. Front. Oncol. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Campbell, J.C.; Botos, L.A.; Sargeant, T.J.; Davidenko, N.; Cameron, R.E.; Watson, C.J. A 3-D in vitro co-culture model of mammary gland involution. Integr. Biol (Camb). 2014, 6, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Laboratory for Synthetic Biology, RIKEN Quantitative Biology Center, Department of Systems Pharmacology, UTokyo Graduate School of Medicine. The CUBIC Clearing Protocol with Reagent-1A (for Whole Mouse Brain). 2015. Available online: http://cubic.riken.jp/data/CUBIC_clearing_protocol_with_Reagent-1A.pdf (accessed on 27 October 2021).
- Monteiro, J.; Gaspar, C.; Richer, W.; Franken, P.F.; Sacchetti, A.; Joosten, R.; Idali, A.; Brandao, J.; Decraene, C.; Fodde, R. Cancer Stemness in Wnt-Driven Mammary Tumorigenesis. Carcinogenesis 2014, 35, 2–13. [Google Scholar] [CrossRef]
- Hemmrich, K.; Von Heimburg, D.; Rendchen, R.; Di, C.; Milella, E.; Pallua, N. Implantation of Preadipocyte-Loaded Hyaluronic Acid-Based Scaffolds into Nude Mice to Evaluate Potential for Soft Tissue Engineering. Biomaterials 2005, 26, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.; Prestwich, G.D.; Semple, J.L.; Woodhouse, K.A. Adipose Tissue Engineering in Vivo with Adipose-Derived Stem Cells on Naturally Derived Scaffolds. J. Biomed. Mater. Res. Part A 2009, 89, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.; Prestwich, G.D.; Semple, J.L.; Woodhouse, K.A. Adipose Tissue Engineering with Naturally Derived Scaffolds and Adipose-Derived Stem Cells. Biomaterials 2007, 28, 3834–3842. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, M.; Skurk, T.; De Luca, C.; Von Heimburg, D.; Hauner, H. Tissue Engineering of White Adipose Tissue Using Hyaluronic Acid-Based Scaffolds. I: In Vitro Differentiation of Human Adipocyte Precursor Cells on Scaffolds. Biomaterials 2003, 24, 3125–3132. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Ashworth, J.C.; Cameron, R.E.; Oyen, M.L. Multi-Scale Mechanical Response of Freeze-Dried Collagen Scaffolds for Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2015, 42, 19–25. [Google Scholar] [CrossRef]
- Hume, R.D.; Pensa, S.; Brown, E.J.; Kreuzaler, P.A.; Hitchcock, J.; Husmann, A.; Campbell, J.J.; Lloyd-thomas, A.O.; Cameron, R.E.; Watson, C.J. Tumour Cell Invasiveness and Response to Chemotherapeutics in Adipocyte Invested 3D Engineered Anisotropic Collagen Scaffolds. Sci. Rep. 2018, 1–15. [Google Scholar] [CrossRef]
- Wondraczek, H.; Elschner, T.; Heinze, T. Synthesis of Highly Functionalized Dextran Alkyl Carbonates Showing Nanosphere Formation. Carbohydr. Polym. 2011, 83, 1112–1118. [Google Scholar] [CrossRef]
- Nowakowska, M.; Zapotoczny, S.; Sterzel, M.; Kot, E. Novel Water-Soluble Photosensitizers from Dextrans. Biomacromolecules 2004, 5, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Li, L.; Wu, H.; Tang, Q.; Sun, B.; Dong, H.; Zhe, J.; Cheng, G. Zwitteration of Dextran: A Facile Route to Integrate Antifouling, Switchability and Optical Transparency into Natural Polymers. Chem. Commun. 2014, 50, 3234–3237. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; Lee, S.; Choi, S.W.; Maruyama, A.; Spencer, N.D. A Biomimetic Alternative to Poly(Ethylene Glycol) as an Antifouling Coating: Resistance to Nonspecific Protein Adsorption of Poly(L-Lysine)-Graft-Dextran. Langmuir 2008, 24, 8850–8856. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Strong Collagen Hydrogels by Oxidized Dextran Modification. ACS Sustain. Chem. Eng. 2014, 2, 1318–1324. [Google Scholar] [CrossRef]
- Berillo, D.; Elowsson, L.; Kirsebom, H. Oxidized Dextran as Crosslinker for Chitosan Cryogel Scaffolds and Formation of Polyelectrolyte Complexes between Chitosan and Gelatin. Macromol. Biosci. 2012, 12, 1090–1099. [Google Scholar] [CrossRef]
- Wang, T.; Nie, J.; Yang, D. Dextran and Gelatin Based Photocrosslinkable Tissue Adhesive. Carbohydr. Polym. 2012, 90, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and Oxidised Dextran as Crosslinker Reagents for Chitosan-Based Scaffolds for Cartilage Tissue Engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable Applications of Chitin and Chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Dash, M.; Federica, C.; Ottenbrite, R.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W. Porous Chitosan Scaffolds for Tissue Engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Hornof, M.D.; Kast, C.E.; Bernkop-Schnürch, A. In Vitro Evaluation of the Viscoelastic Properties of Chitosan-Thioglycolic Acid Conjugates. Eur. J. Pharm. Biopharm. 2003, 55, 185–190. [Google Scholar] [CrossRef]
- Loscalzo, D.E.H.R.C.J. NIH Public Access. Bone 2011, 23, 1–7. [Google Scholar] [CrossRef]
- Rabionet, M.; Yeste, M. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymers 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Sundaresh, S.; Chu, B.; Hadjiargyrou, M. Cdk2 Silencing via a DNA/PCL Electrospun Scaffold Suppresses Proliferation and Increases Death of Breast Cancer Cells. PLoS ONE 2012, 7, e52356. [Google Scholar] [CrossRef]
- Sims-Mourtada, J.; Niamat, R.A.; Samuel, S.; Eskridge, C.; Kmiec, E.B. Enrichment of Breast Cancer Stem-like Cells by Growth on Electrospun Polycaprolactone-Chitosan Nanofiber Scaffolds. Int. J. Nanomed. 2014, 9, 995–1003. [Google Scholar] [CrossRef]
- Ampuja, M.; Jokimäki, R.; Juuti-uusitalo, K.; Rodriguez-martinez, A.; Alarmo, E.; Kallioniemi, A. BMP4 Inhibits the Proliferation of Breast Cancer Cells and Induces an MMP-Dependent Migratory Phenotype in MDA-MB-231 Cells in 3D Environment. BMC Cancer 2013, 13, 429. [Google Scholar] [CrossRef]
- Pradhan, S.; Hassani, I.; Seeto, W.J.; Lipke, E.A. PEG-Fibrinogen Hydrogels for Three-Dimensional Breast Cancer Cell Culture. J. Biomed. Mater. Res. Part A 2016, 105, 236–252. [Google Scholar] [CrossRef]
- Chang, F.-C.; Tsao, C.-T.; Lin, A.; Zhang, M.; Levengood, S.L.; Zhang, M. PEG-Chitosan Hydrogel with Tunable Stiffness for Study of Drug Response of Breast Cancer Cells. Polymers 2016, 8, 112. [Google Scholar] [CrossRef]
- Pathi, S.P.; Lin, D.D.W.; Dorvee, J.R.; Estroff, L.A.; Fischbach, C. Biomaterials Hydroxyapatite Nanoparticle-Containing Scaffolds for the Study of Breast Cancer Bone Metastasis. Biomaterials 2011, 32, 5112–5122. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S.; Lee, D.A.; Bader, D.L. Crosslinking Density Influences the Morphology of Chondrocytes Photoencapsulated in PEG Hydrogels during the Application of Compressive Strain. J. Orthop. Res. 2004, 22, 1143–1149. [Google Scholar] [CrossRef]
- Bryant, S.J.; Chowdhury, T.T.; Lee, D.A.; Bader, D.L.; Anseth, K.S. Crosslinking Density Influences Chondrocyte Metabolism in Dynamically Loaded Photocrosslinked Poly(Ethylene Glycol) Hydrogels. Ann. Biomed. Eng. 2004, 32, 407–417. [Google Scholar] [CrossRef]
- Levenberg, S.; Huang, N.F.; Lavik, E.; Rogers, A.B.; Itskovitz-Eldor, J.; Langer, R. Differentiation of Human Embryonic Stem Cells on Three-Dimensional Polymer Scaffolds. Proc. Natl. Acad. Sci. USA 2003, 100, 12741–12746. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacant, J.P.; Langer, R. Laminated Three-Dimensional Biodegradable Foams for Use in Tissue Engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef]
- Ouyang, A.; Ng, R.; Yang, S.-T. Long-Term Culturing of Undifferentiated Embryonic Stem Cells in Conditioned Media and Three-Dimensional Fibrous Matrices Without Extracellular Matrix Coating. Stem Cells 2007, 25, 447–454. [Google Scholar] [CrossRef]
- Girard, Y.K.; Wang, C.; Ravi, S.; Howell, M.C.; Mallela, J.; Alibrahim, M.; Green, R.; Hellermann, G.; Mohapatra, S.S.; Mohapatra, S. A 3D Fibrous Scaffold Inducing Tumoroids: A Platform for Anticancer Drug Development. PLoS ONE 2013, 8, e75345. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and Their Applications in Tissue Engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Glass-Brudzinski, J.; Perizzolo, D.; Brunette, D.M. Effects of Substratum Surface Topography on the Organization of Cells and Collagen Fibers in Collagen Gel Cultures. J. Biomed. Mater. Res. 2002, 61, 608–618. [Google Scholar] [CrossRef]
- Karuri, N.W.; Liliensiek, S.; Teixeira, A.I.; Abrams, G.; Campbell, S.; Nealey, P.F.; Murphy, C.J. Biological Length Scale Topography Enhances Cell-Substratum Adhesion of Human Corneal Epithelial Cells. J. Cell Sci. 2004, 117, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J. Cellular Response to Low Adhesion Nanotopographies. Int. J. Nanomed. 2007, 2, 373–381. [Google Scholar]
- Maltman, D.J.; Przyborski, S.A. Developments in Three-Dimensional Cell Culture Technology Aimed at Improving the Accuracy of in Vitro Analyses. Biochem. Soc. Trans. 2010, 38, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Murray, B.; Carnachan, R.; Przyborski, S. Alvetex®: Polystyrene Scaffold Technology for Routine Three Dimensional Cell Culture. Methods Mol. Biol. 2011, 695, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Hayman, M.W.; Smith, K.H.; Cameron, N.R.; Przyborski, S.A. Growth of Human Stem Cell-Derived Neurons on Solid Three-Dimensional Polymers. J. Biochem. Biophys. Methods 2005, 62, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hayman, M.W.; Smith, K.H.; Cameron, N.R.; Przyborski, S.A. Enhanced Neurite Outgrowth by Human Neurons Grown on Solid Three-Dimensional Scaffolds. Biochem. Biophys. Res. Commun. 2004, 314, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Alayoubi, A.; Alqahtani, S.; Kaddoumi, A.; Nazzal, S. Effect of PEG Surface Conformation on Anticancer Activity and Blood Circulation of Nanoemulsions Loaded with Tocotrienol-Rich Fraction of Palm Oil. AAPS J. 2013, 15, 1168–1179. [Google Scholar] [CrossRef]
- Han, S.; Nie, K.; Li, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. 3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications. Stem Cells Int. 2021, 2021, 8790143. [Google Scholar] [CrossRef]
- Sancho, A.; Vazquez, L.; De Juan-Pardo, E. Effect of Cold Storage on Collagen-Based Hydrogels for the Three-Dimensional Culture of Adipose-Derived Stem Cells. Biofabrication 2014, 6, 35017. [Google Scholar] [CrossRef]
- Ghaedi, M.; Soleimani, M.; Shabani, I.; Duan, Y.; Lotfi, A. Hepatic Differentiation from Human Mesenchymal Stem Cells on a Novel Nanofiber Scaffold. Cell. Mol. Biol. Lett. 2012, 17, 89–106. [Google Scholar] [CrossRef]
- Puppi, D.; Zhang, X.; Yang, L.; Chiellini, F.; Sun, X.; Chiellini, E. Nano/Microfibrous Polymeric Constructs Loaded with Bioactive Agents and Designed for Tissue Engineering Applications: A Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1562–1579. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk Fibroin Biomaterials for Tissue Regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The Effect of Pore Size on Cell Adhesion in Collagen-GAG Scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Zhu, W.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D Printed Nanocomposite Matrix for the Study of Breast Cancer Bone Metastasis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 69–79. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Zou, J.; Jia, C.; Hu, Y.; Du, H.; Wang, H. Evaluation of Photodynamic Therapy Efficiency Using an In Vitro Three-Dimensional Microfluidic Breast Cancer Tissue Model. Lab Chip 2014, 15, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; DeJesus, J.; Short, A.R.; Otero, J.J.; Sarkar, A.; Winter, J.O. Glioblastoma Behaviors in Three-Dimensional Collagen-Hyaluronan Composite Hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 9276–9284. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Maaser, K.; Klein, C.E.; Niggemann, B.; Krohne, G.; Zã, K.S. Migration of Highly Aggressive MV3 Melanoma Cells in 3-Dimensional Collagen Lattices Results in Local Matrix Reorganization and Shedding of A2 and 131Integrins and CD441. Cancer Res. 1997, 57, 2061–2070. [Google Scholar] [PubMed]
- Nyga, A.; Loizidou, M.; Emberton, M.; Cheema, U. A Novel Tissue Engineered Three-Dimensional in Vitro Colorectal Cancer Model. Acta Biomater. 2013, 9, 7917–7926. [Google Scholar] [CrossRef]
- Heywood, H.K.; Sembi, P.K.; Lee, D.A.; Bader, D.L. Cellular Utilization Determines Viability and Matrix Distribution Profiles in Chondrocyte-Seeded Alginate Constructs. Tissue Eng. 2004, 10, 1467–1479. [Google Scholar] [CrossRef]
- Jongpaiboonkit, L.; King, W.J.; Lyons, G.E.; Paguirigan, A.L.; Warrick, J.W.; Beebe, D.J.; Murphy, W.L. An Adaptable Hydrogel Array Format for 3-Dimensional Cell Culture and Analysis. Biomaterials 2008, 29, 3346–3356. [Google Scholar] [CrossRef]
- Gutowska, A.; Bae, Y.H.; Feijen, J.; Kim, S.W. Heparin Release from Thermosensitive Hydrogels. J. Control. Release 1992, 22, 95–104. [Google Scholar] [CrossRef]
- Ferreira, L.; Vidal, M.M.; Gil, M.H. Evaluation of Poly(2-Hydroxyethyl Methacrylate) Gels as Drug Delivery Systems at Different PH Values. Int. J. Pharm. 2000, 194, 169–180. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Staniforth, J.N. An Electrically Modulated Drug Delivery Device: I. Pharm. Res. 1991, 8, 913–918. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Chiesa, R.; Cigada, A.; Ginebra, M.P.; Leali, P.T.; Lewis, A.L.; Lloyd, A.W.; Merolli, A.; Montufar, E.B.; Pérez, R.A. Biomimetic, bioresponsive, and bioactive materials: An Introduction to Integrating Materials with Tissues; Santin, M., Phillips, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 9780470056714. [Google Scholar]
- Léon, Y. León, C.A. New Perspectives in Mercury Porosimetry. Adv. Colloid Interface Sci. 1998, 76–77, 341–372. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Brauker, J.H.; Carr-Brendel, V.E.; Martinson, L.A.; Crudele, J.; Johnston, W.D.; Johnson, R.C. Neovascularization of Synthetic Membranes Directed by Membrane Microarchitecture. J. Biomed. Mater. Res. 1995, 29, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, J.J.; Hulbert, S.F. Application of Porous Ceramics for the Attachment of Load Bearing Internal Orthopedic Applications. J. Biomed. Mater. Res. 1971, 5, 161–229. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Matsiko, A.; Dickson, G.R.; Brien, F.J.O.; Gleeson, J.P. Acta Biomaterialia A Biomimetic Multi-Layered Collagen-Based Scaffold for Osteochondral Repair. Acta Biomater. 2014, 10, 1996–2004. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; Mckiernan, R.C.; Altenbuchner, C.; Brien, F.J.O. Cell Attachment, Proliferation, and Migration Within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Curtin, C.M.; Cunniffe, G.M.; Lyons, F.G.; Bessho, K.; Dickson, G.R.; Duffy, G.P.; Brien, F.J.O. Innovative Collagen Nano-Hydroxyapatite Scaffolds Offer a Highly Efficient Non-Viral Gene Delivery Platform for Stem Cell-Mediated Bone Formation. Adv. Mater. 2012, 24, 749–754. [Google Scholar] [CrossRef]
- Ueda, H.; Hong, L.; Yamamoto, M.; Shigeno, K.; Inoue, M.; Toba, T.; Yoshitani, M.; Nakamura, T.; Tabata, Y.; Shimizu, Y. Use of Collagen Sponge Incorporating Transforming Growth Factor-Β1 to Promote Bone Repair in Skull Defects in Rabbits. Biomaterials 2002, 23, 1003–1010. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. Understanding Anisotropy and Architecture in Ice-Templated Biopolymer Scaffolds. Mater. Sci. Eng. C 2014, 37, 141–147. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel Freeze-Drying Methods to Produce a Range of Collagen–Glycosaminoglycan Scaffolds with Tailored Mean Pore Sizes. Tissue Eng. Part C Methods 2009, 16, 887–894. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of Freezing Rate on Pore Structure in Freeze-Dried Collagen-GAG Scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Szot, C.S.; Buchanan, C.F.; Gatenholm, P.; Nichole, M.; Freeman, J.W. Investigation of Cancer Cell Behavior on Nano Fi Brous Scaffolds. Mater. Sci. Eng. C 2011, 31, 37–42. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of Porous Carbon Nanofibers with Adjustable Pore Sizes as Electrodes for Supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the Pore Sizes of Bone-Mimetic Electrospun Scaffolds Comprised of Polycaprolactone, Collagen I and Hydroxyapatite to Enhance Cell Infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.; Dean, D.R.; Jun, H. Biomaterials Cell in Fi Ltration and Growth in a Low Density, Uncompressed Three-Dimensional Electrospun Nano Fi Brous Scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Feng, Y.; Huang, Z.-M.; Ramakrishna, S.; Lim, C.T. Fabrication of Porous Electrospun Nanofibres. Nanotechnology 2006, 17, 901–908. [Google Scholar] [CrossRef]
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and Nanofibrous Hyaluronic Acid/Collagen Hybrid Scaffold Fabricated by Concurrent Electrospinning and Deposition/Leaching of Salt Particles. Acta Biomater. 2008, 4, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun Nanofibrous Structure: A Novel Scaffold for Tissue Engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Chen, Z.G.; Wang, P.W.; Wei, B.; Mo, X.M.; Cui, F.Z. Electrospun Collagen–Chitosan Nanofiber: A Biomimetic Extracellular Matrix for Endothelial Cell and Smooth Muscle Cell. Acta Biomater. 2010, 6, 372–382. [Google Scholar] [CrossRef]
- Aras, O.; Kazanci, M. Production of Collagen Micro- and Nanofibers for Potential Drug-Carrier Systems. J. Enzym. Inhib. Med. Chem. 2015, 30, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, A.; Gualandi, C.; Panseri, S.; Montesi, M.; Marcacci, M.; Focarete, M.L.; Bigi, A. Comparative Performance of Collagen Nanofibers Electrospun from Different Solvents and Stabilized by Different Crosslinkers. J. Mater. Sci. Mater. Med. 2014, 25, 2313–2321. [Google Scholar] [CrossRef]
- Dong, B.; Arnoult, O.; Smith, M.E.; Wnek, G.E. Electrospinning of Collagen Nanofiber Scaffolds from Benign Solvents. Macromol. Rapid Commun. 2009, 30, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y.L. An Aligned Nanofibrous Collagen Scaffold by Electrospinning and Its Effects on in Vitro Fibroblast Culture. J. Biomed. Mater. Res. Part A 2006, 79, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hartman, O.; Zhang, C.; Adams, E.L.; Farach-Carson, M.C.; Petrelli, N.J.; Chase, B.D.; Rabolt, J.F. Microfabricated Electrospun Collagen Membranes for 3-D Cancer Models and Drug Screening Applications. Biomacromolecules 2009, 10, 2019–2032. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Creff, J.; Courson, R.; Mangeat, T.; Foncy, J.; Souleille, S.; Thibault, C.; Besson, A.; Malaquin, L. Fabrication of 3D Scaffolds Reproducing Intestinal Epithelium Topography by High-Resolution 3D Stereolithography. Biomaterials 2019, 221, 119404. [Google Scholar] [CrossRef]
- Lee, S.-J.; Nowicki, M.; Harris, B.; Zhang, L.G. Fabrication of a Highly Aligned Neural Scaffold via a Table Top Stereolithography 3D Printing and Electrospinning. Tissue Eng. Part A 2016, 23, 491–502. [Google Scholar] [CrossRef]
- Accardo, A.; Courson, R.; Riesco, R.; Raimbault, V.; Malaquin, L. Direct Laser Fabrication of Meso-Scale 2D and 3D Architectures with Micrometric Feature Resolution. Addit. Manuf. 2018, 22, 440–446. [Google Scholar] [CrossRef]
- Wang, Y.; Gunasekara, D.B.; Reed, M.I.; DiSalvo, M.; Bultman, S.J.; Sims, C.E.; Magness, S.T.; Allbritton, N.L. A Microengineered Collagen Scaffold for Generating a Polarized Crypt-Villus Architecture of Human Small Intestinal Epithelium. Biomaterials 2017, 128, 44–55. [Google Scholar] [CrossRef]
- Yu, J.; Peng, S.; Luo, D.; March, J.C. In Vitro 3D Human Small Intestinal Villous Model for Drug Permeability Determination. Biotechnol. Bioeng. 2012, 109, 2173–2178. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nagrath, S. Microfluidics and Cancer: Are We There Yet? Biomed. Microdevices 2013, 15, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Goh, W.H.; Hashimoto, M.; Miura, S.; Onoe, H. ECM-Based Stretchable Microfluidic System for in Vitro 3D Tissue Culture. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 752–755. [Google Scholar]
- Hutmacher, D.W.; Loessner, D.; Rizzi, S.; Kaplan, D.L.; Mooney, D.J.; Clements, J.A. Can Tissue Engineering Concepts Advance Tumor Biology Research? Trends Biotechnol. 2010, 28, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, C.M.; Bissell, M.J. Tumor Engineering: The Other Face of Tissue Engineering. Tissue Eng. Part A 2010, 16, 2153–2156. [Google Scholar] [CrossRef]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on Chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef]
- Pisano, M.; Triacca, V.; Barbee, K.A.; Swartz, M.A. An in Vitro Model of the Tumor-Lymphatic Microenvironment with Simultaneous Transendothelial and Luminal Flows Reveals Mechanisms of Flow Enhanced Invasion. Integr. Biol. 2015, 7, 525–533. [Google Scholar] [CrossRef]
- Jeon, J.S.; Bersini, S.; Gilardi, M.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Human 3D Vascularized Organotypic Microfluidic Assays to Study Breast Cancer Cell Extravasation. Proc. Natl. Acad. Sci. USA 2015, 112, 214–219. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.; Liu, H.; Lin, S.; Jiang, Y. Drug Cytotoxicity and Signaling Pathway Analysis with Three-Dimensional Tumor Spheroids in a Microwell-Based Microfluidic Chip for Drug Screening. Anal. Chim. Acta 2015, 898, 85–92. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Fluri, D.A.; Marchan, R.; Boonen, K.; Mohanty, S.; Singh, P.; Hammad, S.; Landuyt, B.; Hengstler, J.G.; Kelm, J.M.; et al. 3D Spherical Microtissues and Microfluidic Technology for Multi-Tissue Experiments and Analysis. J. Biotechnol. 2015, 205, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ruppen, J.; Wildhaber, F.D.; Strub, C.; Hall, S.R.R.; Schmid, R.A.; Geiser, T.; Guenat, O.T. Towards Personalized Medicine: Chemosensitivity Assays of Patient Lung Cancer Cell Spheroids in a Perfused Microfluidic Platform. Lab Chip 2015, 15, 3076–3085. [Google Scholar] [CrossRef] [PubMed]
- McMillan, K.S.; Boyd, M.; Zagnoni, M. Transitioning from Multi-Phase to Single-Phase Microfluidics for Long-Term Culture and Treatment of Multicellular Spheroids. Lab Chip 2016, 16, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.-Y.; Tseng, S.-Y.; Yang, S.-M.; Hsu, L.; Liu, C.-H.; Chang, H.-Y. A Microfluidic Chip with a U-Shaped Microstructure Array for Multicellular Spheroid Formation, Culturing and Analysis. Biofabrication 2014, 6, 15009. [Google Scholar] [CrossRef]
- Patra, B.; Peng, C.-C.; Liao, W.-H.; Lee, C.-H.; Tung, Y.-C. Drug Testing and Flow Cytometry Analysis on a Large Number of Uniform Sized Tumor Spheroids Using a Microfluidic Device. Sci. Rep. 2016, 6, 21061. [Google Scholar] [CrossRef]
- Ramanujan, S.; Pluen, A.; McKee, T.D.; Brown, E.B.; Boucher, Y.; Jain, R.K. Diffusion and Convection in Collagen Gels: Implications for Transport in the Tumor Interstitium. Biophys. J. 2002, 83, 1650–1660. [Google Scholar] [CrossRef]
- Lintz, M.; Muñoz, A.; Reinhart-King, C.A. The Mechanics of Single Cell and Collective Migration of Tumor Cells. J. Biomech. Eng. 2017, 139, 210051–210059. [Google Scholar] [CrossRef]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal Design and Fabrication of Scaffolds to Mimic Tissue Properties and Satisfy Biological Constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen Reorganization at the Tumor-Stromal Interface Facilitates Local Invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Brabrand, A.; Kariuki, I.I.; Engstrøm, M.J.; Haugen, O.A.; Dyrnes, L.A.; Åsvold, B.O.; Lilledahl, M.B.; Bofin, A.M. Alterations in Collagen Fibre Patterns in Breast Cancer. A Premise for Tumour Invasiveness? APMIS 2015, 123, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-carreras, I.; Frise, E.; Kaynig, V.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; Tinevez, J.; et al. Fiji—An Open Source Platform for Biological Image Analysis. Nat. Methods 2019, 9, 10–38. [Google Scholar] [CrossRef] [PubMed]
- Hilliou, F.; Pairault, J.; Dominice, J.; Redziniak, G. Growth and Differentiation of 3T3-F442A Preadipocytes in Three-Dimensional Gels of Native Collagen. Exp. Cell Res. 1988, 177, 372–381. [Google Scholar] [CrossRef]
- Huss, F.R.M.; Kratz, G. Mammary Epithelial Cell and Adipocyte Co-Culture in a 3-D Matrix: The First Step towards Tissue-Engineered Human Breast Tissue. Cells Tissues Organs 2001, 169, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.S.; Ayres, C.E.; Bowman, J.R.; Telemeco, T.A.; Sell, S.A.; Bowlin, G.L.; Simpson, D.G. Electrospun Collagen: A Tissue Engineering Scaffold with Unique Functional Properties in a Wide Variety of Applications. J. Nanomater. 2011, 2011, 348268. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable Electrospun Fibers for Drug Delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Chaurey, V.; Chiang, P.-C.; Polanco, C.; Su, Y.-H.; Chou, C.-F.; Swami, N.S. Interplay of Electrical Forces for Alignment of Sub-100 Nm Electrospun Nanofibers on Insulator Gap Collectors. Langmuir 2010, 26, 19022–19026. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and Process Relationship of Electrospun Bioabsorbable Nanofiber Membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of Humidity and Solution Viscosity on Electrospun Fiber Morphology. Tissue Eng. Part C: Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of Polymer Concentration on the Morphology and Mechanical Characteristics of Electrospun Cellulose Acetate and Poly (Vinyl Chloride) Nanofiber Mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the Pore Size of Electrospun Scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.J.; Beckman, M.J.; Bowlin, G.L.; Wayne, J.S. Mechanical Properties and Cellular Proliferation of Electrospun Collagen Type II. Tissue Eng. 2004, 10, 1510–1517. [Google Scholar] [CrossRef]
- Malakpour-Permlid, A.; Buzzi, I.; Hegardt, C.; Johansson, F.; Oredsson, S. Identification of Extracellular Matrix Proteins Secreted by Human Dermal Fibroblasts Cultured in 3D Electrospun Scaffolds. Sci. Rep. 2021, 11, 6655. [Google Scholar] [CrossRef]
- Yamada, D.; Kobayashi, S.; Wada, H.; Kawamoto, K.; Marubashi, S.; Eguchi, H.; Ishii, H.; Nagano, H.; Doki, Y.; Mori, M. Role of Crosstalk between Interleukin-6 and Transforming Growth Factor-Beta 1 in Epithelial–Mesenchymal Transition and Chemoresistance in Biliary Tract Cancer. Eur. J. Cancer 2013, 49, 1725–1740. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a Glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Lee, M.P.; Cooper, G.J.T.; Hinkley, T.; Gibson, G.M.; Padgett, M.J.; Cronin, L. Development of a 3D Printer Using Scanning Projection Stereolithography. Sci. Rep. 2015, 5, 9875. [Google Scholar] [CrossRef]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the Formation, Use and Understanding of Multi-Cellular Spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef]
- Duguay, D.; Foty, R.A.; Steinberg, M.S. Cadherin-Mediated Cell Adhesion and Tissue Segregation: Qualitative and Quantitative Determinants. Dev. Biol. 2003, 253, 309–323. [Google Scholar] [CrossRef]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A.; Moscona, H. The Dissociation and Aggregation of Cells from Organ Rudiments of the Early Chick Embryo. J. Anat. 1952, 86, 287–301. [Google Scholar] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Advanced Cell Culture Techniques for Cancer Drug Discovery. Biology 2014, 3, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Seo, H.I.; Chung, B.G.; Lee, S.-H. Concave Microwell Array-Mediated Three-Dimensional Tumor Model for Screening Anticancer Drug-Loaded Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Zwierzina, M.; Gamerith, G.; Bitsche, M.; Huber, J.M.; Vogel, G.F.; Blumer, M.; Koeck, S.; Pechriggl, E.J.; Kelm, J.M.; et al. Development of an Innovative 3D Cell Culture System to Study Tumour-Stroma Interactions in Non-Small Cell Lung Cancer Cells. PLoS ONE 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Three-Dimensional Cell Cultures: From Molecular Mechanisms to Clinical Applications. Am. J. Physiol. Physiol. 1997, 273, C1109–C1123. [Google Scholar] [CrossRef]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Three-Dimensional Chitosan Scaffold-Based MCF-7 Cell Culture for the Determination of the Cytotoxicity of Tamoxifen. Biomaterials 2005, 26, 979–986. [Google Scholar] [CrossRef]
- Ong, S.-M.; Zhao, Z.; Arooz, T.; Zhao, D.; Zhang, S.; Du, T.; Wasser, M.; van Noort, D.; Yu, H. Engineering a Scaffold-Free 3D Tumor Model for in Vitro Drug Penetration Studies. Biomaterials 2010, 31, 1180–1190. [Google Scholar] [CrossRef]
- Godugu, C.; Patel, A.R.; Desai, U.; Andey, T.; Sams, A.; Singh, M. AlgiMatrixTM Based 3D Cell Culture System as an In-Vitro Tumor Model for Anticancer Studies. PLoS One 2013, 8, e53708. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, J. A Study of the Mechanics of Gastrulation. J. Exp. Zool. 1944, 95, 171–212. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life Is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Neaves, W.B. Normal Stem Cells and Cancer Stem Cells: The Niche Matters. Cancer Res. 2006, 66, 4553–4557. [Google Scholar] [CrossRef]
- Murphy, K.C.; Hoch, A.I.; Harvestine, J.N.; Zhou, D.; Leach, J.K. Mesenchymal Stem Cell Spheroids Retain Osteogenic Phenotype Through A2β1 Signaling. Stem Cells Transl. Med. 2016, 5, 1229–1237. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Costa, E.C.; Gaspar, V.M.; Coutinho, P.; Correia, I.J. Optimization of Liquid Overlay Technique to Formulate Heterogenic 3D Co-Cultures Models. Biotechnol. Bioeng. 2014, 111, 1672–1685. [Google Scholar] [CrossRef]
- Metzger, W.; Sossong, D.; Bächle, A.; Pütz, N.; Wennemuth, G.; Pohlemann, T.; Oberringer, M. The Liquid Overlay Technique Is the Key to Formation of Co-Culture Spheroids Consisting of Primary Osteoblasts, Fibroblasts and Endothelial Cells. Cytotherapy 2011, 13, 1000–1012. [Google Scholar] [CrossRef]
- Wang, X.; Zhen, X.; Wang, J.; Zhang, J.; Wu, W.; Jiang, X. Doxorubicin Delivery to 3D Multicellular Spheroids and Tumors Based on Boronic Acid-Rich Chitosan Nanoparticles. Biomaterials 2013, 34, 4667–4679. [Google Scholar] [CrossRef]
- Gunay, G.; Kirit, H.A.; Kamatar, A.; Baghdasaryan, O.; Hamsici, S.; Acar, H. The Effects of Size and Shape of the Ovarian Cancer Spheroids on the Drug Resistance and Migration. Gynecol. Oncol. 2020, 159, 563–572. [Google Scholar] [CrossRef]
- Torisawa, Y.; Takagi, A.; Nashimoto, Y.; Yasukawa, T.; Shiku, H.; Matsue, T. A Multicellular Spheroid Array to Realize Spheroid Formation, Culture, and Viability Assay on a Chip. Biomaterials 2007, 28, 559–566. [Google Scholar] [CrossRef]
- Sarisozen, C.; Dhokai, S.; Tsikudo, E.G.; Luther, E.; Rachman, I.M.; Torchilin, V.P. Nanomedicine Based Curcumin and Doxorubicin Combination Treatment of Glioblastoma with ScFv-Targeted Micelles: In Vitro Evaluation on 2D and 3D Tumor Models. Eur. J. Pharm. Biopharm. 2016, 108, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Patel, N.R.; Torchilin, V.P. Accumulation and Toxicity of Antibody-Targeted Doxorubicin-Loaded PEG-PE Micelles in Ovarian Cancer Cell Spheroid Model. J. Control. Release 2012, 164, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the Cancer Microenvironment. ACS Biomater. Sci. Eng. 2016, 2, 1710–1721. [Google Scholar] [CrossRef]
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. JoVE 2011, 51, e2720. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, V.; Jernström, S.; Fey, V.; Mpindi, J.-P.; Kleivi Sahlberg, K.; Kallioniemi, O.; Perälä, M. High-Throughput 3D Screening Reveals Differences in Drug Sensitivities between Culture Models of JIMT1 Breast Cancer Cells. PLoS One 2013, 8, e77232. [Google Scholar] [CrossRef]
- Fukuda, J.; Nakazawa, K. Orderly Arrangement of Hepatocyte Spheroids on a Microfabricated Chip. Tissue Eng. 2005, 11, 1254–1262. [Google Scholar] [CrossRef]
- Desroches, B.R.; Zhang, P.; Choi, B.-R.; King, M.E.; Maldonado, A.E.; Li, W.; Rago, A.; Liu, G.; Nath, N.; Hartmann, K.M.; et al. Functional Scaffold-Free 3-D Cardiac Microtissues: A Novel Model for the Investigation of Heart Cells. Am. J. Physiol. Circ. Physiol. 2012, 302, H2031–H2042. [Google Scholar] [CrossRef]
- Ekert, J.E.; Johnson, K.; Strake, B.; Pardinas, J.; Jarantow, S.; Perkinson, R.; Colter, D.C. Three-Dimensional Lung Tumor Microenvironment Modulates Therapeutic Compound Responsiveness In Vitro—Implication for Drug Development. PLoS One 2014, 9, e92248. [Google Scholar] [CrossRef]
- Perets, A.; Baruch, Y.; Weisbuch, F.; Shoshany, G.; Neufeld, G.; Cohen, S. Enhancing the Vascularization of Three-Dimensional Porous Alginate Scaffolds by Incorporating Controlled Release Basic Fibroblast Growth Factor Microspheres. J. Biomed. Mater. Res. 2003, 65, 489–497. [Google Scholar] [CrossRef]
- Huang, G.-S.; Dai, L.-G.; Yen, B.L.; Hsu, S. Spheroid Formation of Mesenchymal Stem Cells on Chitosan and Chitosan-Hyaluronan Membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-C.; Wang, S.; Young, T.-H. The Influence of Spheroid Formation of Human Adipose-Derived Stem Cells on Chitosan Films on Stemness and Differentiation Capabilities. Biomaterials 2012, 33, 1748–1758. [Google Scholar] [CrossRef]
- Maritan, S.M.; Lian, E.Y.; Mulligan, L.M. An Efficient and Flexible Cell Aggregation Method for 3D Spheroid Production. JoVE 2017, 121, 55544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, F.; Pei, M. Creation of an in Vitro Microenvironment to Enhance Human Fetal Synovium-Derived Stem Cell Chondrogenesis. Cell Tissue Res. 2011, 345, 357–365. [Google Scholar] [CrossRef]
- Carpenedo, R.L.; Sargent, C.Y.; McDevitt, T.C. Rotary Suspension Culture Enhances the Efficiency, Yield, and Homogeneity of Embryoid Body Differentiation. Stem Cells 2007, 25, 2224–2234. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Ishiguro, T.; Okumura, M.; Iwanaga, T.; Kadosawa, T.; Fujinaga, T. Chondrogenic Differentiation of Bovine Bone Marrow Mesenchymal Stem Cells in Pellet Cultural System. Exp. Hematol. 2004, 32, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Diaz-Romero, J.; Aigner, T.; Heini, P.; Mainil-Varlet, P.; Nesic, D. Micromass Co-Culture of Human Articular Chondrocytes and Human Bone Marrow Mesenchymal Stem Cells to Investigate Stable Neocartilage Tissue Formation in Vitro. Eur. Cells Mater. 2010, 20, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Qihao, Z.; Xigu, C.; Guanghui, C.; Weiwei, Z. Spheroid Formation and Differentiation into Hepatocyte-like Cells of Rat Mesenchymal Stem Cell Induced by Co-Culture with Liver Cells. DNA Cell Biol. 2007, 26, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H. Preparation of Anti-Inflammatory Mesenchymal Stem/Precursor Cells (MSCs) through Sphere Formation Using Hanging-Drop Culture Technique. Curr. Protoc. Stem Cell Biol. 2014, 28, 2B.6.1–2B.6.23. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.; Ho, M.; Takayama, S. High-Throughput 3D Spheroid Culture and Drug Testing Using a 384 Hanging Drop Array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for Generation of Homogeneous Multicellular Tumor Spheroids Applicable to a Wide Variety of Cell Types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Timmins, N.E.; Nielsen, L.K. Generation of Multicellular Tumor Spheroids by the Hanging-Drop Method. Methods Mol. Med. 2007, 140, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of Multicellular Tumor Spheroids with Microwell-Based Agarose Scaffolds for Drug Testing. PLoS ONE 2015, 10, e0130348. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann, J.; Kensah, G.; Kempf, H.; Skvorc, D.; Gawol, A.; Elliott, D.A.; Dräger, G.; Zweigerdt, R.; Martin, U.; Gruh, I. The Use of Agarose Microwells for Scalable Embryoid Body Formation and Cardiac Differentiation of Human and Murine Pluripotent Stem Cells. Biomaterials 2013, 34, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Yuhas, J.M. Liquid-Overlay Culture of Cellular Spheroids. Recent Results Cancer Res. 1984, 95, 1–23. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef]
- Enmon, R.M.J.; O’Connor, K.C.; Lacks, D.J.; Schwartz, D.K.; Dotson, R.S. Dynamics of Spheroid Self-Assembly in Liquid-Overlay Culture of DU 145 Human Prostate Cancer Cells. Biotechnol. Bioeng. 2001, 72, 579–591. [Google Scholar] [CrossRef]
- Landry, J.; Bernier, D.; Ouellet, C.; Goyette, R.; Marceau, N. Spheroidal Aggregate Culture of Rat Liver Cells: Histotypic Reorganization, Biomatrix Deposition, and Maintenance of Functional Activities. J. Cell Biol. 1985, 101, 914–923. [Google Scholar] [CrossRef]
- Castañeda, F.; Kinne, R.K. Short Exposure to Millimolar Concentrations of Ethanol Induces Apoptotic Cell Death in Multicellular HepG2 Spheroids. J. Cancer Res. Clin. Oncol. 2000, 126, 305–310. [Google Scholar] [CrossRef]
- Foty, R.A.; Steinberg, M.S. The Differential Adhesion Hypothesis: A Direct Evaluation. Dev. Biol. 2005, 278, 255–263. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent Advances in Three-Dimensional Multicellular Spheroid Culture for Biomedical Research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Fussenegger, M. Microscale Tissue Engineering Using Gravity-Enforced Cell Assembly. Trends Biotechnol. 2004, 22, 195–202. [Google Scholar] [CrossRef]
- Nyberg, S.L.; Hardin, J.; Amiot, B.; Argikar, U.A.; Remmel, R.P.; Rinaldo, P. Rapid, Large-Scale Formation of Porcine Hepatocyte Spheroids in a Novel Spheroid Reservoir Bioartificial Liver. Liver Transplant. 2005, 11, 901–910. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Friedrich, J.; Ebner, R.; Kunz-Schughart, L.A. Experimental Anti-Tumor Therapy in 3-D: Spheroids—Old Hat or New Challenge? Int. J. Radiat. Biol. 2007, 83, 849–871. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct Isolation of Human Central Nervous System Stem Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef] [PubMed]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and in Vitro Propagation of Tumorigenic Breast Cancer Cells with Stem/Progenitor Cell Properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar] [CrossRef]
- Smart, C.E.; Morrison, B.J.; Saunus, J.M.; Vargas, A.C.; Keith, P.; Reid, L.; Wockner, L.; Askarian-Amiri, M.; Sarkar, D.; Simpson, P.T.; et al. In Vitro Analysis of Breast Cancer Cell Line Tumourspheres and Primary Human Breast Epithelia Mammospheres Demonstrates Inter- and Intrasphere Heterogeneity. PLoS ONE 2013, 8, e64388. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer Therapy. Ex Vivo Culture of Circulating Breast Tumor Cells for Individualized Testing of Drug Susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Kondo, J.; Endo, H.; Okuyama, H.; Ishikawa, O.; Iishi, H.; Tsujii, M.; Ohue, M.; Inoue, M. Retaining Cell-Cell Contact Enables Preparation and Culture of Spheroids Composed of Pure Primary Cancer Cells from Colorectal Cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 6235–6240. [Google Scholar] [CrossRef] [PubMed]
- Rajcevic, U.; Knol, J.C.; Piersma, S.; Bougnaud, S.; Fack, F.; Sundlisaeter, E.; Søndenaa, K.; Myklebust, R.; Pham, T.V.; Niclou, S.P.; et al. Colorectal Cancer Derived Organotypic Spheroids Maintain Essential Tissue Characteristics but Adapt Their Metabolism in Culture. Proteome Sci. 2014, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Young, S.R.; Saar, M.; Santos, J.; Nguyen, H.M.; Vessella, R.L.; Peehl, D.M. Establishment and Serial Passage of Cell Cultures Derived from LuCaP Xenografts. Prostate 2013, 73, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Richon, S.; Massonnet, G.; Guinebretière, J.-M.; Vacher, S.; Laurendeau, I.; Cottu, P.; Marangoni, E.; Nemati, F.; Validire, P.; et al. A Short-Term Colorectal Cancer Sphere Culture as a Relevant Tool for Human Cancer Biology Investigation. Br. J. Cancer 2013, 108, 1720–1731. [Google Scholar] [CrossRef][Green Version]
- Sundlisæter, E.; Wang, J.; Sakariassen, P.Ø.; Marie, M.; Mathisen, J.R.; Karlsen, B.O.; Prestegarden, L.; Skaftnesmo, K.O.; Bjerkvig, R.; Enger, P.Ø. Primary Glioma Spheroids Maintain Tumourogenicity and Essential Phenotypic Traits after Cryopreservation. Neuropathol. Appl. Neurobiol. 2006, 32, 419–427. [Google Scholar] [CrossRef] [PubMed]


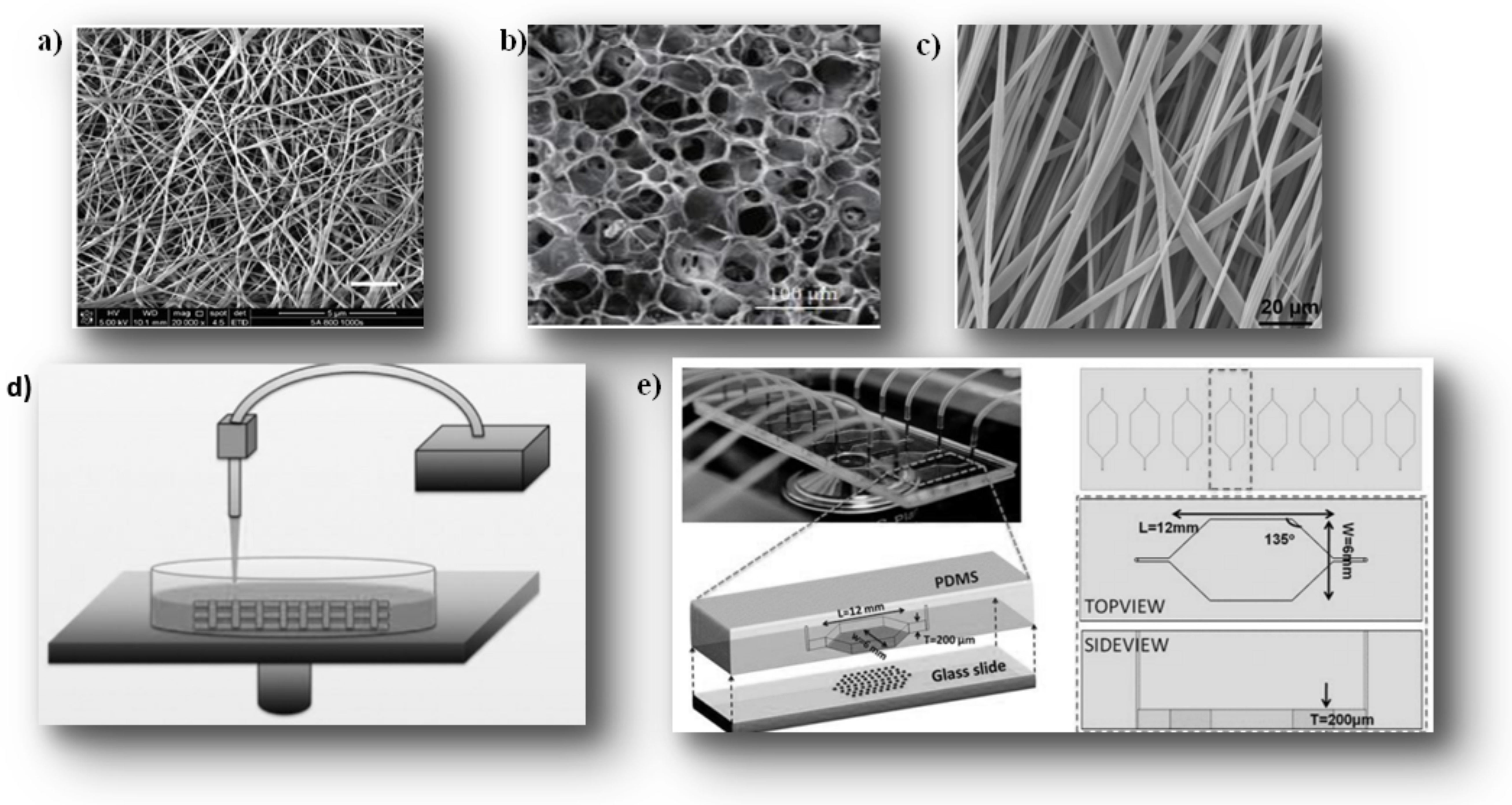
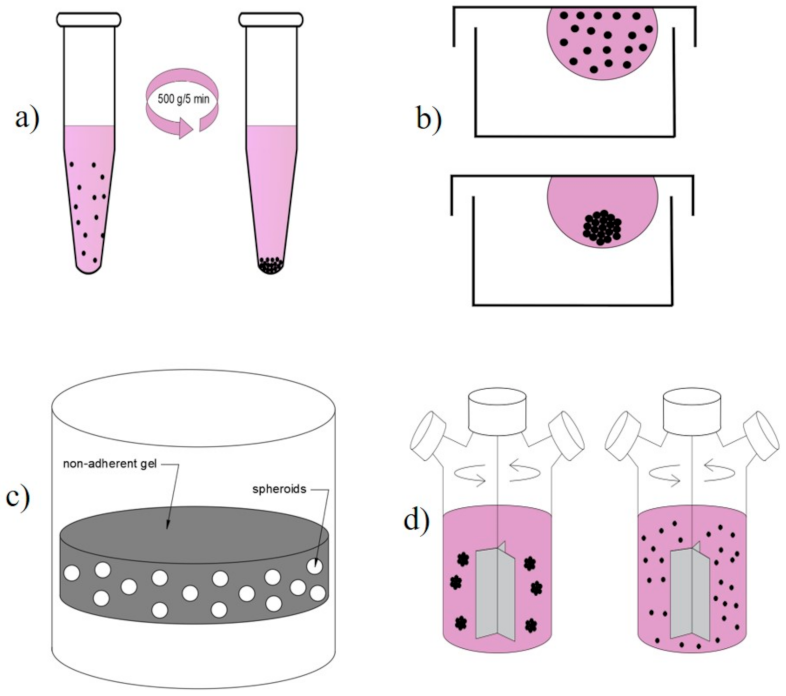
| Characteristic | 2D | 3D | References |
|---|---|---|---|
| Support for cell fixation | Utensils (plastic, polycarbonate) | Extracellular matrix in vitro | [2] |
| Instructions for use | Traditional culture | Imitating the natural microenvironment | [11,12,13,25,26,52] |
| Interaction and communication | Cell-cell (co-culture) | Cell-cell and cell-matrix 3D interactions | [53] |
| Cell forms | Flat and extensible | Natural cellular structure preserved | [23,24,43,45] |
| Media cell interface | Homogeneous exposure of all cells to the media | Heterogeneous exposure (the upper layer is more exposed than the lower layer) | [26] |
| Cell junctions | Less common | More common (cell-cell communication) | [3,10,12,54] |
| Cell differentiation | Moderately and poorly differentiated | Well-differentiated | [43,44] |
| Cell proliferation | Higher proliferation rate than in the natural environment | Medium or high proliferation rate depending on cell type and 3D culture technique | [45,50,51] |
| Treatment sensitivity | Cells more sensitive to treatment | Cells less sensitive to treatment | [46,47] |
| Viability | Sensitive to cytotoxins | High viability and less sensitivity to external factors | [55] |
| Cost | Cheap | Expensive | [7,8] |
| Technique | Protein-Based EMC | Natural Hydrogels | Synthetic Hydrogels | Hard Polymer Scaffold |
|---|---|---|---|---|
| Product description | Matrigel® | Collagen, hyaluronic acid | TrueGel3D (polymers with crosslinkers) | Polystyrene-polycaprolactone Alvetex |
| Biological relevance | Effective +++ | Effective +++ | +/− | +/− |
| Consistency/reproducibility | Low − | High ++ | Very high+++ | Very high +++ |
| Risk of contamination | Low − | High++ | Very high+++ | Very high +++ |
| Modularity/customization | Low − | Moderate + | High ++ | low − |
| Cell recovery | +/− | + | ++ | +++ |
| Downstream analysis (imaging, molecular analysis) | + | ++ | ++ | ++ |
| References | [107,108,109,110] | [111,112,113,114,115] | [116,117,118] | [119,120,121,122,123] |
| Hydrogel | Advantage | Disadvantages |
|---|---|---|
| Matrigel® | -Widely available -Frequently used in cancer research [151,173,174,175,176] | -Unknown and uncontrollable amount growth factors [24] -Lack of control over its exact composition -Variable from batch to batch [178] -Difficulties of handling when it is in a refrigerated liquid state [104] |
| Based on Collagen | -Good adhesion and cell migration support [145,146,147,148,156] -Biocompatibility, mechanical strength, degradability, and limited immunogenicity [157,158] -The most widely used tissue engineering and in tumor culture [11,152,158,199,200,201,202] -Cell signaling patterns [140,141,142,143,144,203] | -Animal origin can potentially transmit pathogens [204] -Biodegradable [159] |
| Hyaluronic acid | -Provide hydration and resistance for cellular mechanisms [33,35,39] -Biodegradable, non-immunogenic, non-inflammatory [205] -Hydrodynamic and swelling [33,35] | -Animal origin can potentially transmit pathogens[204]. -Mechanically poor [38] -Biodegradable [159] |
| Synthetic (PEG), (PCL), (PLA) (PGA) | -Most used in 3D neural culture, bones, cartilaginous, tissue, and kidney tissue [206,207,208,209,210,211,212] -A defined chemical composition and adjustable mechanical properties for cultivation [213,214,215] -Available [119] -Easily modified and formulated with different rigidity only the type of fabric [119] | -Physiologically irrelevant and may release toxic degradation products to cells [216] -Limited applications in in vitro tumor engineering [216] -Contains active chemical groups sensitive to chemical reactions [217] -Irrelevant and may release toxic degradation products to cells [216] -Biophysical parameters (mechanical properties and permeability, stiffness) must be considered [119,206,212] -Loss of cell signaling patterns [203] -Sensitive to pH (PEG) [218] |
| Pure Collagen | Collagen-HA | |
|---|---|---|
| Technique | By lyophilization 1% | By lyophilization 1% |
| Pore size | 100 et 220 μm | 100 et 220 μm |
| Porosity | Similar | Similar |
| Denaturation | Absent | Absent |
| Efficacity | ++ | +++ |
| Resistance of dissolution | + | ++ |
| Dissolution hydrolyte | 19.2% in 7 days | 11.4% to 13.3% in 7 days |
| Cellular proliferation | ++ | +++ |
| Fabrication Method | Method Overview | Scaffolding Morphology | Advantages | Disadvantages |
|---|---|---|---|---|
| Hydrogels [11,54,193,279,280,281] | -Collagen gel solution (usually type 1 collagen and acetic acid) mixed on ice and usually neutralized (NaOH) and then gelled -Physical parameters: collagen, pH, the temperature of desired gelling | -Dense gel network of string-like fibers. The thickness of the fiber depends on the manufacturing parameters | -Easy to apply -Matrigel is widely used in cancer research, so many user guides are available -High level of cell viability | -The least porous -Risk of poor distribution of cells and nutrients. -An architecture is more difficult to control, therefore, has less reproducibility of the exact architectures desired -Poor mechanical properties before cross-linking |
| Lyophilization [153,276,292,293,294,295,296,297,298] | -Creation of a homogeneous suspension of collagen with acid (usually acetic acid) at high speed -Heat treatment (controlled or quenched) for the sublimation of ice crystals under vacuum to the defined freezing point before returning to ~0 °C The dried scaffolding must reach room temperature to complete the process | -Interconnected network -Highly porous -A well-defined pore shape and sizes | -Good control of scaffolding architecture -A wide production range in terms of pore sizes and orientation -High porosity levels. -Inexpensive-High level of cell viability | -Problems in the freezing process affect the final scaffolding architecture from one batch to another -Poor mechanical properties before cross-linking |
| Electrospiding [299,300,301,302,303,304,305,306,307,308,309,310,311,312] | -Collagen solubilized (usually HFIP or TFE) and added to the syringe/injection system -A high-voltage electric field is applied, causing the charge of the solution, the eruption of the polymer fiber of the tip of the needle, and the whip of the liquid jet -The solvent evaporates during the process, leaving a network of dried fibers deposited on the collection plate (non-woven or aligned) | -Dense and tight fiber array (chain-shaped) of nanometric or micro size | -Fibrous network that closely resembles native collagen fibers. -Wide range of size/diameter/achievable fiber pattern -High level of reported cell viability | -Use of harmful solvents (collagen scaffolding) -Solvents are expensive -Dense fiber networks can reduce the level of cellular infiltration. |
| Stereolithography [277,313,314,315,316,317,318,319] | -prints layer by layer a UV-curable material in thin sheets -Installation of a multiresolution 3D printer (Dilase 3D, Kloe France) -Each layer is superimposed after drying the next layer -Use of different light sources (visible, UV, IR) capable of polymerizing photosensitive materials. | -Hard layer set (UV) | -Capable of producing scaffolding of size mm to cm -Can be combined with different components to hydrogels or electro spinning (PCL fibers, PCL /gelatin) -high differentiation rates and adhesion -Imitates complex structures in vitro: as villi of the intestine | -Specific equipment -Expensive -Manufactured scaffolding is usually limited to a few tens of microns of resolution |
| Micro fluid [278,320,321,322,323,324,325,326,327,328,329,330,331,332,333] | Support consisting of silicon/elastomer-based devices having microchannels with proportions from 1 to 1000 μm that exploit a small volume of fluids (10-9 to 10-18 L). These fluids are continuous flows of nutrients and therapeutic agents, establish a physiological profile such as that of blood circulation and intravenous injections | -Matrix that has micro channels- which can be either strictly laminar (in parallel layers) or turbulent (parallel and strong numbers) | -Labor-saving -Microenvironment dynamics (fluid flow) -Generate aggregates of different forms Co-culture of several cells -Simulates cell-cell contacts and biological signals controlled by spatial and temporal gradients of soluble biological factors -Study tumor progression, invasion, angiogenesis as well as treatment tests -Low reagent consumption and low cell utilization | -Requiring professional equipment and special design -Complexity. -High cost |
| Advantage [371,372,373,376,377,378,379,380,381] | Disadvantage [359,366,376,377,378,379,380,381] |
|---|---|
| Variable diameter and size Intense work Diffusion gradient depends on the size (oxygen nutrient, paracrine factor) that decreases inwards Self-disassembly is affected by the rate of production and consumption of factors |
| Technical Methods | Means of Application | Mode of Operation | Advantages and Disadvantages | References |
|---|---|---|---|---|
| Pellet Culture | Concentrate the cells at the conical bottom of a tube by centrifugal force (500 g/5 min) | -Remove the supernatants to collect the cell cap -Capus resuspended in a culture medium to form the spheroids -To optimize: the suspension can be incubated on an agitator for one hour before centrifugation | -Maximized cell-to-cell adhesions -Suitable for the differentiation of mesenchymal cells, chondrogenesis, and bone formation -Disadvantage: Shear stress due to centrifugation can damage cells | [359,361,391,392,393,394,395,396] |
| Hanging drop | Use of surface tension and gravitational force to form spheroids in the form of droplets that rely on gravity self-disassembly | -Preparation of a cell suspension at desired density distribution in the wells of a mini-plateau -Placed a lid on the mini-tray, and the entire mini-tray is overturned upside down -The drop remains fixed on the mini-tray on the inverted surface (surface tension) | -Most commonly used -Defined and controlled size of the spheroid (drop volume and suspension density) -Coefficient of variation narrow size distribution from 10 to 15% -Inexpensive equipment -A large amount can be produced-Heterotypic spheroids (’to 384 spheroids in a single network) | [359,361,383,397,398,399,400] |
| The cultivation of molded lozenges | Non-adhesive gel (agarose) usually prepared in molds | -Cells are forced to aggregate by continuous agitation -Can be accelerated by centrifugation | -Removes restrictions on spheroid size -Increases production rate -High centrifugation can disrupt spheroids (function) | [361,401,402] |
| Liquid overlay (static suspension | Materials that do not adhere to cells that inhibit cell attachment, such as agarose (agar) gel or pHEMA | Cell bindings to the support are inhibited; cells spontaneously form spheroids | -Coefficient of variation narrow size distribution from 40% to 60% -Easy to monitor the formation and growth of spheroids in a plate 96 wells -Simple Method -Heterogeneous spheroids in size and shape | [359,361,376,399,403,404,405,406] |
| Spinner Culture | Use of convection force by stirring the bar in centrifugal flask bioreactor containers generated by a magnetic stirring wheel or bar | Add the uniform and well-mixed single-celled suspension with constant continuous stirring | -The spheroid depends on the size of the bioreactor container -Speed must be constant -A high stirring speed affects the spheroids and a slow speed makes the cells sink to the bottom of the container (blocks the spheroids) -Forms heterotypic spheroids -May not be useful for cells with low cohesion (risk of apoptosis) -it is difficult to follow the spheroids during formation | [26,359,361,407,408,409,410,411] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. https://doi.org/10.3390/ijms222212200
Habanjar O, Diab-Assaf M, Caldefie-Chezet F, Delort L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. International Journal of Molecular Sciences. 2021; 22(22):12200. https://doi.org/10.3390/ijms222212200
Chicago/Turabian StyleHabanjar, Ola, Mona Diab-Assaf, Florence Caldefie-Chezet, and Laetitia Delort. 2021. "3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages" International Journal of Molecular Sciences 22, no. 22: 12200. https://doi.org/10.3390/ijms222212200
APA StyleHabanjar, O., Diab-Assaf, M., Caldefie-Chezet, F., & Delort, L. (2021). 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. International Journal of Molecular Sciences, 22(22), 12200. https://doi.org/10.3390/ijms222212200






