The Evolution of Fluoroquinolone Resistance in Salmonella under Exposure to Sub-Inhibitory Concentration of Enrofloxacin
Abstract
:1. Introduction
2. Results
2.1. Resistance Development of S. Enteritidis under Exposure to sub-MICs of ENR In Vitro
2.2. Mutations in the QRDRs of the Mutants with Reduced Susceptibility to ENR
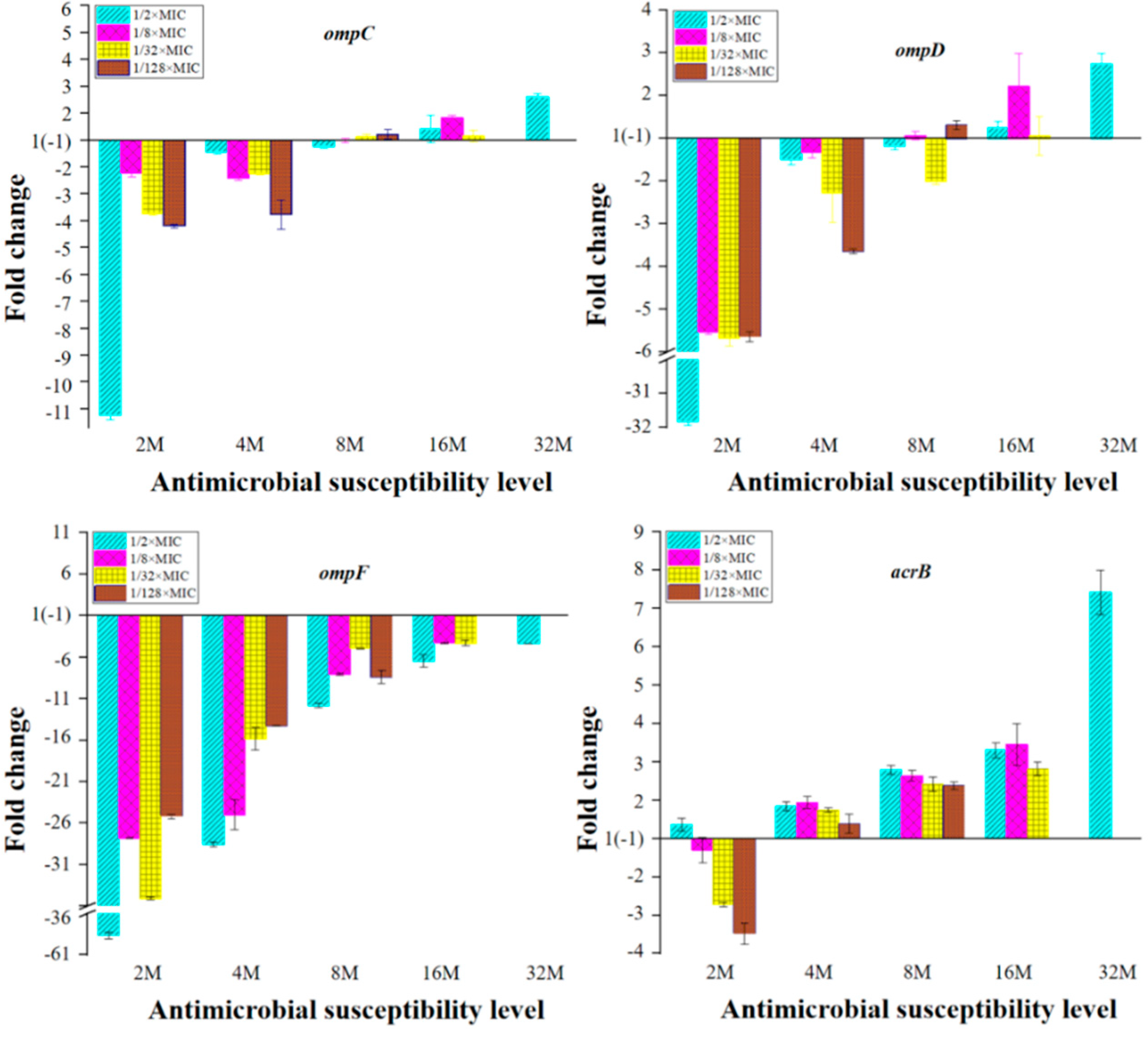
2.3. Expression of OMPs and MDR Efflux Pump Transporters of the Mutants with Reduced Susceptibility to ENR
2.4. Transcriptomic Profiles of S. Enteritidis Mutants Induced by sub-MICs of ENR
3. Discussion
4. Materials and Methods
4.1. Bacteria, Drugs, and Reagents
4.2. Antimicrobial Susceptibility Testing
4.3. In Vitro Selection of Mutants under sub-MICs of ENR
4.4. Sequence Analysis of QRDR Region in gyrA, gyrB, parC, and parE Genes
4.5. Examination of the Expression Levels of OMPs and MDR Efflux Pump Transporters
4.6. RNA Sequencing and Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, M.; Zhao, S.; Pettengill, J.; Luo, Y.; Monday, S.R.; Abbott, J.; Ayers, S.L.; Cinar, H.N.; Tim Muruvanda, T.; Li, C.; et al. Comparative Genomic Analysis and Virulence Differences in Closely Related Salmonella enterica Serotype Heidelberg Isolates from Humans, Retail Meats, and Animals. Genome Biol. Evol. 2014, 6, 1046–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo Fo Wong, D.M.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Mcdermott, P.; Walker, R. Pharmacology of the fluoroquinolones: A perspective for the use in domestic animals. Vet. J. 2006, 172, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agent 2005, 25, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.W.S.; Chu, M.T.; Ling, J.M. Increasing quinolone resistance and multidrug resistant isolates among Salmonella enterica in Hong Kong. J. Infect. 2012, 65, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [Green Version]
- Rushdy, A.A.; Mabrouk, M.I.; Abu-Sef FA, H.; Kheiralla, Z.H.; Abdel-All, S.M.; Saleh, N.M. Contribution of different mechanisms to the resistance to fluoroquinolones in clinical isolates of Salmonella enterica. Braz. J. Infect. Dis. 2013, 17, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, K.; Latifi, T.; Groisman, E.A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2010, 59, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Nagasawa, S.; Fukushima, A.; Hayashi-Nishino, M.; Nishino, K. Cooperation of the multidrug efflux pump and lipopolysaccharides in the intrinsic antibiotic resistance of Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 2013, 68, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. 2021, 99, 21–27. [Google Scholar] [CrossRef]
- Li, L.; Dai, X.; Wang, Y.; Yang, Y.; Zhao, X.; Wang, L.; Zeng, M. RNA-seq-based analysis of drug-resistant Salmonella enterica serovar Typhimurium selected in vivo and in vitro. PLoS ONE 2017, 12, e0175234. [Google Scholar] [CrossRef] [Green Version]
- van der Horst, M.A.; Schuurmans, J.M.; Smid, M.C.; Koenders, B.B.; ter Kuile, B.H. De novo acquisition of resistance to three antibiotics by Escherichia coli. Microb. Drug Resist. 2011, 17, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thi, T.D.; López, E.; Rodríguez-Rojas, A.; Rodríguez-Beltrán, J.; Couce, A.; Guelfo, J.R.; Castañeda-García, A.; Blázquez, J. Effect of recA inactivation on mutagenesis of Escherichia coli exposed to sublethal concentrations of antimicrobials. J. Antimicrob. Chemother. 2011, 66, 531. [Google Scholar] [CrossRef] [Green Version]
- Kohanski, M.A.; Depristo, M.A.; Collins, J.J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balashov, S.; Humayun, M.Z. Mistranslation induced by streptomycin provokes a RecABC/RuvABC-dependent mutator phenotype in Escherichia coli cells. J. Mol. Biol. 2002, 315, 513–527. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Elez, M.; Matic, I.; Blázquez, J. Antibiotic-mediated recombination: Ciprofloxacin stimulates SOS-independent recombination of divergent sequences in Escherichia coli. Mol. Microbiol. 2010, 64, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Beaber, J.W.; Hochhut, B.; Waldor, M.K. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 2004, 427, 72–74. [Google Scholar] [CrossRef]
- Wistrand-Yuen, E.; Knopp, M.; Hjort, K.; Koskiniemi, S.; Berg, O.G.; Andersson, D.I. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 2018, 9, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Z.; Ren, S.Q.; Chang, M.X.; Chen, P.X.; Ding, H.Z.; Jiang, H.X. Resistance mechanisms and fitness of Salmonella Typhimurium and Salmonella Enteritidis mutants evolved under selection with ciprofloxacin in vitro. Sci. Rep. 2017, 7, 9113. [Google Scholar] [CrossRef] [Green Version]
- Dawan, J.; Uddin, M.J.; Ahn, J. Development of de novo resistance in Salmonella Typhimurium treated with antibiotic combinations. FEMS Microbiol. Lett. 2019, 366, fnz127. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.K.; Park, M.S.; Na, H.T. Analysis of the Fluoroquinolone Antibiotic Resistance Mechanism of Salmonella enterica Isolates. J. Microbiol. Biotechnol. 2016, 26, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, T.; Dashti, A.A.; Albaksami, O.; Udo, E.E.; Jadaon, M.M.; Albert, M.J. Ciprofloxacin-resistant Salmonella enterica serovar typhi from Kuwait with novel mutations in gyrA and parC genes. J. Clin. Microbiol. 2009, 47, 208–211. [Google Scholar] [CrossRef] [Green Version]
- O’Regan, E.; Quinn, T.; Pagès, J.M.; McCusker, M.; Piddock, L.; Fanning, S. Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar enteritidis: Involvement of RamA and other global regulators. Antimicrob. Agents Chemother. 2009, 53, 1080–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, H.; Cheng, S.; Liu, G.; Tan, Z.; Dong, C.; Bao, J.; Hong, J.; Jin, D.; Bao, C.; Gu, B. Discovery of seven novel mutations of gyrB, parC and parE in Salmonella Typhi and Paratyphi strains from Jiangsu Province of China. Sci. Rep. 2020, 10, 7359. [Google Scholar] [CrossRef]
- Baucheron, S.; Chaslus-Dancla, E.; Cloeckaert, A.; Chiu, C.H.; Butaye, P. High-Level Resistance to Fluoroquinolones Linked to Mutations in gyrA, parC, and parE in Salmonella enterica Serovar Schwarzengrund Isolates from Humans in Taiwan. Antimicrob. Agents Chemother. 2005, 49, 862–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, S.; Duy, P.T.; Nga, T.V.T.; Dung, T.T.N.; Phat, V.V.; Chau, T.T.; Turner, A.K.; Farrar, J.; Boni, M.F. Fitness benefits in fluoroquinolone-resistant Salmonella Typhi in the absence of antimicrobial pressure. eLife 2013, 2, e01229. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Woo, G.J. Increase of multidrug efflux pump expression in fluoroquinolone-resistant Salmonella mutants induced by ciprofloxacin selective pressure. Res. Vet. Sci. 2014, 97, 182–186. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Sun, X.; Zhao, J.; Wang, J.; Li, J.; Liu, X.; Wang, J.; Li, Y. The mRNA expression of ompF, invA and invE was associated with the ciprofloxacin-resistance in Salmonella. Arch. Microbiol. 2020, 202, 2263–2268. [Google Scholar] [CrossRef]
- Tsukasa, H.; Akihito, Y.; Kunihiko, N. TolC dependency of multidrug efflux systems in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 2010, 65, 1372–1376. [Google Scholar]
- Singh, R.; Swick, M.C.; Ledesma, K.R.; Yang, Z.; Hu, M.; Zechiedrich, L.; Tam, V.H. Temporal Interplay between Efflux Pumps and Target Mutations in Development of Antibiotic Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2012, 56, 1680–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.M.; Lu, P.L.; Li, H.H.; Chang, C.Y.; Chen, T.C.; Chang, L.L. Characterization of Fluoroquinolone Resistance Mechanisms and Their Correlation with the Degree of Resistance to Clinically Used Fluoroquinolones among Escherichia coli Isolates. J. Chemother. 2007, 19, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Chen, P.X.; Yang, L.; Li, W.; Chang, M.X.; Jiang, H.X. Coordinated Expression of acrAB-tolC and Eight Other Functional Efflux Pumps through Activating ramA and marA in Salmonella enterica serovar Typhimurium. Microb. Drug Resist. 2018, 24, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Smith, H.E.; Ricci, V.; Lawler, A.J.; Thompson, L.J.; Piddock, L.J. Expression of homologous RND efflux pump genes is dependent upon AcrB expression: Implications for efflux and virulence inhibitor design. J. Antimicrob. Chemother. 2014, 2, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Nikaido, E.; Nakashima, R.; Sakurai, K.; Fujiwara, D.; Fujii, I.; Nishino, K. The crystal structure of multidrug-resistance regulator RamR with multiple drugs. Nat. Commun. 2013, 4, 2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Switzer, R.L.; Zalkin, H.; Saxild, H.H. Purine, Pyrimidine, and Pyridine Nucleotide Metabolism; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Le Breton, Y.; Mistry, P.; Valdes, K.M.; Quigley, J.; Kumar, N.; Tettelin, H.; McIver, K.S. Genome-wide identification of genes required for fitness of group A Streptococcus in human blood. Infect. Immun. 2013, 81, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Am Jang, H.; Won, Y.J.; Kikuchi, Y.; Han, S.H.; Kim, C.H.; Nikoh, N.; Fukatsu, T.; Lee, B.L. Purine biosynthesis-deficient Burkholderia mutants are incapable of symbiotic accommodation in the stinkbug. ISME J. 2014, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Wang, S.; Huang, L.; Sa, W.; Li, J.; Huang, J.; Dai, M.; Cheng, G. Development of Resistance in Escherichia coli ATCC25922 under Exposure of Sub-Inhibitory Concentration of Olaquindox. Antibiotics 2020, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Du, G.F.; Zheng, Y.D.; Chen, J.; He, Q.Y.; Sun, X. Novel Mechanistic Insights into Bacterial Fluoroquinolone Resistance. J. Proteome Res. 2019, 18, 3955–3966. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Four Informational Supplement. CLSI document M100-S24; CLSI: Wayne, NJ, USA, 2011; Volume 34. [Google Scholar]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Rovira, A.; Davies, P.; Ahlstrom, C.; Muellner, P.; Rendahl, A.; Olsen, K.; Bender, J.B.; Wells, S.; Perez, A.; et al. Serotypes and Antimicrobial Resistance in Salmonella enterica Recovered from Clinical Samples from Cattle and Swine in Minnesota, 2006 to 2015. PLoS ONE 2016, 11, e0168016. [Google Scholar] [CrossRef] [PubMed]







| Strain Number | MIC (μg/mL) | Substitutions in QRDR Amino Acid Residues | |||
|---|---|---|---|---|---|
| gyrA | gyrB | parC | parE | ||
| 2M (1/2M) | 0.125 | wt | wt | wt | wt |
| 2M (1/8M) | 0.125 | Asp87Gly | wt | wt | wt |
| 2M (1/32M) | 0.125 | wt | wt | wt | wt |
| 2M (1/128M) | 0.125 | Ser83Tyr | wt | wt | wt |
| 4M (1/2M) | 0.25 | wt | wt | wt | wt |
| 4M (1/8M) | 0.25 | Ser83Phe | wt | wt | wt |
| 4M (1/32M) | 0.25 | Ser83Tyr | wt | wt | wt |
| 4M (1/128M) | 0.25 | Ser83Tyr | wt | wt | wt |
| 8M (1/2M) | 0.5 | wt | wt | wt | wt |
| 8M (1/8M) | 0.5 | Ser83Phe | wt | wt | wt |
| 8M (1/32M) | 0.5 | Ser83Phe | wt | wt | wt |
| 8M (1/128M) | 0.5 | Asp87Gly | wt | wt | wt |
| 16M (1/2M) | 1 | Ser83Phe | wt | wt | wt |
| 16M (1/8M) | 1 | Ser83Phe | wt | wt | wt |
| 16M (1/32M) | 1 | Ser83Tyr | wt | wt | wt |
| 32M (1/2M) | 2 | Ser83Tyr | wt | wt | wt |
| MCODE | GO | Description | False Discovery Rate |
|---|---|---|---|
| MCODE-1 | GO:0006189 | ‘de novo’ IMP biosynthetic process | 4.08 × e−10 |
| MCODE-1/MCODE-2 | GO:0009168 | Purine ribonucleoside monophosphate biosynthetic process | 4.92 × e−9 |
| MCODE-1/MCODE-2 | GO:0009152 | Purine ribonucleotide biosynthetic process | 1.15 × e−8 |
| MCODE-1/MCODE-2 | GO:0034641 | Cellular nitrogen compound metabolic process | 1.55 × e−8 |
| MCODE-1/MCODE-2 | GO:0044271 | Cellular nitrogen compound biosynthetic process | 1.77 × e−8 |
| Gene Name | Fold Change | Non-Redundant Protein Sequence Description | ||
|---|---|---|---|---|
| C vs. B | D vs. B | E vs. B | ||
| ramA | 14.52 | 6.24 | 47.54 | Transcriptional activator RamA |
| rpoB | 10.30 | 5.45 | 3.48 | Hypothetical protein SARI_03509 |
| fusA | 9.80 | 5.49 | 4.45 | Elongation factor G |
| rpsL | 9.62 | 3.20 | 2.84 | 30S ribosomal protein S12 |
| typA | 6.54 | 2.89 | 3.31 | Ribosome-dependent GTPase TypA |
| lepB | 6.52 | 3.86 | 3.66 | Signal peptidase I |
| acrB | 6.16 | 4.83 | 4.66 | Multidrug efflux RND transporter permease subunit |
| rplU | 5.80 | 2.88 | 5.38 | 50S ribosomal protein L21 |
| gyrA | 4.98 | 3.38 | 2.24 | DNA topoisomerase (ATP-hydrolyzing) subunit A |
| rpoC | 4.75 | 3.78 | 3.94 | DNA-directed RNA polymerase subunit beta |
| ompC | 4.66 | 4.67 | 3.93 | Porin OmpC |
| acrE | 4.52 | 9.16 | 7.65 | Efflux RND transporter periplasmic adaptor subunit |
| rsxE | 3.95 | 2.19 | 2.39 | Electron transport complex subunit E |
| gyrB | 3.91 | 2.26 | 2.12 | DNA gyrase subunit B |
| soxR | 3.58 | −3.45 | −3.57 | Redox-sensitive transcriptional activator SoxR |
| betI | 2.77 | 2.69 | 4.50 | TetR/AcrR family transcriptional regulator |
| acrA | 2.46 | 2.15 | 2.32 | Multidrug efflux RND transporter periplasmic adaptor subunit AcrA |
| acrR | 2.02 | −5.26 | −4.17 | TetR-family transcriptional regulator |
| 1_00145 | −5.56 | −3.85 | −2.50 | Cryptic aminoglycoside N-acetyltransferase AAC(6′)-Iy/Iaa |
| Gene Name | Fold Change | Non-Redundant Protein Sequence Description | ||
|---|---|---|---|---|
| C vs. B | D vs. B | E vs. B | ||
| ompA | 1.75 | 2.40 | 2.08 | Membrane protein |
| ompC | 4.66 | 4.67 | 3.93 | Porin OmpC |
| ompD | 1.17 | 15.41 | 6.80 | Porin OmpD |
| ompF | −2.38 | 1.06 | −3.45 | Porin OmpF |
| mdsC | −1.20 | −1.33 | −1.96 | Multidrug efflux transporter outer membrane subunit MdsC |
| acrA | 2.46 | 2.15 | 2.32 | Multidrug efflux RND transporter Periplasmic adaptor subunit AcrA |
| acrB | 6.16 | 4.83 | 4.66 | Multidrug efflux RND transporter permease subunit |
| tolC | 1.03 | 2.00 | 2.48 | Outer membrane protein TolC |
| acrD | 1.80 | 2.99 | 4.42 | Multidrug efflux RND transporter permease AcrD |
| acrE | 4.52 | 9.16 | 7.65 | Efflux RND transporter periplasmic adaptor subunit |
| acrF | −1.59 | −1.59 | −1.28 | Multidrug efflux RND transporter permease subunit |
| emrA | 1.22 | 1.34 | 1.12 | Multidrug efflux MFS transporter periplasmic adaptor subunit EmrA |
| emrB | 1.97 | 2.68 | 2.60 | Multidrug efflux MFS transporter permease subunit EmrB |
| mdfA | 1.33 | 1.88 | 2.98 | MFS transporter |
| mdtK | −1.49 | −1.19 | 1.35 | Multidrug efflux MATE transporter MdtK |
| mdsA | −1.79 | −2.22 | −2.08 | Multidrug efflux RND transporter periplasmic adaptor subunit MdsA |
| mdsB | −2.00 | −2.08 | −2.44 | Multidrug efflux RND transporter permease subunit MdsB |
| mdtA | 1.19 | −1.37 | 1.13 | Multidrug efflux RND transporter subunit MdtA |
| mdtB | 1.64 | 1.52 | 2.63 | Multidrug efflux RND transporter permease subunit MdtB |
| mdtC | −1.05 | 1.18 | 1.59 | Multidrug efflux RND transporter permease subunit MdtC |
| macA | −1.08 | 1.02 | 1.50 | Macrolide transporter subunit MacA |
| macB | 1.04 | 1.47 | 1.53 | Macrolide ABC transporter ATP-binding protein/permease MacB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Huang, L.; Wu, C.; Huang, J.; Hao, H.; Yuan, Z.; Cheng, G. The Evolution of Fluoroquinolone Resistance in Salmonella under Exposure to Sub-Inhibitory Concentration of Enrofloxacin. Int. J. Mol. Sci. 2021, 22, 12218. https://doi.org/10.3390/ijms222212218
Gu Y, Huang L, Wu C, Huang J, Hao H, Yuan Z, Cheng G. The Evolution of Fluoroquinolone Resistance in Salmonella under Exposure to Sub-Inhibitory Concentration of Enrofloxacin. International Journal of Molecular Sciences. 2021; 22(22):12218. https://doi.org/10.3390/ijms222212218
Chicago/Turabian StyleGu, Yufeng, Lulu Huang, Cuirong Wu, Junhong Huang, Haihong Hao, Zonghui Yuan, and Guyue Cheng. 2021. "The Evolution of Fluoroquinolone Resistance in Salmonella under Exposure to Sub-Inhibitory Concentration of Enrofloxacin" International Journal of Molecular Sciences 22, no. 22: 12218. https://doi.org/10.3390/ijms222212218
APA StyleGu, Y., Huang, L., Wu, C., Huang, J., Hao, H., Yuan, Z., & Cheng, G. (2021). The Evolution of Fluoroquinolone Resistance in Salmonella under Exposure to Sub-Inhibitory Concentration of Enrofloxacin. International Journal of Molecular Sciences, 22(22), 12218. https://doi.org/10.3390/ijms222212218







