The Post-Storage Performance of RBCs from Beta-Thalassemia Trait Donors Is Related to Their Storability Profile
Abstract
:1. Introduction
2. Results
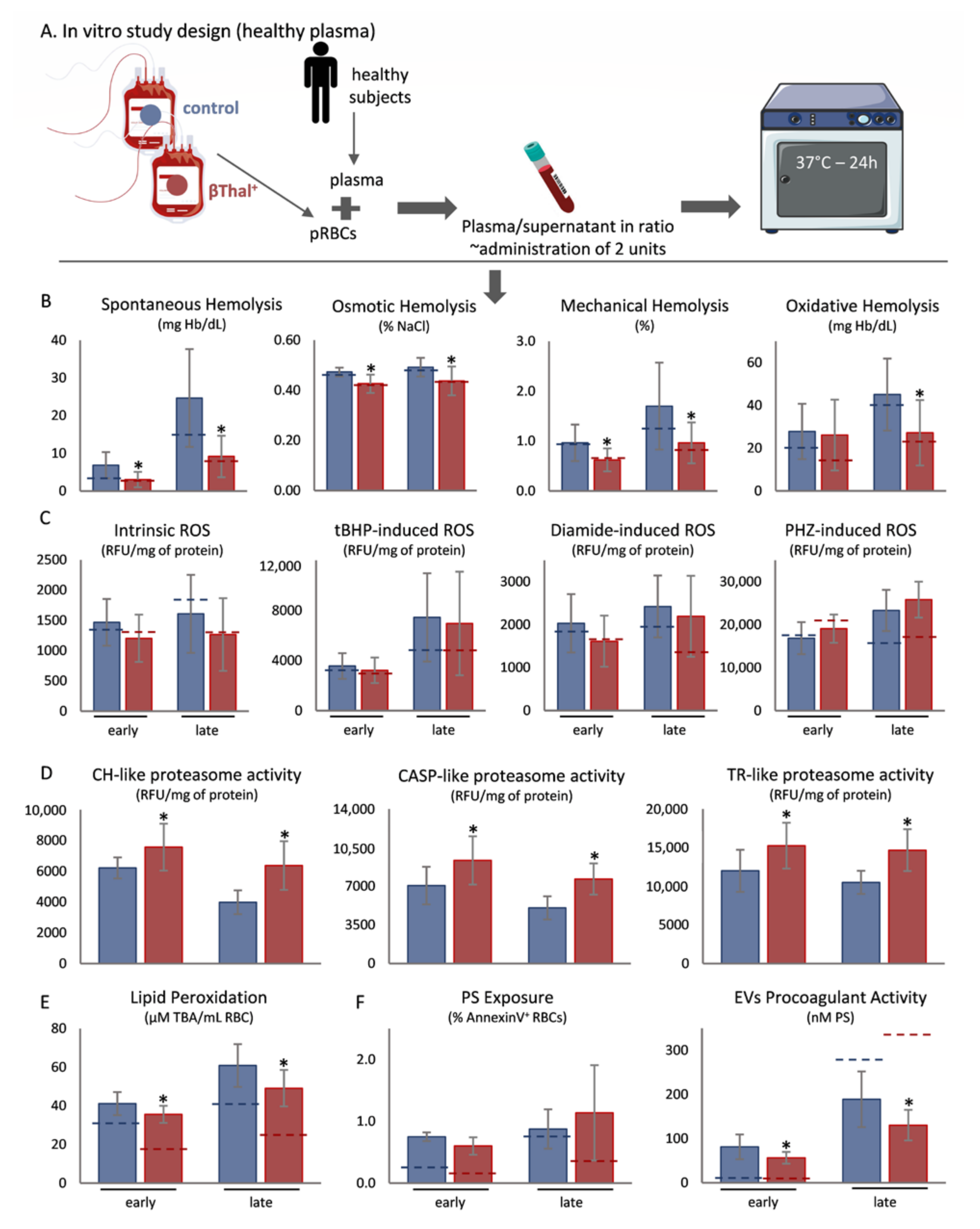
2.1. Exposure of Stored RBCs to Plasma at Body Temperature
2.2. Correlations between Storage and Post-Storage Metrics
2.3. Transfusion to Animal Models
2.4. Correlations of Recovery with Storage Physiology
3. Discussion
3.1. Stored RBC Features in Recipient Plasma and Temperature
3.2. Transfusion to Animal Models
4. Materials and Methods
4.1. Biological Samples and Blood Unit Preparation
4.2. Exposure of Stored RBCs to Human Plasma at Body Temperature
4.3. Animal Model of Transfusion
4.4. Hemolysis Parameters
4.5. Oxidative Stress-Related Parameters
4.6. Proteasome Activity
4.7. Phosphatidylserine Exposure on RBCs and Extracellular Vesicles (EVs)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzounakas, V.L.; Anastasiadi, A.T.; Stefanoni, D.; Cendali, F.; Bertolone, L.; Gamboni, F.; Dzieciatkowska, M.; Rousakis, P.; Vergaki, A.; Soulakis, V.; et al. beta-thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 2021. [Google Scholar] [CrossRef]
- Nuinoon, M.; Kruachan, K.; Sengking, W.; Horpet, D.; Sungyuan, U. Thalassemia and hemoglobin e in southern thai blood donors. Adv. Hematol. 2014, 2014, 932306. [Google Scholar] [CrossRef] [Green Version]
- Haj Khelil, A.; Laradi, S.; Miled, A.; Omar Tadmouri, G.; Ben Chibani, J.; Perrin, P. Clinical and molecular aspects of haemoglobinopathies in Tunisia. Clin. Chim. Acta 2004, 340, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Schrier, S.L.; Rachmilewitz, E.; Mohandas, N. Cellular and membrane properties of alpha and beta thalassemic erythrocytes are different: Implication for differences in clinical manifestations. Blood 1989, 74, 2194–2202. [Google Scholar] [CrossRef] [Green Version]
- Teran, M.M.; Monaco, M.E.; Lazarte, S.S.; Haro, C.; Ledesma Achem, E.; Asensio, N.A.; Isse, B.A. Genetic Regulation of Redox Balance in beta-Thalassemia Trait. Hemoglobin 2020, 44, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Anastasiadi, A.T.; Dzieciatkowska, M.; Karadimas, D.G.; Stamoulis, K.; Papassideri, I.S.; Hansen, K.C.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Proteome of Stored RBC Membrane and Vesicles from Heterozygous Beta Thalassemia Donors. Int. J. Mol. Sci. 2021, 22, 3369. [Google Scholar] [CrossRef] [PubMed]
- Page, G.P.; Kanias, T.; Guo, Y.J.; Lanteri, M.C.; Zhang, X.; Mast, A.E.; Cable, R.G.; Spencer, B.R.; Kiss, J.E.; Fang, F.; et al. Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Tzounakas, V.L.; Arvaniti, V.Z.; Dzieciatkowska, M.; Stamoulis, K.; Lekka, M.E.; Papassideri, I.S.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Red Blood Cell Proteasome in Beta-Thalassemia Trait: Topology of Activity and Networking in Blood Bank Conditions. Membranes 2021, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Roubinian, N.H.; Plimier, C.; Woo, J.P.; Lee, C.; Bruhn, R.; Liu, V.X.; Escobar, G.J.; Kleinman, S.H.; Triulzi, D.J.; Murphy, E.L.; et al. Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood 2019, 134, 1003–1013. [Google Scholar] [CrossRef]
- Kanias, T.; Lanteri, M.C.; Page, G.P.; Guo, Y.; Endres, S.M.; Stone, M.; Keating, S.; Mast, A.E.; Cable, R.G.; Triulzi, D.J.; et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: Results of the REDS-III RBC-Omics study. Blood Adv. 2017, 1, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Tzounakas, V.L.; Anastasiadi, A.T.; Drossos, P.V.; Karadimas, D.G.; Valsami, S.E.; Stamoulis, K.E.; Papassideri, I.S.; Politou, M.; Antonelou, M.H.; Kriebardis, A.G. Sex-related aspects of the red blood cell storage lesion. Blood Transfus. Trasfus. Sangue 2021, 19, 224–236. [Google Scholar] [CrossRef]
- Zeller, M.P.; Rochwerg, B.; Jamula, E.; Li, N.; Hillis, C.; Acker, J.P.; Runciman, R.J.R.; Lane, S.J.; Ahmed, N.; Arnold, D.M.; et al. Sex-mismatched red blood cell transfusions and mortality: A systematic review and meta-analysis. Vox Sang. 2019, 114, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic. Biol. Med. 2016, 96, 152–165. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Data on how several physiological parameters of stored red blood cells are similar in glucose 6-phosphate dehydrogenase deficient and sufficient donors. Data Brief 2016, 8, 618–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, A.; Fu, X.; Kanias, T.; Reisz, J.A.; Culp-Hill, R.; Guo, Y.; Gladwin, M.T.; Page, G.; Kleinman, S.; Lanteri, M.; et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica 2021, 106, 1290–1302. [Google Scholar] [CrossRef] [Green Version]
- Francis, R.O.; D’Alessandro, A.; Eisenberger, A.; Soffing, M.; Yeh, R.; Coronel, E.; Sheikh, A.; Rapido, F.; La Carpia, F.; Reisz, J.A.; et al. Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J. Clin. Investig. 2020, 130, 2270–2285. [Google Scholar] [CrossRef]
- Hazegh, K.; Fang, F.; Bravo, M.D.; Tran, J.Q.; Muench, M.O.; Jackman, R.P.; Roubinian, N.; Bertolone, L.; D’Alessandro, A.; Dumont, L.; et al. Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion 2021, 61, 435–448. [Google Scholar] [CrossRef]
- Bordbar, A.; Johansson, P.I.; Paglia, G.; Harrison, S.J.; Wichuk, K.; Magnusdottir, M.; Valgeirsdottir, S.; Gybel-Brask, M.; Ostrowski, S.R.; Palsson, S.; et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion 2016, 56, 852–862. [Google Scholar] [CrossRef] [Green Version]
- Tzounakas, V.L.; Anastasiadi, A.T.; Valsami, S.I.; Stamoulis, K.E.; Papageorgiou, E.G.; Politou, M.; Papassideri, I.S.; Kriebardis, A.G.; Antonelou, M.H. Osmotic hemolysis is a donor-specific feature of red blood cells under various storage conditions and genetic backgrounds. Transfusion 2021. [Google Scholar] [CrossRef]
- Barshtein, G.; Pries, A.R.; Goldschmidt, N.; Zukerman, A.; Orbach, A.; Zelig, O.; Arbell, D.; Yedgar, S. Deformability of transfused red blood cells is a potent determinant of transfusion-induced change in recipient’s blood flow. Microcirculation 2016, 23, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Kriebardis, A.G.; Seghatchian, J.; Papassideri, I.S.; Antonelou, M.H. Unraveling the Gordian knot: Red blood cell storage lesion and transfusion outcomes. Blood Transfus. Trasfus. Sangue 2017, 15, 126–130. [Google Scholar] [CrossRef]
- Osei-Hwedieh, D.O.; Kanias, T.; Croix, C.S.; Jessup, M.; Xiong, Z.; Sinchar, D.; Franks, J.; Xu, Q.; Novelli, E.M.; Sertorio, J.T.; et al. Sickle Cell Trait Increases Red Blood Cell Storage Hemolysis and Post-Transfusion Clearance in Mice. EBioMedicine 2016, 11, 239–248. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, A.; Hansen, K.C.; Eisenmesser, E.Z.; Zimring, J.C. Protect, repair, destroy or sacrifice: A role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus. Trasfus. Sangue 2019, 17, 281–288. [Google Scholar] [CrossRef]
- Cantu Rajnoldi, A.; Ferrari, M.; Pietri, S.; Travi, M. Glycerol lysis time for screening for beta-thalassaemia trait. Lancet 1980, 2, 638. [Google Scholar] [CrossRef]
- Gunn, R.B.; Silvers, D.N.; Rosse, W.F. Potassium permeability in -thalassemia minor red blood cells. J. Clin. Investig. 1972, 51, 1043–1050. [Google Scholar] [CrossRef]
- Issaian, A.; Hay, A.; Dzieciatkowska, M.; Roberti, D.; Perrotta, S.; Darula, Z.; Redzic, J.; Busch, M.P.; Page, G.P.; Rogers, S.C.; et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica 2021. [Google Scholar] [CrossRef] [PubMed]
- Risinger, M.; Kalfa, T.A. Red cell membrane disorders: Structure meets function. Blood 2020, 136, 1250–1261. [Google Scholar] [CrossRef]
- Orbach, A.; Zelig, O.; Yedgar, S.; Barshtein, G. Biophysical and Biochemical Markers of Red Blood Cell Fragility. Transfus. Med. Hemotherapy Off. Organ Der Dtsch. Ges. Fur Transfus. Immunhamatol. 2017, 44, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barvitenko, N.; Aslam, M.; Lawen, A.; Saldanha, C.; Skverchinskaya, E.; Uras, G.; Manca, A.; Pantaleo, A. Two Motors and One Spring: Hypothetic Roles of Non-Muscle Myosin II and Submembrane Actin-Based Cytoskeleton in Cell Volume Sensing. Int. J. Mol. Sci. 2021, 22, 7967. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Nowak, R.B.; Zhou, S.; Giannetto, M.; Gokhin, D.S.; Papoin, J.; Ghiran, I.C.; Blanc, L.; Wan, J.; Fowler, V.M. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc. Natl. Acad. Sci. USA 2018, 115, E4377–E4385. [Google Scholar] [CrossRef] [Green Version]
- Fujii, J.; Homma, T.; Kobayashi, S.; Warang, P.; Madkaikar, M.; Mukherjee, M.B. Erythrocytes as a preferential target of oxidative stress in blood. Free Radic. Res. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Delobel, J.; Prudent, M.; Tissot, J.D.; Lion, N. Proteomics of the red blood cell carbonylome during blood banking of erythrocyte concentrates. Proteom. Clin. Appl. 2016, 10, 257–266. [Google Scholar] [CrossRef]
- Goodman, S.R.; Kurdia, A.; Ammann, L.; Kakhniashvili, D.; Daescu, O. The human red blood cell proteome and interactome. Exp. Biol. Med. 2007, 232, 1391–1408. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Righetti, P.G.; Zolla, L. The red blood cell proteome and interactome: An update. J. Proteome Res. 2010, 9, 144–163. [Google Scholar] [CrossRef]
- Abi Habib, J.; De Plaen, E.; Stroobant, V.; Zivkovic, D.; Bousquet, M.P.; Guillaume, B.; Wahni, K.; Messens, J.; Busse, A.; Vigneron, N.; et al. Efficiency of the four proteasome subtypes to degrade ubiquitinated or oxidized proteins. Sci. Rep. 2020, 10, 15765. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. Trasfus. Sangue 2019, 17, 27–52. [Google Scholar] [CrossRef]
- de Wolski, K.; Fu, X.; Dumont, L.J.; Roback, J.D.; Waterman, H.; Odem-Davis, K.; Howie, H.L.; Zimring, J.C. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica 2016, 101, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Fortier, N.; Snyder, L.M.; Garver, F.; Kiefer, C.; McKenney, J.; Mohandas, N. The relationship between in vivo generated hemoglobin skeletal protein complex and increased red cell membrane rigidity. Blood 1988, 71, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Stolwijk, J.M.; Stefely, J.A.; Veling, M.T.; van ’t Erve, T.J.; Wagner, B.A.; Raife, T.J.; Buettner, G.R. Red blood cells contain enzymatically active GPx4 whose abundance anticorrelates with hemolysis during blood bank storage. Redox Biol. 2021, 46, 102073. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Karadimas, D.G.; Anastasiadi, A.T.; Georgatzakou, H.T.; Kazepidou, E.; Moschovas, D.; Velentzas, A.D.; Kriebardis, A.G.; Zafeiropoulos, N.E.; Avgeropoulos, A.; et al. Donor-specific individuality of red blood cell performance during storage is partly a function of serum uric acid levels. Transfusion 2018, 58, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Lim, G. Pyridoxine and pyridoxamine inhibits superoxide radicals and prevents lipid peroxidation, protein glycosylation, and (Na+ + K+)-ATPase activity reduction in high glucose-treated human erythrocytes. Free Radic. Biol. Med. 2001, 30, 232–237. [Google Scholar] [CrossRef]
- Paglia, G.; D’Alessandro, A.; Rolfsson, O.; Sigurjonsson, O.E.; Bordbar, A.; Palsson, S.; Nemkov, T.; Hansen, K.C.; Gudmundsson, S.; Palsson, B.O. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016, 128, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Heaton, A.; Keegan, T.; Holme, S. In vivo regeneration of red cell 2,3-diphosphoglycerate following transfusion of DPG-depleted AS-1, AS-3 and CPDA-1 red cells. Br. J. Haematol. 1989, 71, 131–136. [Google Scholar] [CrossRef]
- Dern, R.J.; Brewer, G.J.; Wiorkowski, J.J. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J. Lab. Clin. Med. 1967, 69, 968–978. [Google Scholar]
- Heaton, W.A. Evaluation of posttransfusion recovery and survival of transfused red cells. Transfus. Med. Rev. 1992, 6, 153–169. [Google Scholar] [CrossRef]
- Nakao, K.; Wada, T.; Kamiyama, T.; Nakao, M.; Nagano, K. A direct relationship between adenosine triphosphate-level and in vivo viability of erythrocytes. Nature 1962, 194, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Van’t Erve, T.J.; Wagner, B.A.; Martin, S.M.; Knudson, C.M.; Blendowski, R.; Keaton, M.; Holt, T.; Hess, J.R.; Buettner, G.R.; Ryckman, K.K.; et al. The heritability of hemolysis in stored human red blood cells. Transfusion 2015, 55, 1178–1185. [Google Scholar] [CrossRef] [Green Version]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Song, A.; Yoshida, T.; Dunham, A.; Wither, M.J.; Francis, R.O.; Roach, R.C.; Dzieciatkowska, M.; et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018, 103, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Blessinger, S.A.; Tran, J.Q.; Jackman, R.P.; Gilfanova, R.; Rittenhouse, J.; Gutierrez, A.G.; Heitman, J.W.; Hazegh, K.; Kanias, T.; Muench, M.O. Immunodeficient mice are better for modeling the transfusion of human blood components than wild-type mice. PLoS ONE 2020, 15, e0237106. [Google Scholar] [CrossRef]
- Deplaine, G.; Safeukui, I.; Jeddi, F.; Lacoste, F.; Brousse, V.; Perrot, S.; Biligui, S.; Guillotte, M.; Guitton, C.; Dokmak, S.; et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood 2011, 117, e88–e95. [Google Scholar] [CrossRef]
- Garcia-Herreros, A.; Yeh, Y.-T.; Peng, Z.; del Álamo, J.C. Cyclic mechanical stresses alter erythrocyte membrane composition and microstructure and trigger macrophage phagocytosis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Roussel, C.; Morel, A.; Dussiot, M.; Marin, M.; Colard, M.; Fricot-Monsinjon, A.; Martinez, A.; Chambrion, C.; Henry, B.; Casimir, M.; et al. Rapid clearance of storage-induced microerythrocytes alters transfusion recovery. Blood 2021, 137, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Weiss, L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood 1973, 41, 529–537. [Google Scholar] [CrossRef]
- Hod, E.A.; Zhang, N.; Sokol, S.A.; Wojczyk, B.S.; Francis, R.O.; Ansaldi, D.; Francis, K.P.; Della-Latta, P.; Whittier, S.; Sheth, S.; et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010, 115, 4284–4292. [Google Scholar] [CrossRef] [Green Version]
- Harboe, M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand. J. Clin. Lab. Investig. 1959, 11, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Tzounakas, V.L.; Dzieciatkowska, M.; Anastasiadi, A.T.; Karadimas, D.G.; Vergaki, A.; Siourounis, P.; Stamoulis, K.; Papassideri, I.S.; Kriebardis, A.G.; D’Alessandro, A.; et al. Red cell proteasome modulation by storage, redox metabolism and transfusion. Blood Transfus. Trasfus. Sangue 2020. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Gevi, F.; Georgatzakou, H.T.; Zolla, L.; Papassideri, I.S.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H. Redox Status, Procoagulant Activity, and Metabolome of Fresh Frozen Plasma in Glucose 6-Phosphate Dehydrogenase Deficiency. Front. Med. 2018, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiadi, A.T.; Paronis, E.C.; Arvaniti, V.-Z.; Velentzas, A.D.; Apostolidou, A.C.; Balafas, E.G.; Dzieciatkowska, M.; Kostomitsopoulos, N.G.; Stamoulis, K.; Papassideri, I.S.; et al. The Post-Storage Performance of RBCs from Beta-Thalassemia Trait Donors Is Related to Their Storability Profile. Int. J. Mol. Sci. 2021, 22, 12281. https://doi.org/10.3390/ijms222212281
Anastasiadi AT, Paronis EC, Arvaniti V-Z, Velentzas AD, Apostolidou AC, Balafas EG, Dzieciatkowska M, Kostomitsopoulos NG, Stamoulis K, Papassideri IS, et al. The Post-Storage Performance of RBCs from Beta-Thalassemia Trait Donors Is Related to Their Storability Profile. International Journal of Molecular Sciences. 2021; 22(22):12281. https://doi.org/10.3390/ijms222212281
Chicago/Turabian StyleAnastasiadi, Alkmini T., Efthymios C. Paronis, Vasiliki-Zoi Arvaniti, Athanasios D. Velentzas, Anastasia C. Apostolidou, Evangelos G. Balafas, Monika Dzieciatkowska, Nikolaos G. Kostomitsopoulos, Konstantinos Stamoulis, Issidora S. Papassideri, and et al. 2021. "The Post-Storage Performance of RBCs from Beta-Thalassemia Trait Donors Is Related to Their Storability Profile" International Journal of Molecular Sciences 22, no. 22: 12281. https://doi.org/10.3390/ijms222212281
APA StyleAnastasiadi, A. T., Paronis, E. C., Arvaniti, V.-Z., Velentzas, A. D., Apostolidou, A. C., Balafas, E. G., Dzieciatkowska, M., Kostomitsopoulos, N. G., Stamoulis, K., Papassideri, I. S., D’Alessandro, A., Kriebardis, A. G., Antonelou, M. H., & Tzounakas, V. L. (2021). The Post-Storage Performance of RBCs from Beta-Thalassemia Trait Donors Is Related to Their Storability Profile. International Journal of Molecular Sciences, 22(22), 12281. https://doi.org/10.3390/ijms222212281










