Aesculetin Accelerates Osteoblast Differentiation and Matrix-Vesicle-Mediated Mineralization
Abstract
:1. Introduction
2. Results
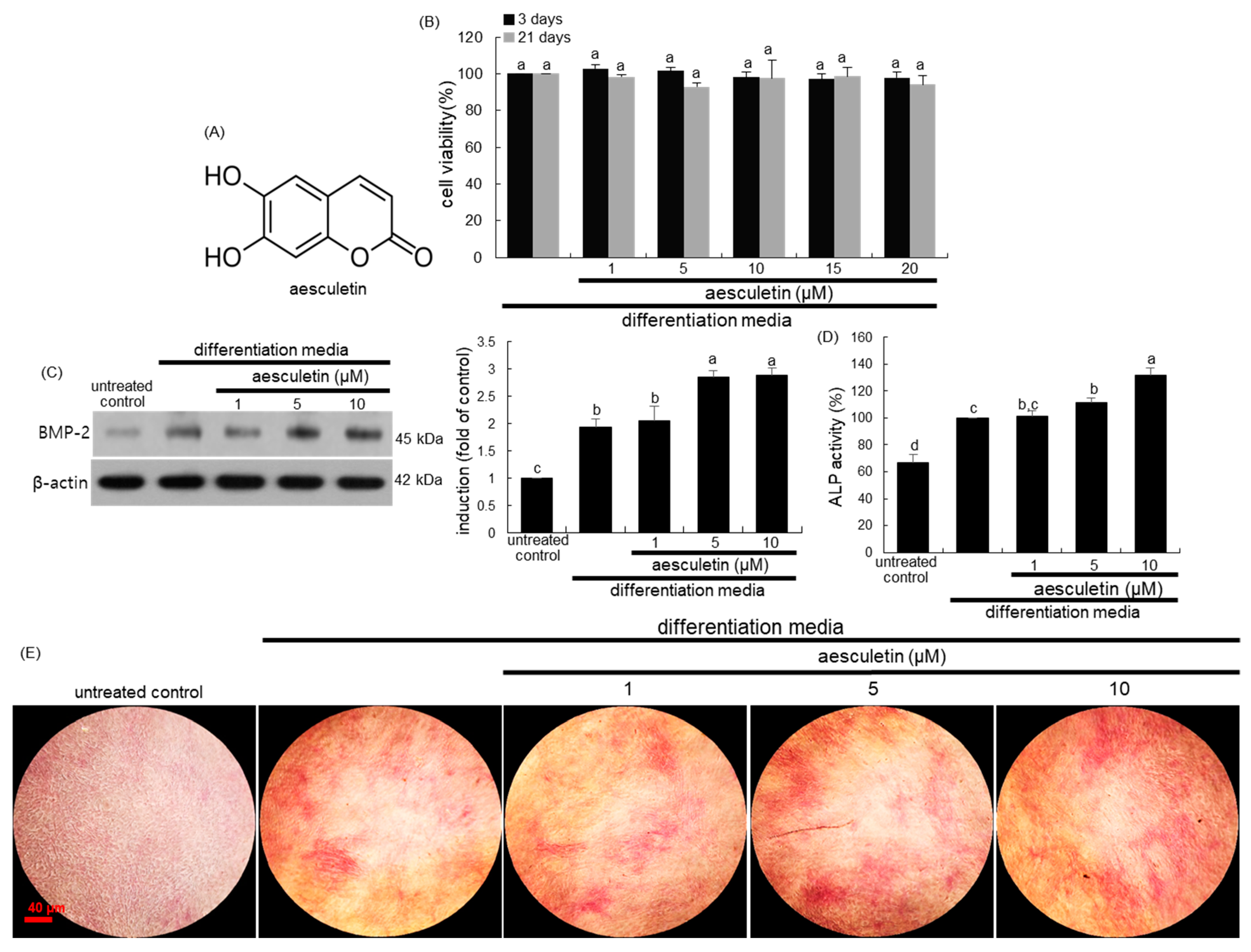
2.1. Initiation of Osteoblastic Differentiation by Aesculetin
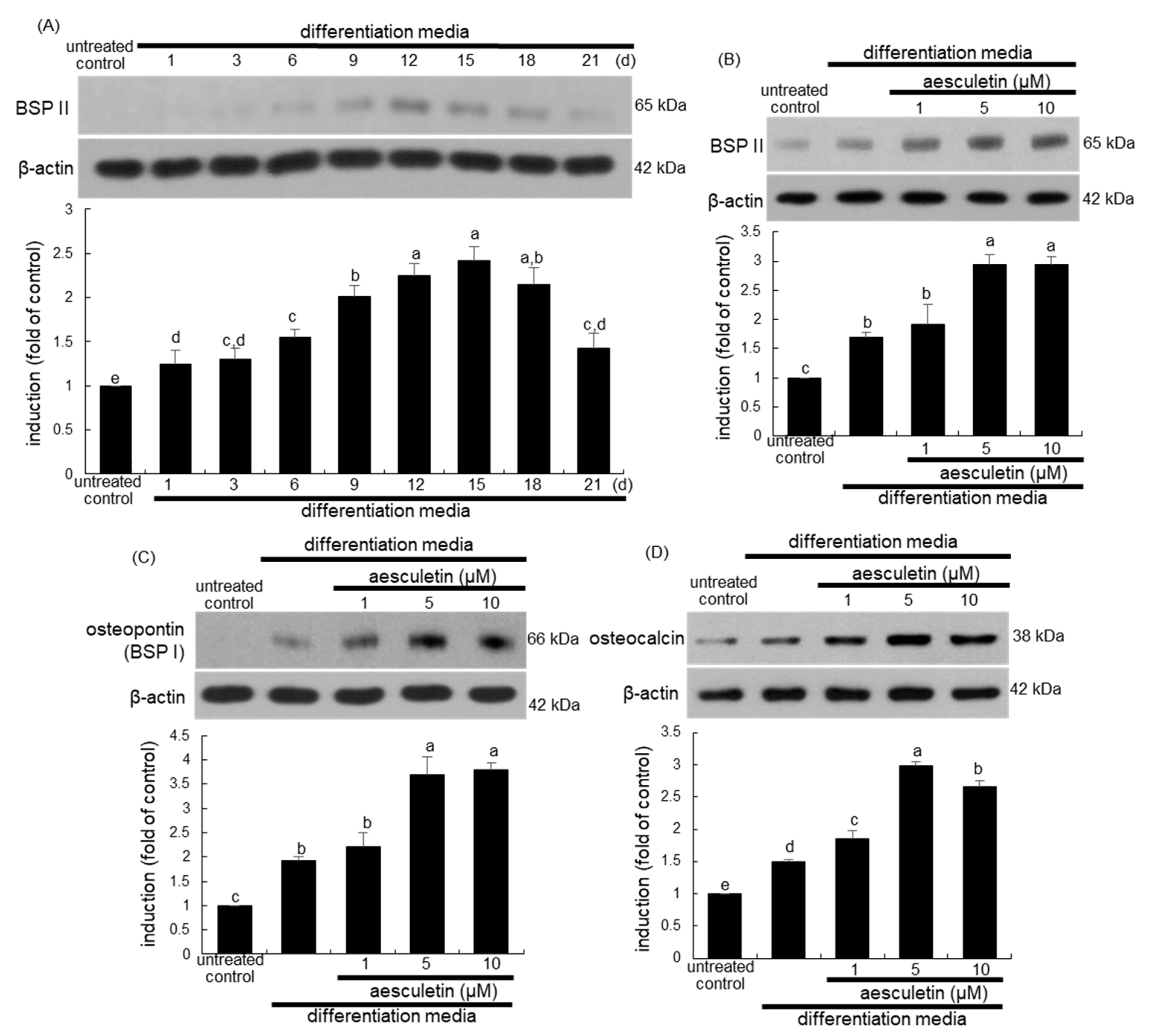
2.2. Induction of Mid-to-Late Stage of Osteoblastic Differentiation by Aesculetin
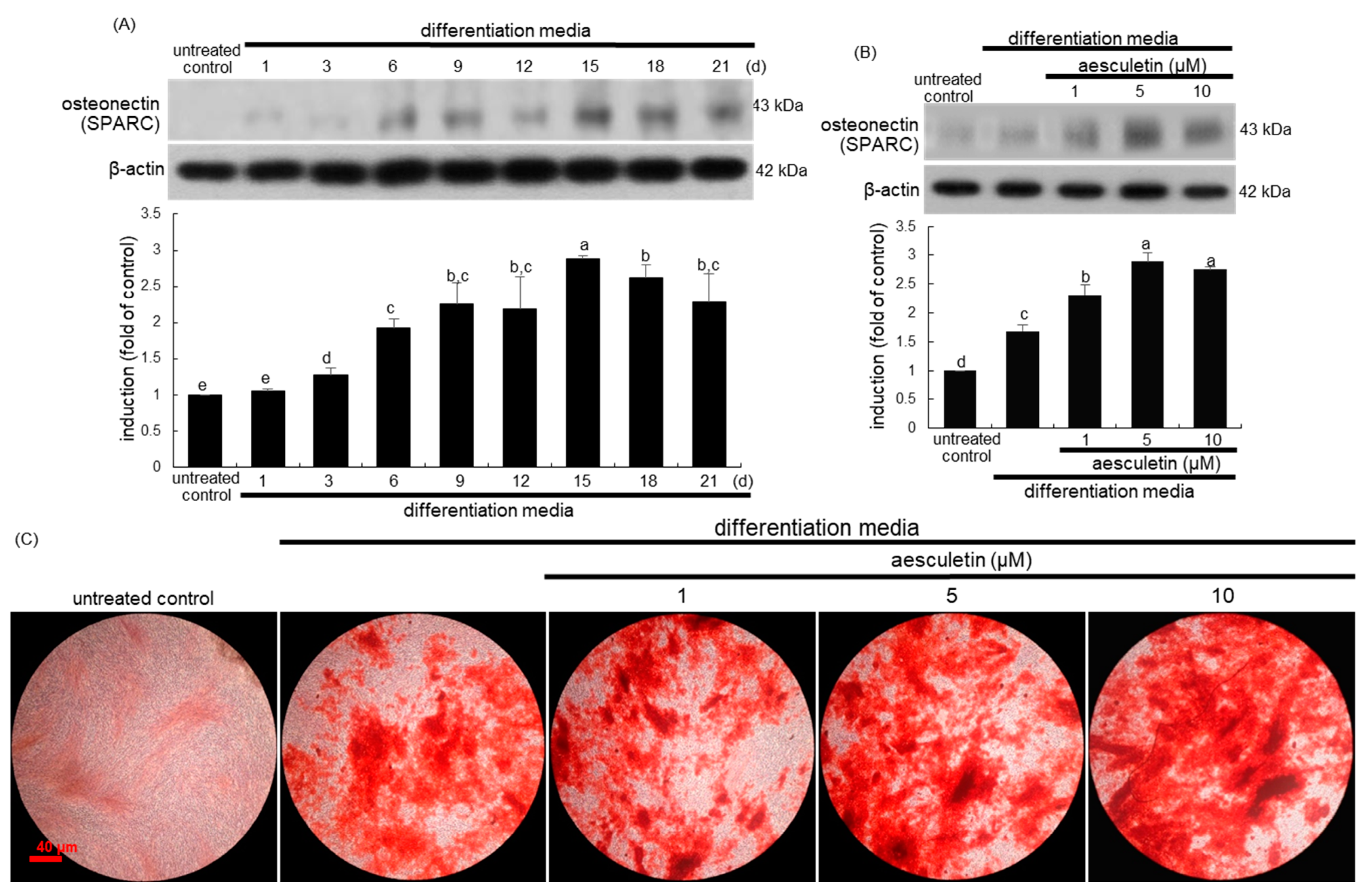
2.3. Upregulation of Later Stages of Osteoblastic Differentiation by Aesculetin
2.4. Increased Terminal Differentiation Leading to Mineralization by Aesculetin
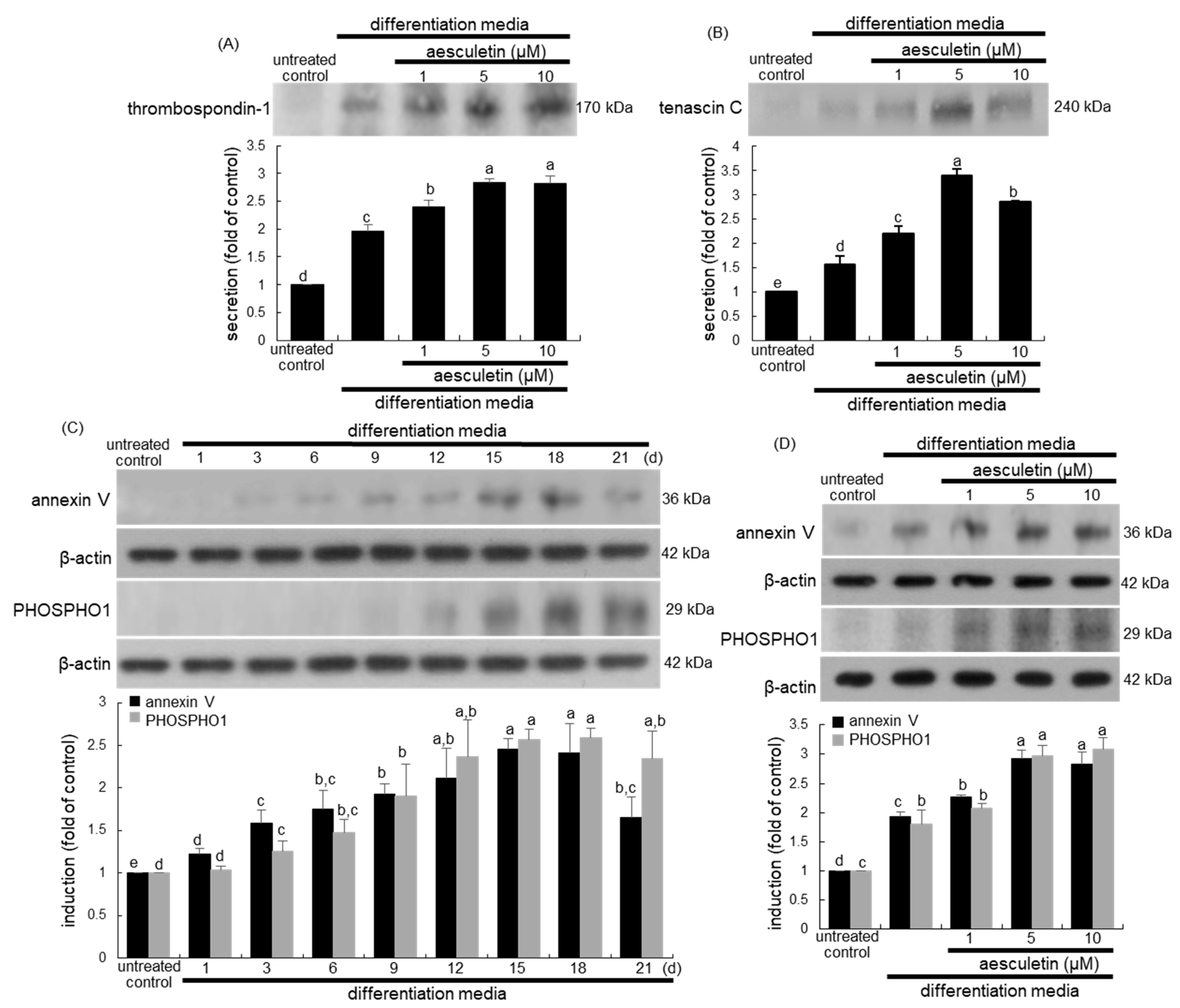
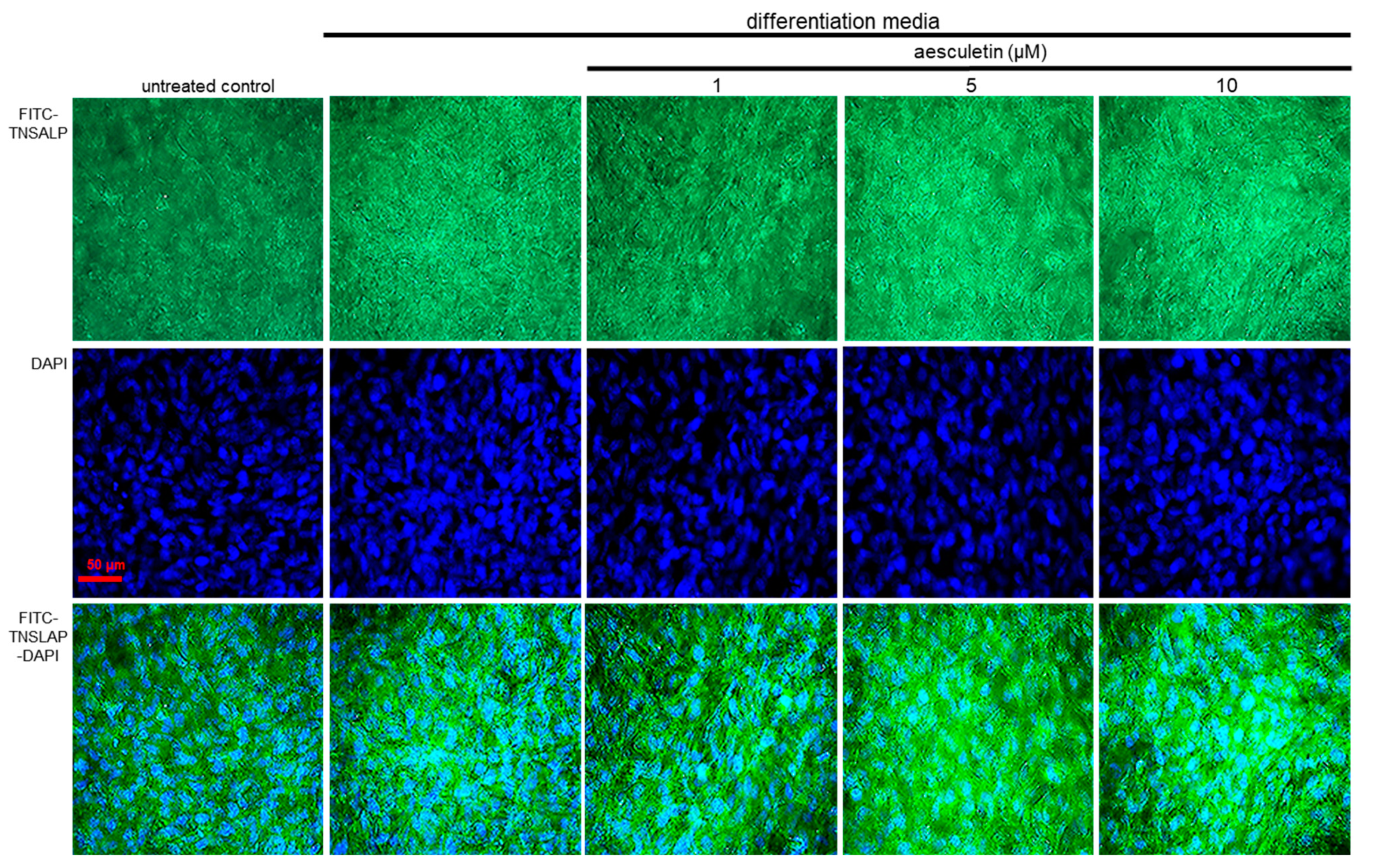
2.5. Incorporation of Osteoblasts into the Matrix by Aesculetin
2.6. Formation of Matrix Vesicles for Bone Mineralization by Aesculetin
2.7. Induction of Collagen Mineralization by Aesculetin
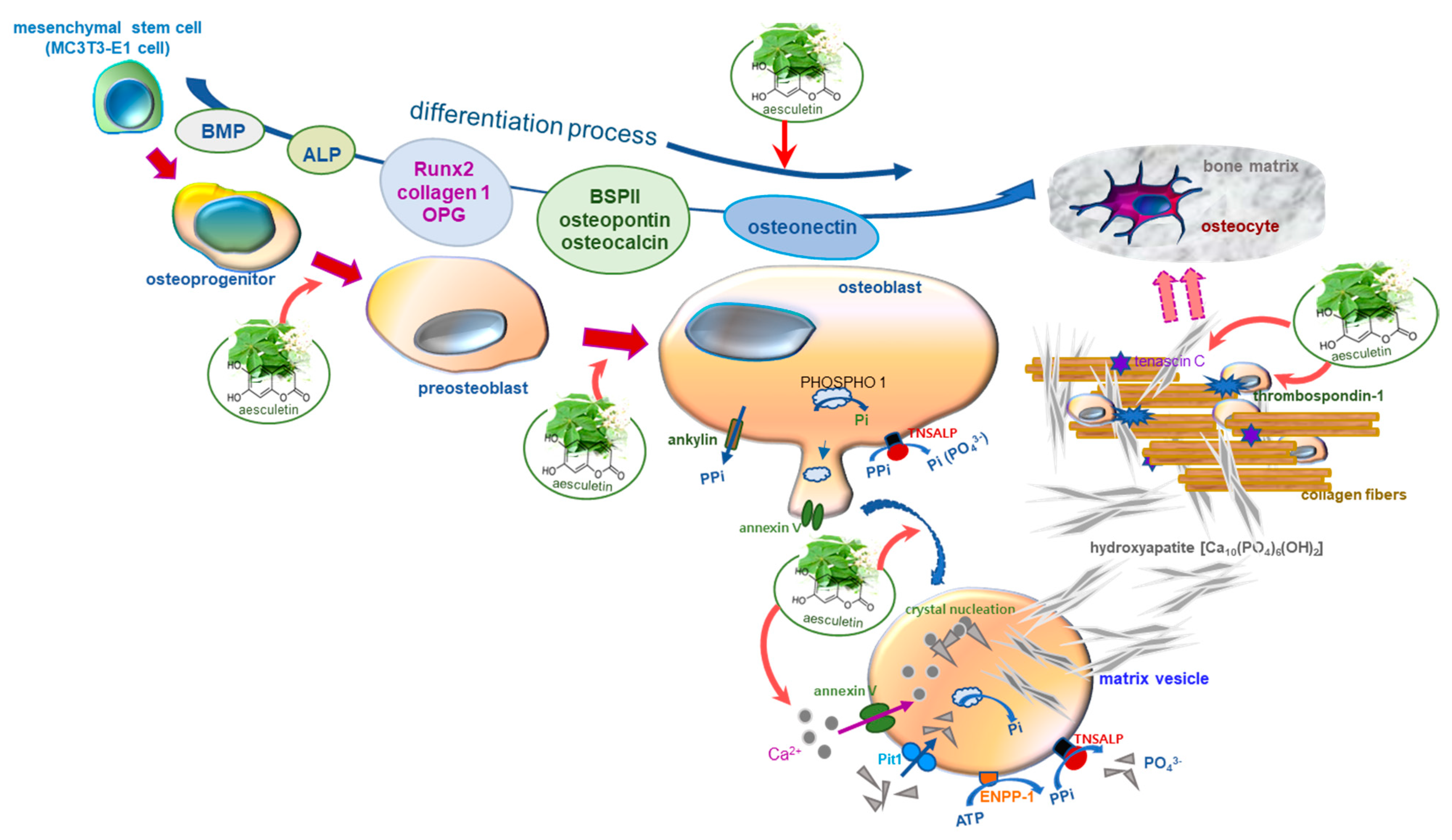
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. MC3T3-E1 Cell Culture and Osteoblastic Differentiation
4.3. Western Blot Analysis
4.4. Measurement of ALP Activity and ALP Staining
4.5. Real-Time Polymerase Chain Reaction (PCR) Analysis
4.6. Alizarin Red S Staining
4.7. Immunofluorocytochemical Staining of TNSALP and Collagen Type 1
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [Green Version]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The Osteocyte: An Endocrine Cell and More. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, N.; Rosenberg, O.; Soudry, M. Osteoblasts in bone physiology-mini review. Rambam Maimonides Med. J. 2012, 3, e0013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, F.; Turner, W.; Belibasakis, G.; Martuscelli, G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol. 2000 2006, 41, 48–72. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, P.H.; Blair, H.C.; Stolz, D.B.; Riazanski, V.; Ray, E.C.; Tourkova, I.L.; Nelson, D.J. Cellular and extracellular matrix of bone, with principles of synthesis and dependency of mineral deposition on cell membrane transport. Am. J. Physiol. Cell Physiol. 2020, 318, C111–C124. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Hasegawa, T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem. Cell Biol. 2018, 149, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M. Mechanism of Bone Mineralization. Cold Spring Harb. Perspect. Med. 2018, 8, a031229. [Google Scholar] [CrossRef]
- Bonjour, J.-P. Calcium and Phosphate: A Duet of Ions Playing for Bone Health. J. Am. Coll. Nutr. 2011, 30, 438S–448S. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.R. The Mineral–Collagen Interface in Bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golub, E.E. Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1592–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, S.; Michikami, I.; Kitagaki, J.; Hata, K.; Kiyonari, H.; Abe, T.; Amano, A.; Wakisaka, S. Syntaxin 4α regulates matrix vesicle-mediated bone matrix production by osteoblasts. J. Bone Miner. Res. 2017, 32, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Croes, M.; Oner, F.C.; Kruyt, M.C.; Blokhuis, T.J.; Bastian, O.; Dhert, W.; Alblas, J. Proinflammatory Mediators Enhance the Osteogenesis of Human Mesenchymal Stem Cells after Lineage Commitment. PLoS ONE 2015, 10, e0132781. [Google Scholar] [CrossRef]
- Ghorbaninejad, M.; Khademi-Shirvan, M.; Hosseini, S.; Eslaminejad, M.B. Epidrugs: Novel epigenetic regulators that open a new window for targeting osteoblast differentiation. Stem Cell Res. Ther. 2020, 11, 456. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Mu, S.; Guo, R.; Zhou, L.; Fu, Q. Paeoniflorin Attenuates Dexamethasone-Induced Apoptosis of Osteoblast Cells and Promotes Bone Formation via Regulating AKT/mTOR/Autophagy Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 6623464. [Google Scholar] [CrossRef]
- Jian, J.; Sun, L.; Cheng, X.; Hu, X.; Liang, J.; Chen, Y. Calycosin-7-O-β-d-glucopyranoside stimulates osteoblast differentiation through regulating the BMP/WNT signaling pathways. Acta Pharm. Sin. B 2015, 5, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Antika, L.D.; Lee, E.-J.; Kim, Y.-H.; Kang, M.-K.; Park, S.-H.; Kim, D.Y.; Oh, H.; Choi, Y.-J.; Kang, Y.-H. Dietary phlorizin enhances osteoblastogenic bone formation through enhancing β-catenin activity via GSK-3β inhibition in a model of senile osteoporosis. J. Nutr. Biochem. 2017, 49, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Lee, E.-J.; Kang, M.-K.; Kim, Y.-H.; Kim, D.Y.; Oh, H.; Kim, S.-I.; Oh, S.Y.; Kang, Y.-H. Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking. Int. J. Mol. Sci. 2020, 21, 8581. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix vesicles: Role in bone miner-alization and potential use as therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Orimo, H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Roach, H.I. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol. Int. 1994, 18, 617–628. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E. Thrombospondin 1 and Its Diverse Roles as a Regulator of Extracellular Matrix in Fibrotic Disease. J. Histochem. Cytochem. 2019, 67, 683–699. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.; Wong, A.; Yellowley, C.E.; Genetos, D.C. Regulation of tenascin expression in bone. J. Cell. Biochem. 2011, 112, 3354–3363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Bandorowicz-Pikuła, J.; Pikuła, S.; et al. Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Narisawa, S.; Harmey, D.; Millán, J.L.; Farquharson, C. Functional involvement of PHOSPHO1 in matrix vesi-cle-mediated skeletal mineralization. J. Bone Miner. Res. 2007, 22, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2005, 17, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Soltanoff, C.S.; Yang, S.; Chen, W.; Li, Y.-P. Signaling Networks that Control the Lineage Commitment and Differentiation of Bone Cells. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 1–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.; Hata, K.; Matsubara, T.; Wakabayashi, M.; Yoneda, T. Regulation of bone and cartilage development by net-work between BMP signalling and transcription factors. J. Biochem. 2012, 151, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Termine, J.D. Non-Collagen Proteins in Bone. Ciba Found Symp. 1988, 136, 178–202. [Google Scholar]
- Licini, C.; Vitale-Brovarone, C.; Mattioli-Belmonte, M. Collagen and non-collagenous proteins molecular crosstalk in the pathophysiology of osteoporosis. Cytokine Growth Factor Rev. 2019, 49, 59–69. [Google Scholar] [CrossRef]
- Jia, M.; Nie, Y.; Cao, D.-P.; Xue, Y.-Y.; Wang, J.-S.; Zhao, L.; Rahman, K.; Zhang, Q.-Y.; Qin, L.-P. Potential Antiosteoporotic Agents from Plants: A Comprehensive Review. Evid. Based Complement. Altern. Med. 2012, 2012, 1–28. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.L.; Lee, E.J.; Park, S.H.; Han, S.Y.; Kang, S.A.; Kang, Y.H. Fisetin antagonizes cell fusion, cytoskeletal or-ganization and bone resorption in RANKL-differentiated murine macrophages. J. Nutr. Biochem. 2014, 25, 295–303. [Google Scholar] [CrossRef]
- Kim, J.L.; Kang, M.K.; Gong, J.H.; Park, S.H.; Han, S.Y.; Kang, Y.H. Novel antiosteoclastogenic activity of phloretin antago-nizing RANKL-induced osteoclast differentiation of murine macrophages. Mol. Nutr. Food Res. 2012, 56, 1223–1233. [Google Scholar] [CrossRef]
- Kim, J.-L.; Park, S.-H.; Jeong, D.; Nam, J.-S.; Kang, Y.-H. Osteogenic activity of silymarin through enhancement of alkaline phosphatase and osteocalcin in osteoblasts and tibia-fractured mice. Exp. Biol. Med. 2012, 237, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, N.; King, K.; Ayers, R. Biologic potential of calcium phosphate biopowders produced via decomposition combustion synthesis. Ceram. Int. 2015, 41, 7735–7744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emami, A.; Talaei-Khozani, T.; Tavanafar, S.; Zareifard, N.; Azarpira, N.; Vojdani, Z. Synergic effects of decellularized bone matrix, hydroxyapatite, and extracellular vesicles on repairing of the rabbit mandibular bone defect model. J. Transl. Med. 2020, 18, 361. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Loftus, A.; Muraca, M.; Maurizi, A.; Rucci, N.; Teti, A. Osteoblast-Derived Extracellular Vesicles Are Biological Tools for the Delivery of Active Molecules to Bone. J. Bone Miner. Res. 2018, 33, 517–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Z.; Greven, J.; Horst, K.; Pfeifer, R.; Kobbe, P.; Pape, H.-C.; Hildebrand, F. Fracture Healing and the Underexposed Role of Extracellular Vesicle-Based Cross Talk. Shock 2018, 49, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Kliemt, S.; Lange, C.; Otto, W.; Hintze, V.; Möller, S.; Von Bergen, M.; Hempel, U.; Kalkhof, S. Sulfated Hyaluronan Containing Collagen Matrices Enhance Cell-Matrix-Interaction, Endocytosis, and Osteogenic Differentiation of Human Mesenchymal Stromal Cells. J. Proteome Res. 2012, 12, 378–389. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, W.; Kang, M.-K.; Park, S.-H.; Kim, D.Y.; Oh, S.Y.; Oh, M.-S.; Park, S.; Kang, I.-J.; Kang, Y.-H. Aesculetin Accelerates Osteoblast Differentiation and Matrix-Vesicle-Mediated Mineralization. Int. J. Mol. Sci. 2021, 22, 12391. https://doi.org/10.3390/ijms222212391
Na W, Kang M-K, Park S-H, Kim DY, Oh SY, Oh M-S, Park S, Kang I-J, Kang Y-H. Aesculetin Accelerates Osteoblast Differentiation and Matrix-Vesicle-Mediated Mineralization. International Journal of Molecular Sciences. 2021; 22(22):12391. https://doi.org/10.3390/ijms222212391
Chicago/Turabian StyleNa, Woojin, Min-Kyung Kang, Sin-Hye Park, Dong Yeon Kim, Su Yeon Oh, Moon-Sik Oh, Sohyun Park, II-Jun Kang, and Young-Hee Kang. 2021. "Aesculetin Accelerates Osteoblast Differentiation and Matrix-Vesicle-Mediated Mineralization" International Journal of Molecular Sciences 22, no. 22: 12391. https://doi.org/10.3390/ijms222212391
APA StyleNa, W., Kang, M.-K., Park, S.-H., Kim, D. Y., Oh, S. Y., Oh, M.-S., Park, S., Kang, I.-J., & Kang, Y.-H. (2021). Aesculetin Accelerates Osteoblast Differentiation and Matrix-Vesicle-Mediated Mineralization. International Journal of Molecular Sciences, 22(22), 12391. https://doi.org/10.3390/ijms222212391





