Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts
Abstract
:1. Introduction
2. Results
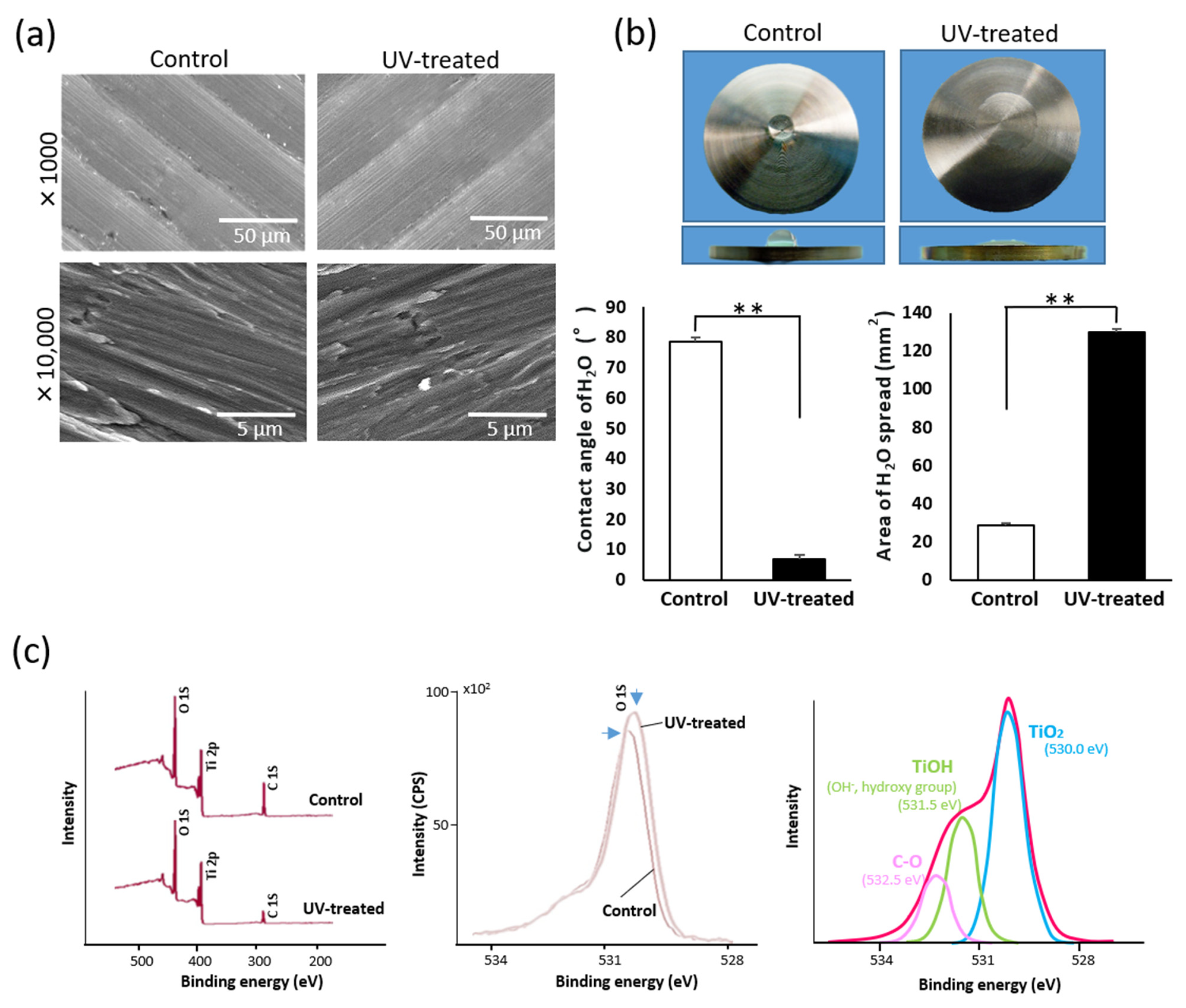
2.1. Surface Properties
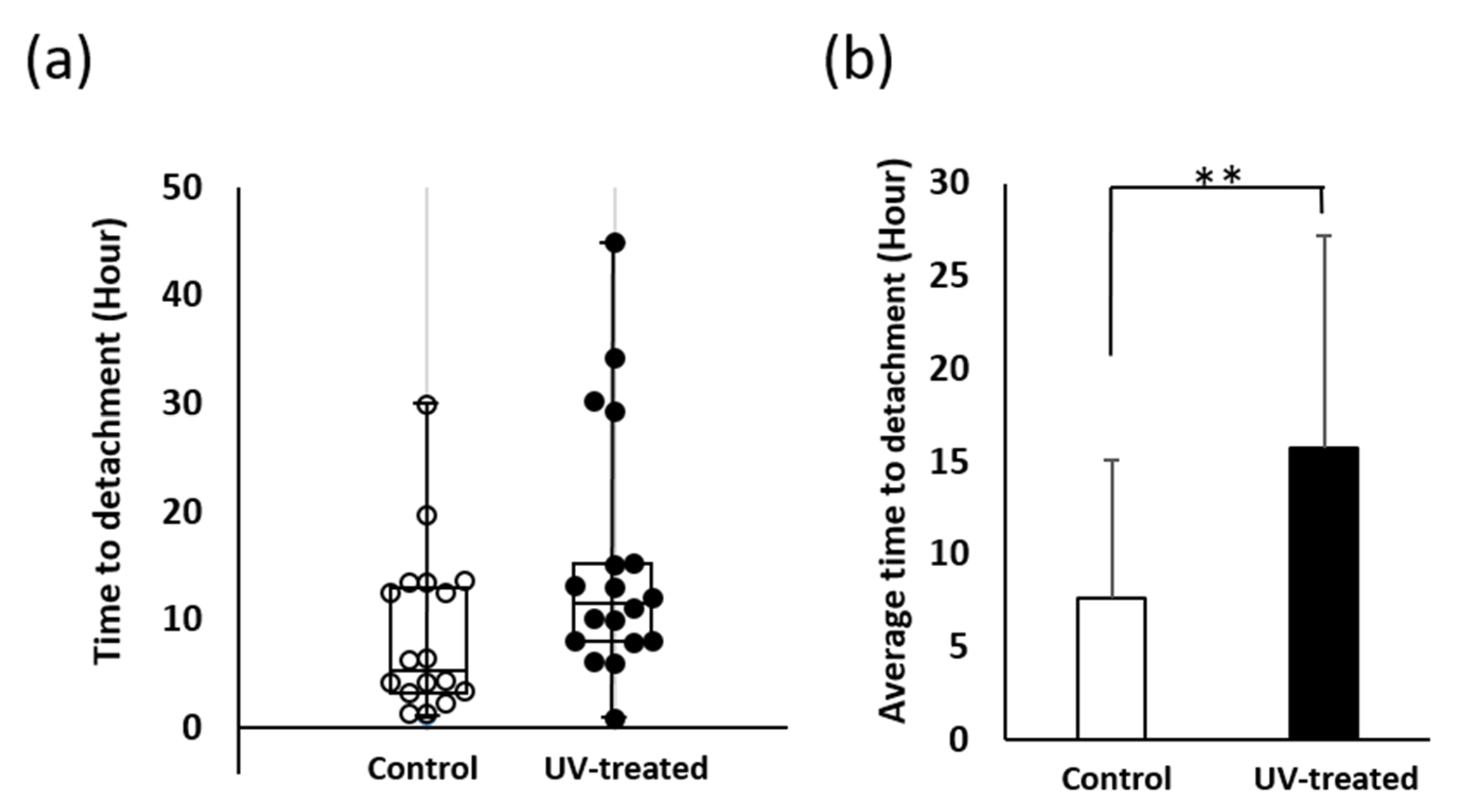
2.2. Time to Detachment during Agitation
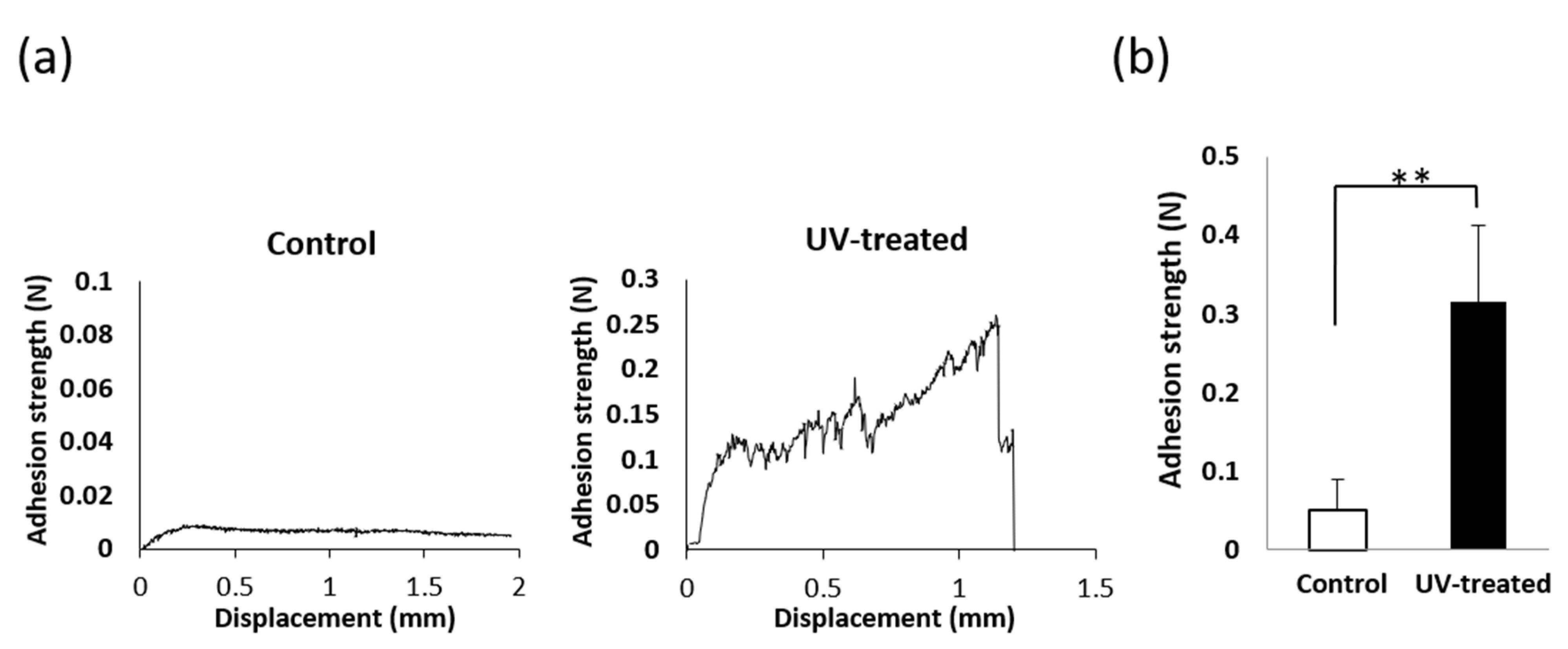
2.3. Mucosa Connective Tissue Adhesion Strength
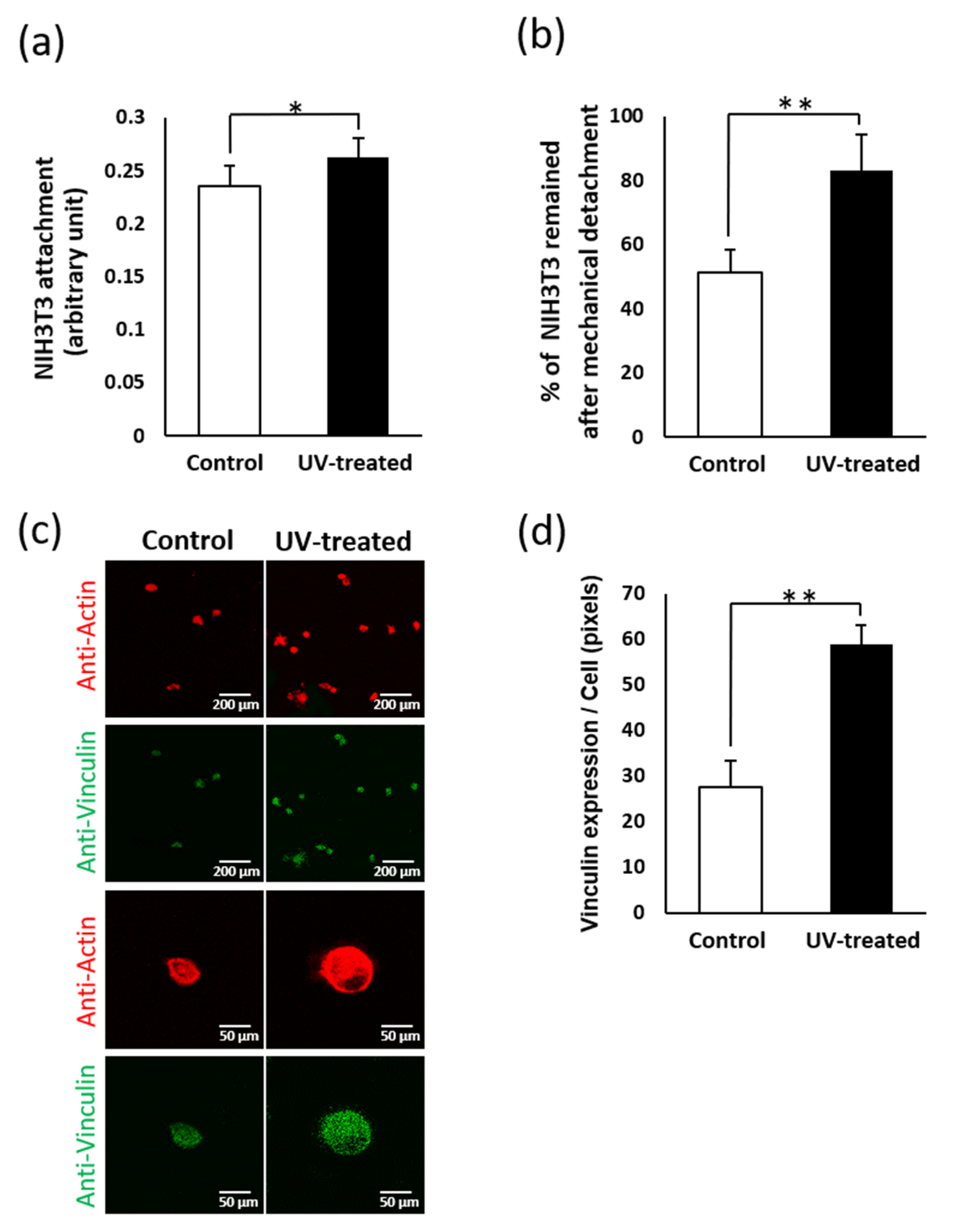
2.4. Fibroblast Attachment
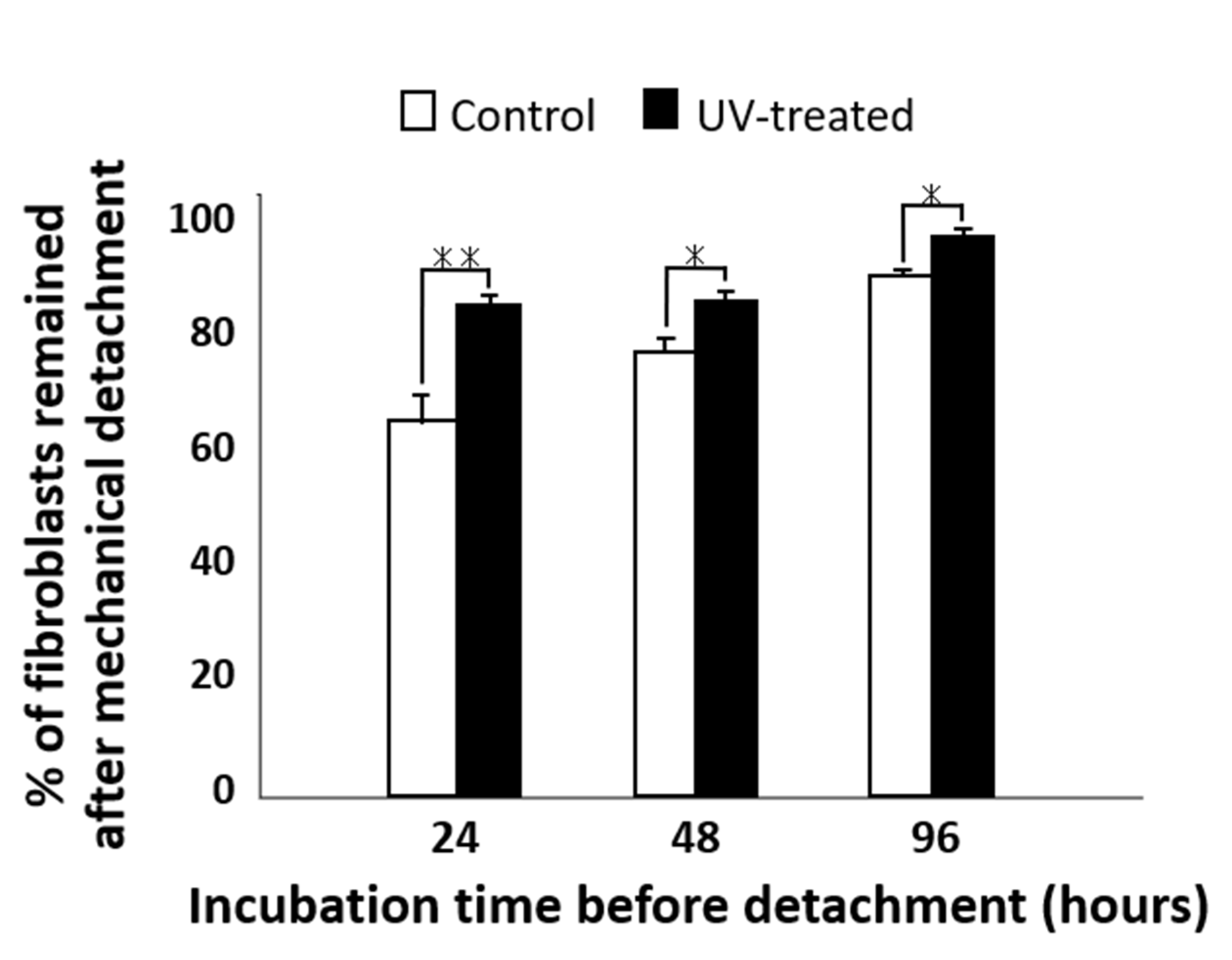
2.5. Remaining Fibroblasts after Mechanical Detachment
2.6. Remaining Fibroblasts after Chemical Detachment by Trypsin
2.7. Validation of Vinculin Expression Using NIH3T3 Cells after Mechanical Detachment
3. Discussion
4. Materials and Methods
4.1. Titanium Disks and UV Treatment
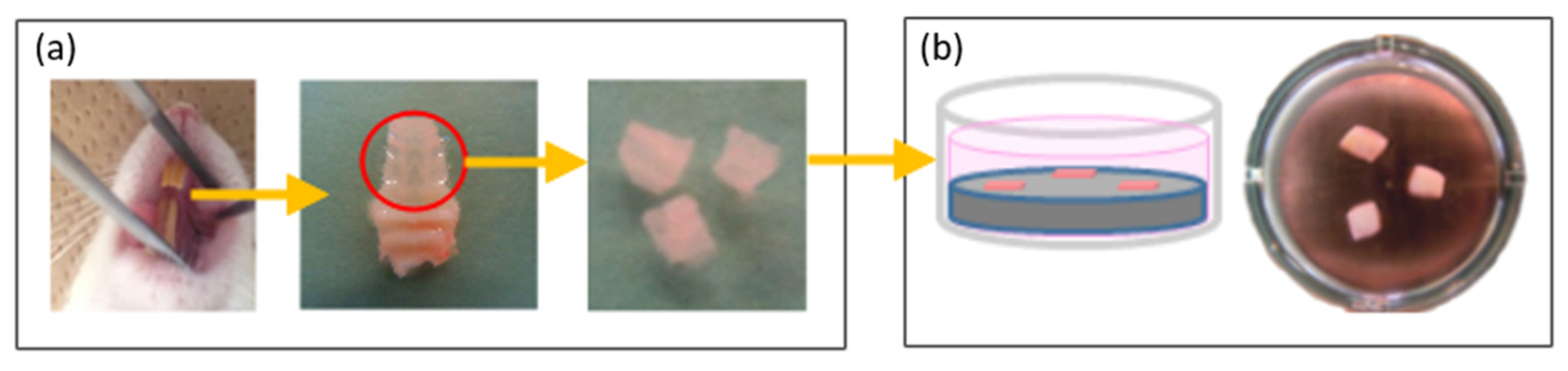
4.2. Keratinized Mucosa Connective Tissue Attachment
4.3. Adhesion Time Measurement
4.4. Adhesion Strength Assay
4.5. Fibroblasts and NIH3T3 Cell Culture
4.6. Cell Adhesion Assay
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saruwatari, L.; Aita, H.; Butz, F.; Nakamura, H.K.; Ouyang, J.; Yang, Y.; Chiou, W.-A.; Ogawa, T. Osteoblasts generate harder, stiffer, and more delamination-resistant mineralized tissue on titanium than on polystyrene, associated with distinct tissue micro- and ultrastructure. J. Bone Miner. Res. 2005, 20, 2002–2016. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Genes Differentially Expressed in Titanium Implant Healing. J. Dent. Res. 2006, 85, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Ozawa, S.; Miyata, Y.; Hasegawa, H.; Tanaka, Y.; Ogawa, T. High-throughput gene expression analysis in bone healing around titanium implants by DNA microarray. Clin. Oral Implant. Res. 2008, 19, 173–181. [Google Scholar] [CrossRef]
- Jokstad, A.; Sanz, M.; Ogawa, T.; Bassi, F.; Levin, L.; Wennerberg, A.; Romanos, G.E. A Systematic Review of the Role of Implant Design in the Rehabilitation of the Edentulous Maxilla. Int. J. Oral Maxillofac. Implant. 2017, 31, s43–s99. [Google Scholar] [CrossRef]
- Ozawa, S.; Ogawa, T.; Iida, K.; Sukotjo, C.; Hasegawa, H.; Nishimura, R.; Nishimura, I. Ovariectomy hinders the early stage of bone-implant integration: Histomorphometric, biomechanical, and molecular analyses. Bone 2002, 30, 137–143. [Google Scholar] [CrossRef]
- Hasegawa, H.; Ozawa, S.; Hashimoto, K.; Takeichi, T.; Ogawa, T. Type 2 diabetes impairs implant osseointegration capacity in rats. Int. J. Oral Maxillofac. Implant. 2008, 23, 237–246. [Google Scholar]
- Ueno, T.; Takeuchi, M.; Hori, N.; Iwasa, F.; Minamikawa, H.; Igarashi, Y.; Anpo, M.; Ogawa, T. Gamma ray treatment enhances bioactivity and osseointegration capability of titanium. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 2279–2287. [Google Scholar] [CrossRef]
- Minamikawa, H.; Att, W.; Ikeda, T.; Hirota, M.; Ogawa, T. Long-Term Progressive Degradation of the Biological Capability of Titanium. Materials 2016, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Kanuru, R.K.; Sugita, Y.; Ikeda, T.; Shinwari, H.; Ishijima, M.; Honda, Y.; Maeda, H.; Ogawa, T. Titanium Delivery of Osteoblastic Cell Sheets: An In Vitro Study. J. Hard Tissue Biol. 2018, 27, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ogawa, T. The Biological Aging of Titanium Implants. Implant. Dent. 2012, 21, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ozawa, S.; Shih, J.-H.; Ryu, K.; Sukotjo, C.; Yang, J.-M.; Nishimura, I. Biomechanical Evaluation of Osseous Implants Having Different Surface Topographies in Rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implant. 2003, 18, 200–210. [Google Scholar]
- Ogawa, T.; Sukotjo, C.; Nishimura, I. Modulated bone matrix-related gene expression is associated with differences in interfacial strength of different implant surface roughness. J. Prosthodont. 2002, 11, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ueno, T.; Minamikawa, H.; Ikeda, T.; Nakagawa, K.; Ogawa, T. Early-stage osseointegration capability of a submicrofeatured titanium surface created by microroughening and anodic oxidation. Clin. Oral Implant. Res. 2012, 24, 991–1001. [Google Scholar] [CrossRef]
- Ogawa, T.; Iwasa, F.; Tsukimura, N.; Att, W.; Kodali-Kanuru, R.; Kubo, K.; Hasnain, H. TiO2 micro-nano-hybrid surface to alleviate biological aging of UV-photofunctionalized titanium. Int. J. Nanomed. 2011, 6, 1327–1341. [Google Scholar] [CrossRef] [Green Version]
- Att, W.; Kubo, K.; Yamada, M.; Maeda, H.; Ogawa, T. Biomechanical properties of jaw periosteum-derived mineralized culture on different titanium topography. Int. J. Oral Maxillofac. Implant. 2009, 24, 831–841. [Google Scholar]
- Butz, F.; Aita, H.; Wang, C.; Ogawa, T. Harder and Stiffer Bone Osseointegrated to Roughened Titanium. J. Dent. Res. 2006, 85, 560–565. [Google Scholar] [CrossRef]
- Ueno, T.; Tsukimura, N.; Yamada, M.; Ogawa, T. Enhanced bone-integration capability of alkali- and heat-treated nanopolymorphic titanium in micro-to-nanoscale hierarchy. Biomaterials 2011, 32, 7297–7308. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Iwasa, F.; Ueno, T.; Takeuchi, K.; Tsukimura, N.; Yamada, M.; Hattori, M.; Yamamoto, A.; Ogawa, T. Selective cell affinity of biomimetic micro-nano-hybrid structured TiO2 overcomes the biological dilemma of osteoblasts. Dent. Mater. 2010, 26, 275–287. [Google Scholar] [CrossRef]
- Tsukimura, N.; Kojima, N.; Kubo, K.; Att, W.; Takeuchi, K.; Kameyama, Y.; Maeda, H.; Ogawa, T. The effect of superficial chemistry of titanium on osteoblastic function. J. Biomed. Mater. Res. Part A 2008, 84A, 108–116. [Google Scholar] [CrossRef]
- Tsukimura, N.; Ueno, T.; Iwasa, F.; Minamikawa, H.; Sugita, Y.; Ishizaki, K.; Ikeda, T.; Nakagawa, K.; Yamada, M.; Ogawa, T. Bone integration capability of alkali- and heat-treated nanobimorphic Ti–15Mo–5Zr–3Al. Acta Biomater. 2011, 7, 4267–4277. [Google Scholar] [CrossRef]
- Sugita, Y.; Ishizaki, K.; Iwasa, F.; Ueno, T.; Minamikawa, H.; Yamada, M.; Suzuki, T.; Ogawa, T. Effects of pico-to-nanometer-thin TiO2 coating on the biological properties of microroughened titanium. Biomaterials 2011, 32, 8374–8384. [Google Scholar] [CrossRef]
- Nakamura, H.; Shim, J.; Butz, F.; Aita, H.; Gupta, V.; Ogawa, T. Glycosaminoglycan degradation reduces mineralized tissue–titanium interfacial strength. J. Biomed. Mater. Res. Part A 2006, 77A, 478–486. [Google Scholar] [CrossRef]
- Takeuchi, K.; Saruwatari, L.; Nakamura, H.K.; Yang, J.-M.; Ogawa, T. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J. Biomed. Mater. Res. Part A 2005, 72A, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Saruwatari, L.; Aita, H.; Takeuchi, K.; Ogawa, T. Molecular and Biomechanical Characterization of Mineralized Tissue by Dental Pulp Cells on Titanium. J. Dent. Res. 2005, 84, 515–520. [Google Scholar] [CrossRef]
- Nakamura, H.; Butz, F.; Saruwatari, L.; Ogawa, T. A role for proteoglycans in mineralized tissue-titanium adhesion. J. Dent. Res. 2007, 86, 147–152. [Google Scholar] [CrossRef]
- Att, W.; Tsukimura, N.; Suzuki, T.; Ogawa, T. Effect of supramicron roughness characteristics produced by 1- and 2-step acid etching on the osseointegration capability of titanium. Int. J. Oral Maxillofac. Implant. 2007, 22, 719–728. [Google Scholar]
- Rezaei, N.M.; Hasegawa, M.; Ishijima, M.; Nakhaei, K.; Okubo, T.; Taniyama, T.; Ghassemi, A.; Tahsili, T.; Park, W.; Hirota, M.; et al. Biological and osseointegration capabilities of hierarchically (meso-/micro-/nano-scale) roughened zirconia. Int. J. Nanomed. 2018, 13, 3381–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Kubo, K.; Yamada, M.; Hori, N.; Suzuki, T.; Maeda, H.; Ogawa, T. Osteoblast Mechanoresponses on Ti with Different Surface Topographies. J. Dent. Res. 2009, 88, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Yamada, M.; Ogawa, T. Effect of titanium surface characteristics on the behavior and function of oral fibroblasts. Int. J. Oral Maxillofac. Implant. 2009, 24, 419–431. [Google Scholar]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A newly created meso-, micro-, and nano-scale rough titanium surface promotes bone-implant integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruta, J.; Sato, N.; Ishijima, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20, 4027. [Google Scholar] [CrossRef] [Green Version]
- Uno, M.; Hayashi, M.; Ozawa, R.; Saruta, J.; Ishigami, H.; Ogawa, T. Mechanical Interlocking Capacity of Titanium with Respect to Surface Morphology and Topographical Parameters. J. Dent. Oral Biol. 2020, 5, 1163. [Google Scholar]
- Uno, M.; Ozawa, R.; Hamajima, K.; Saruta, J.; Ishigami, H.; Ogawa, T. Variation in Osteoblast Retention Ability of Titanium Surfaces with Different Topographies. J. Dent. Oral Biol. 2020, 5, 1169. [Google Scholar]
- Saruta, J.; Ozawa, R.; Okubo, T.; Taleghani, S.; Ishijima, M.; Kitajima, H.; Hirota, M.; Ogawa, T. Biomimetic Zirconia with Cactus-Inspired Meso-Scale Spikes and Nano-Trabeculae for Enhanced Bone Integration. Int. J. Mol. Sci. 2021, 22, 7969. [Google Scholar] [CrossRef]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.-A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef] [PubMed]
- Butz, F.; Ogawa, T.; Chang, T.-L.; Nishimura, I. Three-dimensional bone-implant integration profiling using micro-computed tomography. Int. J. Oral Maxillofac. Implant. 2006, 21, 687–695. [Google Scholar]
- Butz, F.; Ogawa, T.; Nishimura, I. Interfacial shear strength of endosseous implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 746–751. [Google Scholar]
- Ogawa, T.; Saruwatari, L.; Takeuchi, K.; Aita, H.; Ohno, N. Ti Nano-nodular Structuring for Bone Integration and Regeneration. J. Dent. Res. 2008, 87, 751–756. [Google Scholar] [CrossRef]
- Nishimura, I.; Huang, Y.; Butz, F.; Ogawa, T.; Lin, A.; Wang, C.J. Discrete deposition of hydroxyapatite nanoparticles on a titanium implant with predisposing substrate microtopography accelerated osseointegration. Nanotechnology 2007, 18, 245101. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, F.; Sabóia-Gomes, R.; Alves, B.E.S.; De Oliveira, R.P.; Araújo, M.G. Prevalence, extent and severity of peri-implant diseases. A cross-sectional study based on a university setting in Brazil. J. Periodontal Res. 2018, 53, 910–915. [Google Scholar] [CrossRef]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef]
- Ogata, Y.; Nakayama, Y.; Tatsumi, J.; Kubota, T.; Sato, S.; Nishida, T.; Takeuchi, Y.; Onitsuka, T.; Sakagami, R.; Nozaki, T.; et al. Prevalence and risk factors for peri-implant diseases in Japanese adult dental patients. J. Oral Sci. 2017, 59, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Romandini, M.; Lima, C.; Pedrinaci, I.; Araoz, A.; Soldini, M.C.; Sanz, M. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study. Clin. Oral Implant. Res. 2021, 32, 112–122. [Google Scholar] [CrossRef]
- Shimchuk, A.A.; Weinstein, B.F.; Daubert, D.M. The impact of a change in classification criteria on the prevalence of peri-implantitis: A cross-sectional analysis. J. Periodontol. 2021, 92, 1339–1346. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Xing, R.; Ramis, J.M.; Taxt-Lamolle, S.; Haugen, H.; Lyngstadaas, S.; Monjo, M. Human gingival fibroblasts function is stimulated on machined hydrided titanium zirconium dental implants. J. Dent. 2014, 42, 30–38. [Google Scholar] [CrossRef]
- Marín-Pareja, N.; Salvagni, E.; Guillem-Marti, J.; Aparicio, C.; Ginebra, M.-P. Collagen-functionalised titanium surfaces for biological sealing of dental implants: Effect of immobilisation process on fibroblasts response. Colloids Surfaces B Biointerfaces 2014, 122, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Att, W.; Ogawa, T. Biological aging of implant surfaces and their restoration with ultraviolet light treatment: A novel understanding of osseointegration. Int. J. Oral Maxillofac. Implant. 2012, 27, 753–761. [Google Scholar]
- Ogawa, T. UV-photofunctionalization of titanium implants. Oral Craniofacial Tissue Eng. 2012, 2, 151–158. [Google Scholar]
- Ogawa, T. Photofunctionalization of TiO2 for optimal integration of titanium with bone. In Benign Photocatalysts; Applications of Titanium Oxide-Based Materials; Kamat, P., Anpo, M., Eds.; Springer: New York, NY, USA, 2010; pp. 699–713. [Google Scholar]
- Liu, X.; Lim, J.Y.; Donahue, H.J.; Dhurjati, R.; Mastro, A.; Vogler, E.A. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials 2007, 28, 4535–4550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Tsukimura, N.; Taniyama, T.; Ishijima, M.; Nakhaei, K.; Rezaei, N.M.; Hirota, M.; Park, W.; Akita, D.; Tateno, A.; et al. Ultraviolet treatment restores bioactivity of titanium mesh plate degraded by contact with medical gloves. J. Oral Sci. 2018, 60, 567–573. [Google Scholar] [CrossRef]
- Suzuki, T.; Kubo, K.; Hori, N.; Yamada, M.; Kojima, N.; Sugita, Y.; Maeda, H.; Ogawa, T. Nonvolatile buffer coating of titanium to prevent its biological aging and for drug delivery. Biomaterials 2010, 31, 4818–4828. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent Degradation of the Protein Adsorption Capacity of Titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Hirota, M.; Ikeda, T.; Sugita, Y.; Ishijima, M.; Hirota, S.; Ogawa, T. Impaired osteoblastic behavior and function on saliva-contaminated titanium and its restoration by UV treatment. Mater. Sci. Eng. 2019, 100, 165–177. [Google Scholar] [CrossRef]
- Hori, N.; Ueno, T.; Minamikawa, H.; Iwasa, F.; Yoshino, F.; Kimoto, K.; Lee, M.C.-I.; Ogawa, T. Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater. 2010, 6, 4175–4180. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium–cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone–titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef]
- Ogawa, T.; Saita, M.; Ikeda, T.; Yamada, M.; Kimoto, K.; Lee, M.C.-I. UV photofunctionalization promotes nano-biomimetic apatite deposition on titanium. Int. J. Nanomed. 2016, 11, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, T.; Yamada, M.; Hori, N.; Suzuki, T.; Ogawa, T. Effect of ultraviolet photoactivation of titanium on osseointegration in a rat model. Int. J. Oral Maxillofac. Implant. 2010, 25, 287–294. [Google Scholar]
- Ishijima, M.; Ghassemi, A.; Soltanzadeh, P.; Tanaka, M.; Nakhaei, K.; Park, W.; Hirota, M.; Tsukimura, N.; Ogawa, T. Effect of UV Photofunctionalization on Osseointegration in Aged Rats. Implant. Dent. 2016, 25, 744–750. [Google Scholar] [CrossRef]
- Pyo, S.-W.; Park, Y.B.; Moon, H.S.; Lee, J.-H.; Ogawa, T. Photofunctionalization Enhances Bone-Implant Contact, Dynamics of Interfacial Osteogenesis, Marginal Bone Seal, and Removal Torque Value of Implants. Implant. Dent. 2013, 22, 666–675. [Google Scholar] [CrossRef]
- Iwasaki, C.; Hirota, M.; Tanaka, M.; Kitajima, H.; Tabuchi, M.; Ishijima, M.; Park, W.; Sugita, Y.; Miyazawa, K.; Goto, S.; et al. Tuning of Titanium Microfiber Scaffold with UV-Photofunctionalization for Enhanced Osteoblast Affinity and Function. Int. J. Mol. Sci. 2020, 21, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, Y.; Saruta, J.; Taniyama, T.; Kitajima, H.; Hirota, M.; Ikeda, T.; Ogawa, T. UV-Pre-Treated and Protein-Adsorbed Titanium Implants Exhibit Enhanced Osteoconductivity. Int. J. Mol. Sci. 2020, 21, 4194. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Hamajima, K.; Tanaka, M.; Sekiya, T.; Hirota, M.; Ogawa, T. UV Light-Generated Superhydrophilicity of a Titanium Surface Enhances the Transfer, Diffusion and Adsorption of Osteogenic Factors from a Collagen Sponge. Int. J. Mol. Sci. 2021, 22, 6811. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, T.; Saruta, J.; Rezaei, N.M.; Nakhaei, K.; Ghassemi, A.; Hirota, M.; Okubo, T.; Ikeda, T.; Sugita, Y.; Hasegawa, M.; et al. UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model. Int. J. Mol. Sci. 2020, 21, 1235. [Google Scholar] [CrossRef] [Green Version]
- Hirota, M.; Ozawa, T.; Iwai, T.; Ogawa, T.; Tohnai, I. Implant Stability Development of Photofunctionalized Implants Placed in Regular and Complex Cases: A Case-Control Study. Int. J. Oral Maxillofac. Implant. 2016, 31, 676–686. [Google Scholar] [CrossRef]
- Sugita, Y.; Honda, Y.; Kato, I.; Kubo, K.; Maeda, H.; Ogawa, T. Role of Photofunctionalization in Mitigating Impaired Osseointegration Associated with Type 2 Diabetes in Rats. Int. J. Oral Maxillofac. Implant. 2014, 29, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Ikeda, T.; Hirota, M.; Nakagawa, K.; Park, W.; Miyazawa, K.; Goto, S.; Ogawa, T. Effect of UV Photofunctionalization on Biologic and Anchoring Capability of Orthodontic Miniscrews. Int. J. Oral Maxillofac. Implant. 2015, 30, 868–879. [Google Scholar] [CrossRef] [Green Version]
- Park, W.; Ishijima, M.; Hirota, M.; Soltanzadeh, P.; Ogawa, T. Engineering bone-implant integration with photofunctionalized titanium microfibers. J. Biomater. Appl. 2016, 30, 1242–1250. [Google Scholar] [CrossRef]
- Hirota, M.; Ikeda, T.; Tabuchi, M.; Iwai, T.; Tohnai, I.; Ogawa, T. Effect of Ultraviolet-Mediated Photofunctionalization for Bone Formation Around Medical Titanium Mesh. J. Oral Maxillofac. Surg. 2014, 72, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, P.; Ghassemi, A.; Ishijima, M.; Tanaka, M.; Park, W.; Iwasaki, C.; Hirota, M.; Ogawa, T. Success rate and strength of osseointegration of immediately loaded UV-photofunctionalized implants in a rat model. J. Prosthet. Dent. 2017, 118, 357–362. [Google Scholar] [CrossRef]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet Treatment Overcomes Time-Related Degrading Bioactivity of Titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef]
- Ghassemi, A.; Ishijima, M.; Hasegawa, M.; Rezaei, N.M.; Nakhaei, K.; Sekiya, T.; Torii, Y.; Hirota, M.; Park, W.; Miley, D.D.; et al. Biological and Physicochemical Characteristics of 2 Different Hydrophilic Surfaces Created by Saline-Storage and Ultraviolet Treatment. Implant. Dent. 2018, 27, 405–414. [Google Scholar] [CrossRef]
- Funato, A.; Ogawa, T. Photofunctionalized Dental Implants: A Case Series in Compromised Bone. Int. J. Oral Maxillofac. Implant. 2013, 28, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Funato, A.; Yamada, M.; Ogawa, T. Success Rate, Healing Time, and Implant Stability of Photofunctionalized Dental Implants. Int. J. Oral Maxillofac. Implant. 2013, 28, 1261–1271. [Google Scholar] [CrossRef]
- Funato, A.; Tonotsuka, R.; Murabe, H.; Hirota, M.; Ogawa, T. A Novel Strategy for Bone Integration and Regeneration: Case Studies. J. Cosmet. Dent. 2014, 29, 74–86. [Google Scholar]
- Ishikawa, T.; Vela, X.; Kida, K.; Moroi, H.; Kitajima, H.; Ogawa, T. Restoration of optimum esthetics in complex clinical situations using an interdisciplinary strategy in combination with advanced techniques and technologies in regenerative medicine. J. Cosmetic Dent. 2014, 29, 60–72. [Google Scholar]
- Kitajima, H.; Ogawa, T. The Use of Photofunctionalized Implants for Low or Extremely Low Primary Stability Cases. Int. J. Oral Maxillofac. Implant. 2016, 31, 439–447. [Google Scholar] [CrossRef]
- Hirota, M.; Ozawa, T.; Iwai, T.; Ogawa, T.; Tohnai, I. Effect of Photofunctionalization on Early Implant Failure. Int. J. Oral Maxillofac. Implant. 2018, 33, 1098–1102. [Google Scholar] [CrossRef]
- Hirota, M.; Ozawa, T.; Iwai, T.; Mitsudo, K.; Ogawa, T. UV-Mediated Photofunctionalization of Dental Implant: A Seven-Year Results of a Prospective Study. J. Clin. Med. 2020, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Tanaka, M.; Ishijima, M.; Iwasaki, C.; Park, W.; Ogawa, T. Effect of Photofunctionalization on Ti6Al4V Screw Stability Placed in Segmental Bone Defects in Rat Femurs. J. Oral Maxillofac. Surg. 2016, 74, 861.e1–861.e16. [Google Scholar] [CrossRef]
- de Avila, E.; Lima, B.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, M.; De Avila, E.D.; Nakhaei, K.; Shi, W.; Lux, R.; Ogawa, T. Ultraviolet Light Treatment of Titanium Suppresses Human Oral Bacterial Attachment and Biofilm Formation: A Short-Term In Vitro Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kobayashi, H.; Ogawa, T. Implant Stability Change and Osseointegration Speed of Immediately Loaded Photofunctionalized Implants. Implant. Dent. 2013, 22, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Miyauchi, T.; Yamamoto, A.; Iwasa, F.; Takeuchi, M.; Anpo, M.; Sakurai, K.; Baba, K.; Ogawa, T. Enhancement of adhesion strength and cellular stiffness of osteoblasts on mirror-polished titanium surface by UV-photofunctionalization. Acta Biomater. 2010, 6, 4578–4588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, T.; Ikeda, T.; Tsukimura, N.; Ishijima, M.; Minamikawa, H.; Sugita, Y.; Yamada, M.; Wakabayashi, N.; Ogawa, T. Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials 2016, 108, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Ueno, T.; Suzuki, T.; Yamada, M.; Att, W.; Okada, S.; Ohno, A.; Aita, H.; Kimoto, K.; Ogawa, T. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int. J. Oral Maxillofac. Implant. 2010, 25, 49–62. [Google Scholar]
- Ikeda, T.; Okubo, T.; Saruta, J.; Hirota, M.; Kitajima, H.; Yanagisawa, N.; Ogawa, T. Osteoblast Attachment Compromised by High and Low Temperature of Titanium and Its Restoration by UV Photofunctionalization. Materials 2021, 14, 5493. [Google Scholar] [CrossRef]
- Okubo, T.; Ikeda, T.; Saruta, J.; Tsukimura, N.; Hirota, M.; Ogawa, T. Compromised Epithelial Cell Attachment after Polishing Titanium Surface and Its Restoration by UV Treatment. Materials 2020, 13, 3946. [Google Scholar] [CrossRef]
- Ishijima, M.; Hirota, M.; Park, W.; Honda, M.J.; Tsukimura, N.; Isokawa, K.; Ishigami, T.; Ogawa, T. Osteogenic cell sheets reinforced with photofunctionalized micro-thin titanium. J. Biomater. Appl. 2015, 29, 1372–1384. [Google Scholar] [CrossRef]
- Ishijima, M.; Soltanzadeh, P.; Hirota, M.; Tsukimura, N.; Shigami, T.; Ogawa, T. Enhancing osteoblast-affinity of titanium scaffolds for bone engineering by use of ultraviolet light treatment. Biomed. Res. 2015, 36, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, F.; Baba, K.; Ogawa, T. Enhanced intracellular signaling pathway in osteoblasts on ultraviolet lighttreated hydrophilic titanium. Biomed. Res. 2016, 37, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hori, N.; Iwasa, F.; Tsukimura, N.; Sugita, Y.; Ueno, T.; Kojima, N.; Ogawa, T. Effects of UV photofunctionalization on the nanotopography enhanced initial bioactivity of titanium. Acta Biomater. 2011, 7, 3679–3691. [Google Scholar] [CrossRef]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontology 2000 2018, 76, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Lemons, J.E.; Liu, J.; Raikar, G.N.; Weimer, J.J.; Vohra, Y. Cleaning and heat-treatment effects on unalloyed titanium implant surfaces. Int. J. Oral Maxillofac. Implant. 2000, 15, 219–230. [Google Scholar]
- Serro, A.; Saramago, B. Influence of sterilization on the mineralization of titanium implants induced by incubation in various biological model fluids. Biomaterials 2003, 24, 4749–4760. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Wu, D.; Wang, Q.-L.; Yan, J.; Buekens, A.G.; Cen, K.-F. Photocatalytic decomposition on nano-TiO2: Destruction of chloroaromatic compounds. Chemosphere 2011, 82, 1215–1224. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakamoto, K.; Martra, G.; Coluccia, S.; Anpo, M. Mechanism of Photoinduced Superhydrophilicity on the TiO2 Photocatalyst Surface. J. Phys. Chem. B 2005, 109, 15422–15428. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yamamoto, K.; Hayasaka, Y.; Matsumoto, K. Surface OH group governing wettability of commercial glasses. J. Non-Crystalline Solids 1999, 249, 41–46. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Kim, W.-J.; Kim, S.; Lee, B.S.; Kim, A.; Ah, C.S.; Huh, C.; Sung, G.Y.; Yun, W.S. Enhanced Protein Immobilization Efficiency on a TiO2 Surface Modified with a Hydroxyl Functional Group. Langmuir 2009, 25, 11692–11697. [Google Scholar] [CrossRef]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon Deposition Attenuates Osteoblast Activity on Titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, I.-S.; Berglundh, T.; Abrahamsson, I.; Linder, E.; Lindhe, J. The barrier between the keratinized mucosa and the dental implant. J. Clin. Periodontol. 1999, 26, 658–663. [Google Scholar] [CrossRef]
- Bailly, M. Connecting cell adhesion to the actin polymerization machinery: Vinculin as the missing link? Trends Cell Biol. 2003, 13, 163–165. [Google Scholar] [CrossRef]
- Goldmann, W.H.; Ingber, D.E. Intact Vinculin Protein Is Required for Control of Cell Shape, Cell Mechanics, and rac-Dependent Lamellipodia Formation. Biochem. Biophys. Res. Commun. 2002, 290, 749–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, J.D.; Wang, P.; Streuli, C.; Geiger, B.; Humphries, M.J.; Ballestrem, C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007, 179, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Wen, K.-K.; Rubenstein, P.; DeMali, K.A. Vinculin Nucleates Actin Polymerization and Modifies Actin Filament Structure. J. Biol. Chem. 2009, 284, 30463–30473. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ma, Q.; Chu, P.; Mei, S.; Ji, K.; Jin, L. Concentration- and time-dependent response of human gingival fibroblasts to fibroblast growth factor 2 immobilized on titanium dental implants. Int. J. Nanomed. 2012, 7, 1965–1976. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.W., 3rd; Culbertson, K.C.; D’Angelo, A.M. Fibronectin and laminin enhance gingival cell attachment to dental implant surfaces in vitro. Int. J. Oral Maxillofac. Implant. 1995, 10, 721–728. [Google Scholar]
- Kim, E.-C.; Lee, Y.; Lee, M.-H.; Lee, H.J.; Kim, K.-H.; Leesungbok, R.; Ahn, S.-J.; Park, S.-J.; Yoon, J.-H.; Jee, Y.-J.; et al. The Effect of Fibronectin-Immobilized Microgrooved Titanium Substrata on Cell Proliferation and Expression of Genes and Proteins in Human Gingival Fibroblasts. Tissue Eng. Regen. Med. 2018, 15, 615–627. [Google Scholar] [CrossRef]
- Middleton, C.; Pendegrass, C.; Gordon, D.; Jacob, J.; Blunn, G. Fibronectin silanized titanium alloy: A bioinductive and durable coating to enhance fibroblast attachmentin vitro. J. Biomed. Mater. Res. Part A 2007, 83A, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Kim, D.M.; Lee, C.; Da Silva, J.; Nagai, S.; Nojiri, T.; Nagai, M. Biological efficacy of perpendicular type-I collagen protruded from TiO2-nanotubes. Int. J. Oral Sci. 2020, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, K.; Ishijima, M.; Ikeda, T.; Ghassemi, A.; Saruta, J.; Ogawa, T. Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization. Materials 2020, 14, 151. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, T.; Ueno, T.; Saruta, J.; Hirota, M.; Park, W.; Ogawa, T. Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 12396. https://doi.org/10.3390/ijms222212396
Ikeda T, Ueno T, Saruta J, Hirota M, Park W, Ogawa T. Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts. International Journal of Molecular Sciences. 2021; 22(22):12396. https://doi.org/10.3390/ijms222212396
Chicago/Turabian StyleIkeda, Takayuki, Takeshi Ueno, Juri Saruta, Makoto Hirota, Wonhee Park, and Takahiro Ogawa. 2021. "Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts" International Journal of Molecular Sciences 22, no. 22: 12396. https://doi.org/10.3390/ijms222212396
APA StyleIkeda, T., Ueno, T., Saruta, J., Hirota, M., Park, W., & Ogawa, T. (2021). Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts. International Journal of Molecular Sciences, 22(22), 12396. https://doi.org/10.3390/ijms222212396






