Evaluation of Olive Oil Quality with Electrochemical Sensors and Biosensors: A Review
Abstract
1. Introduction
2. Olive Oil—Determination of Quality Parameters
2.1. Phenolic-Biomarker Compounds for Olive Oil
- (a)
- Phenolic acids;
- (b)
- Phenolic alcohols, such as 3,4-dihydroxipheniletanol (3,4-DHPEA or hydroxytyrosol) (3,4-dihydroxyphenyl ethanol) and 4-hydroxyphenil ethanol (p-(hydroxyphenyl) ethanol) (4-HPEA or tyrosol);
- (c)
- Secoiridoids, such as the dialdehyde form of elenolic dicarboxymethyl acid linked to hydroxytyrosol (3,4-DHPEA-EDA) (dicarboxymethyl 21 elenolic acid linked to hydroxytyrosol), called oleacein, and the dialdehyde form of tyrosol elenoliclegate dicarboxymethyl acid (p-HPEA-EDA), called oleocanthal [60], 3,4-(dihydroxyphenyl ethanol) elenolic acid (3,4-DHPEA-EA), called isomer of aglycon oleuropeine, and p-(hydroxyphenyl)ethanol elenolic acid (p-HPEA-EA) or aglycon ligstroside;
- (d)
- Lignans, such as (+)-1-acetoxypinorezinol and (+)-pinorezinol;
- (e)
- Flavones, such as apigenin and luteolin [82].
2.2. Other Bioactive Compounds
3. Olive Oil Adulteration
4. Electrochemical Methods for Evaluating the Quality of Olive Oil
4.1. Electrochemical Sensors for the Detection of Phenolic Compounds in Olive Oil
4.2. Electrochemical Sensors for Evaluating the Acidity Index and the Peroxide Index of Olive Oil
4.3. Electrochemical Sensors for the Sensory Evaluation of the Bitterness and Purgency of Olive Oil
4.4. Sensor Arrays. E-Tongue for the Detection of Phenolic Compounds in Olive Oil
4.5. Sensor Arrays: E-Tongue for Evaluating the Acidity Index and the Peroxide Index of Olive Oil
4.6. Sensor Arrays. Electronic Tongues for the Sensory Evaluation of the Bitterness and Purgency of Olive Oil
4.7. Biosensors for the Detection of Phenolic Compounds in Olive Oil
4.8. Biosensors for the Sensory Evaluation of the Bitterness and Pungency of Olive Oil
4.9. Biosensors for Determining the Degree of Olive Oil Rancidity
4.10. Biosensors for Detecting Contaminants in Olive Oil
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz, F.; Julca, I.; Gómez-Garrido, J.; Loska, D.; Marcet-Houben, M.; Cano, E.; Galán, B.; Frias, L.; Ribeca, P.; Derdak, S.; et al. Genome sequence of the olive tree, Olea europaea. GigaScience 2016, 5, 29. [Google Scholar] [CrossRef]
- Garcia-Martinez, O.; Ruiz, C.; Gutierrez-Ibanez, A.; Illescas-Montes, R.; Melguizo-Rodriguez, L. Benefits of Olive Oil Phenolic Compounds in Disease Prevention. Endocr. Metab. Immune Disord.-Drug Targets 2018, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.D.; Cramer, H.; Michalsen, A.; Kessler, C.; Steckhan, N.; Choi, K.; Dobos, G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine 2015, 22, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, C.; Masella, R. Role of polyphenols in cell death control. Nutr. Neurosci. 2012, 15, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vitallini, S. Health-Promoting Effects of Traditional Mediterranean Diets—A Review. Pol. J. Food Nutr. Sci. 2012, 62, 71–76. [Google Scholar] [CrossRef]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition 2020, 69, 110559. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Ali, M.A.; Gong, S.; Sun, Q.; Bi, X.; Liu, S.; Guo, L. Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control. 2018, 94, 289–294. [Google Scholar] [CrossRef]
- Da Silva, R.M.A. Evaluation of the Antimicrobial Potential of Natural Extracts on Helicobacter pylori. Master’s Thesis, University of Coimbra, Coimbra, Portugal, October 2020. [Google Scholar]
- Frontiers|miRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells|Pharmacology. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2020.574317/full (accessed on 27 October 2021).
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates: Antitumor perspectives of oleuropei. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Semenov, V.; Volkov, S.; Khaydukova, M.; Fedorov, A.; Lisitsyna, I.; Kirsanov, D.; Legin, A. Determination of three quality parameters in vegetable oils using potentiometric e-tongue. J. Food Compos. Anal. 2019, 75, 75–80. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable oil blending: A review of physicochemical, nutritional and health effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Aparicio-Ruiz, R.; Tena, N.; García-González, D.L. Authenticity of olive oil: Mapping and comparing official methods and promising alternatives. Food Res. Int. 2013, 54, 2025–2038. [Google Scholar] [CrossRef]
- Bajoub, A.; Bendini, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Olive oil authentication: A comparative analysis of regulatory frameworks with especial emphasis on quality and authenticity indices, and recent analytical techniques developed for their assessment. A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 832–857. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. Prediction of various chemical parameters of olive oils with Fourier transform infrared spectroscopy. LWT-Food Sci. Technol. 2015, 63, 978–984. [Google Scholar] [CrossRef]
- Mabood, F.; Boqué, R.; Folcarelli, R.; Busto, O.; Jabeen, F.; Al-Harrasi, A.; Hussain, J. The effect of thermal treatment on the enhancement of detection of adulteration in extra virgin olive oils by synchronous fluorescence spectroscopy and chemometric analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 161, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Poulli, K.I.; Mousdis, G.A.; Georgiou, C.A. Synchronous fluorescence spectroscopy for quantitative determination of virgin olive oil adulteration with sunflower oil. Anal. Bioanal. Chem. 2006, 386, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Sayago, A.; Morales, M.T.; Aparicio, R. Detection of hazelnut oil in virgin olive oil by a spectrofluorimetric method. Eur. Food Res. Technol. 2004, 218, 480–483. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Evaluation of the overall quality of olive oil using fluorescence spectroscopy. Food Chem. 2015, 173, 927–934. [Google Scholar] [CrossRef]
- De Luca, M.; Restuccia, D.; Clodoveo, M.L.; Puoci, F.; Ragno, G. Chemometric analysis for discrimination of extra virgin olive oils from whole and stoned olive pastes. Food Chem. 2016, 202, 432–437. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Daun, J.K.; Przybylski, R. FT-IR based methodology for quantitation of total tocopherols, tocotrienols and plastochromanol-8 in vegetable oils. J. Food Compos. Anal. 2005, 18, 359–364. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; Osorio, M.T.; Koidis, A.; González-Casado, A.; Cuadros-Rodríguez, L. Chemometric classification and quantification of olive oil in blends with any edible vegetable oils using FTIR-ATR and Raman spectroscopy. LWT 2017, 86, 174–184. [Google Scholar] [CrossRef]
- Tay, A.; Singh, R.K.; Krishnan, S.S.; Gore, J.P. Authentication of Olive Oil Adulterated with Vegetable Oils Using Fourier Transform Infrared Spectroscopy. LWT-Food Sci. Technol. 2002, 35, 99–103. [Google Scholar] [CrossRef]
- Kelly, S.; Parker, I.; Sharman, M.; Dennis, J.; Goodall, I. Assessing the authenticity of single seed vegetable oils using fatty acid stable carbon isotope ratios (13C12C). Food Chem. 1997, 59, 181–186. [Google Scholar] [CrossRef]
- Jakab, A.; Héberger, K.; Forgács, E. Comparative analysis of different plant oils by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2002, 976, 255–263. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Gómez-Romero, M.; Ajal, E.A.; Bagur-González, M.G.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Assessing the varietal origin of extra-virgin olive oil using liquid chromatography fingerprints of phenolic compound, data fusion and chemometrics. Food Chem. 2017, 215, 245–255. [Google Scholar] [CrossRef]
- Alarcón Flores, M.I.; Romero-González, R.; Garrido Frenich, A.; Martínez Vidal, J.L. Analysis of phenolic compounds in olive oil by solid-phase extraction and ultra high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2012, 134, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Capannesi, C.; Palchetti, I.; Mascini, M.; Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000, 71, 553–562. [Google Scholar] [CrossRef]
- Apetrei, C. Novel method based on polypyrrole-modified sensors and emulsions for the evaluation of bitterness in extra virgin olive oils. Food Res. Int. 2012, 48, 673–680. [Google Scholar] [CrossRef]
- Harzalli, U.; Rodrigues, N.; Veloso, A.C.A.; Dias, L.G.; Pereira, J.A.; Oueslati, S.; Peres, A.M. A taste sensor device for unmasking admixing of rancid or winey-vinegary olive oil to extra virgin olive oil. Comput. Electron. Agric. 2018, 144, 222–231. [Google Scholar] [CrossRef]
- Apetrei, C.; Gutierez, F.; Rodríguez-Méndez, M.L.; de Saja, J.A. Novel method based on carbon paste electrodes for the evaluation of bitterness in extra virgin olive oils. Sens. Actuators B Chem. 2007, 121, 567–575. [Google Scholar] [CrossRef]
- Fernández, E.; Vidal, L.; Canals, A. Rapid determination of hydrophilic phenols in olive oil by vortex-assisted reversed-phase dispersive liquid-liquid microextraction and screen-printed carbon electrodes. Talanta 2018, 181, 44–51. [Google Scholar] [CrossRef]
- Martini, E.; Tomassetti, M.; Campanella, L.; Fortuna, A. Reducing the Pollutant Load of Olive Mill Wastewater by Photocatalytic Membranes and Monitoring the Process Using Both Tyrosinase Biosensor and COD Test. Front. Chem. 2013, 1, 36. [Google Scholar] [CrossRef]
- Escuderos, M.E.; García, M.; Jiménez, A.; Horrillo, M.C. Edible and non-edible olive oils discrimination by the application of a sensory olfactory system based on tin dioxide sensors. Food Chem. 2013, 136, 1154–1159. [Google Scholar] [CrossRef]
- Oliveri, P.; Baldo, M.A.; Daniele, S.; Forina, M. Development of a voltammetric electronic tongue for discrimination of edible oils. Anal. Bioanal. Chem. 2009, 395, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Evaluation of extra-virgin olive oils shelf life using an electronic tongue—Chemometric approach. Eur. Food Res. Technol. 2017, 243, 597–607. [Google Scholar] [CrossRef]
- Dias, L.G.; Fernandes, A.; Veloso, A.C.; Machado, A.A.; Pereira, J.A.; Peres, A.M. Single-cultivar extra virgin olive oil classification using a potentiometric electronic tongue. Food Chem. 2014, 160, 321–329. [Google Scholar] [PubMed]
- Apetrei, I.M.; Apetrei, C. Voltammetric e-tongue for the quantification of total polyphenol content in olive oils. Food Res. Int. 2013, 54, 2075–2082. [Google Scholar] [CrossRef]
- Baldo, M.A.; Oliveri, P.; Simonetti, R.; Daniele, S. A novel electroanalytical approach based on the use of a room temperature ionic liquid for the determination of olive oil acidity. Talanta 2016, 161, 881–887. [Google Scholar] [CrossRef]
- Cano Marchal, P.; Martínez Gila, D.; Gámez García, J.; Gómez Ortega, J. Expert system based on computer vision to estimate the content of impurities in olive oil samples. J. Food Eng. 2013, 119, 220–228. [Google Scholar] [CrossRef]
- Haddi, Z.; Alami, H.; El Bari, N.; Tounsi, M.; Barhoumi, H.; Maaref, A.; Jaffrezic-Renault, N.; Bouchikhi, B. Electronic nose and tongue combination for improved classification of Moroccan virgin olive oil profiles. Food Res. Int. 2013, 54, 1488–1498. [Google Scholar] [CrossRef]
- Apetrei, C.; Apetrei, I.M.; Villanueva, S.; de Saja, J.A.; Gutierrez-Rosales, F.; Rodriguez-Mendez, M.L. Combination of an e-nose, an e-tongue and an e-eye for the characterisation of olive oils with different degree of bitterness. Anal. Chim. Acta 2010, 663, 91–97. [Google Scholar] [CrossRef]
- Buratti, S.; Malegori, C.; Benedetti, S.; Oliveri, P.; Giovanelli, G. E-nose, e-tongue and e-eye for edible olive oil characterization and shelf life assessment: A powerful data fusion approach. Talanta 2018, 182, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Amine, A.; Brett, C.M.A.; Oliveira-Brett, A.M. Virgin olive oil ortho-phenols—Electroanalytical quantification. Talanta 2013, 105, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, I.; Eremia, S.A.V.; Albu, C.; Radoi, A.; Litescu, S.-C.; Radu, G.-L. Determination of the antiradical properties of olive oils using an electrochemical method based on DPPH radical. Food Chem. 2015, 166, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.; Sadun, C.; Gontrani, L.; Dini, D.; Antonelli, M.L. A new electrochemical sensor for extra-virgin olive oils classification. Food Control 2020, 109, 106903. [Google Scholar] [CrossRef]
- Rojas, D.; Della Pelle, F.; Del Carlo, M.; Fratini, E.; Escarpa, A.; Compagnone, D. Nanohybrid carbon black-molybdenum disulfide transducers for preconcentration-free voltammetric detection of the olive oil o-diphenols hydroxytyrosol and oleuropein. Microchim. Acta 2019, 186, 363. [Google Scholar] [CrossRef]
- Della Pelle, F.; Di Battista, R.; Vázquez, L.; Palomares, F.J.; Del Carlo, M.; Sergi, M.; Compagnone, D.; Escarpa, A. Press-transferred carbon black nanoparticles for class-selective antioxidant electrochemical detection. Appl. Mater. Today 2017, 9, 29–36. [Google Scholar] [CrossRef]
- Morozova, K.; Aprea, E.; Cantini, C.; Migliorini, M.; Gasperi, F.; Scampicchio, M. Determination of Bitterness of Extra Virgin Olive Oils by Amperometric Detection. Electroanalysis 2016, 28, 2196–2204. [Google Scholar] [CrossRef]
- Nadifiyine, S.; Haddam, M.; Mandli, J.; Chadel, S.; Blanchard, C.C.; Marty, J.L.; Amine, A. Amperometric Biosensor Based on Tyrosinase Immobilized on to a Carbon Black Paste Electrode for Phenol Determination in Olive Oil. Anal. Lett. 2013, 46, 2705–2726. [Google Scholar] [CrossRef]
- Hammami, A.; Kuliček, J.; Raouafi, N. A naphthoquinone/SAM-mediated biosensor for olive oil polyphenol content. Food Chem. 2016, 209, 274–278. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L. Electrochemical sensor for hydroperoxides determination based on Prussian blue film modified electrode. Sens. Actuators B Chem. 2008, 133, 588–592. [Google Scholar] [CrossRef]
- Galeano-Diaz, T.; Espinosa-Mansilla, A.; Murillo, B.R.; Salinas, F. Voltammetric Behavior and Determination of Nordihydroguaiaretic Acid in Presence of Other Antioxidants Using PLS Calibration. Electroanalysis 2003, 15, 646–651. [Google Scholar] [CrossRef]
- Del Carlo, M.; Amine, A.; Haddam, M.; della Pelle, F.; Fusella, G.C.; Compagnone, D. Selective Voltammetric Analysis of o-Diphenols from Olive Oil Using Na2MoO4 as Electrochemical Mediator. Electroanalysis 2012, 24, 889–896. [Google Scholar] [CrossRef]
- Zaroual, H.; Chénè, C.; El Hadrami, E.M.; Karoui, R. Application of new emerging techniques in combination with classical methods for the determination of the quality and authenticity of olive oil: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Ortega, J.; Martínez Gila, D.M.; Aguilera Puerto, D.; Gámez García, J.; Gómez Ortega, J. Novel technologies for monitoring the in-line quality of virgin olive oil during manufacturing and storage: Monitoring the quality of olive oil. J. Sci. Food Agric. 2016, 96, 4644–4662. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Sarni-Manchado, P.; Quideau, S. (Eds.) Recent Advances in Polyphenol Research: Cheynier/Recent Advances in Polyphenol Research; Wiley-Blackwell: Oxford, UK, 2012; ISBN 978-1-118-29975-3. [Google Scholar]
- Visioli, F.; Bernardini, E. Extra Virgin Olive Oil’s Polyphenols: Biological Activities. Curr. Pharm. Des. 2011, 17, 786–804. [Google Scholar] [CrossRef]
- Hardin-Fanning, F. The Effects of a Mediterranean-Style Dietary Pattern on Cardiovascular Disease Risk. Nurs. Clin. N. Am. 2008, 43, 105–115. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Arranz, S.; Silván, J.M.; Saura-Calixto, F. Nonextractable polyphenols, usually ignored, are the major part of dietary polyphenols: A study on the Spanish diet. Mol. Nutr. Food Res. 2010, 54, 1646–1658. [Google Scholar] [CrossRef]
- Marx, Í.; Veloso, A.; Dias, L.; Casal, S.; Pereira, J.; Peres, A. Electrochemical Sensor-Based Devices for Assessing Bioactive Compounds in Olive Oils: A Brief Review. Electronics 2018, 7, 387. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Fagnani, R.; Galli, G.V.; Maschi, O.; Gilardi, F.; Bellosta, S.; Crestani, M.; Bosisio, E.; De Fabiani, E.; Caruso, D. Olive Oil Phenols Modulate the Expression of Metalloproteinase 9 in THP-1 Cells by Acting on Nuclear Factor-κB Signaling. J. Agric. Food Chem. 2010, 58, 2246–2252. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar]
- Sangiovanni, E.; Colombo, E.; Fumagalli, M.; Abbiati, F.; Caruso, D.; Dell’Agli, M. Inhibition of NF-κB Activity by Minor Polar Components of Extra-Virgin Olive Oil at Gastric Level: Olive Oil Phenols and NF- κ B Activity in Gastric Cells. Phytother. Res. 2012, 26, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Dabbou, S.; Selvaggini, R.; Urbani, S.; Taticchi, A.; Servili, M.; Hammami, M. Comparison of the Chemical Composition and the Organoleptic Profile of Virgin Olive Oil from Two Wild and Two Cultivated Tunisian Olea europaea. Chem. Biodivers. 2011, 8, 189–202. [Google Scholar] [CrossRef]
- El Riachy, M.; Priego-Capote, F.; León, L.; Rallo, L.; Luque de Castro, M.D. Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. Eur. J. Lipid Sci. Technol. 2011, 113, 692–707. [Google Scholar] [CrossRef]
- Tovar, M.J.; Motilva, M.J.; Romero, M.P. Changes in the Phenolic Composition of Virgin Olive Oil from Young Trees (Olea europaea L. cv. Arbequina) Grown under Linear Irrigation Strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef]
- Bucelli, P.; Costantini, E.A.C.; Barbetti, R.; Franchini, E. Soil Water Availability in Rainfed Cultivation Affects More than Cultivar Some Nutraceutical Components and the Sensory Profile of Virgin Olive Oil. J. Agric. Food Chem. 2011, 59, 8304–8313. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Del Carlo, M.; Compagnone, D.; Cichelli, A. Effects of Fly Attack (Bactrocera oleae) on the Phenolic Profile and Selected Chemical Parameters of Olive Oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef]
- Anastasopoulos, E.; Kalogeropoulos, N.; Kaliora, A.C.; Kountouri, A.; Andrikopoulos, N.K. The influence of ripening and crop year on quality indices, polyphenols, terpenic acids, squalene, fatty acid profile, and sterols in virgin olive oil (Koroneiki cv.) produced by organic versus non-organic cultivation method: Quality and bioactive microconstituents of organic and non-organic olive oil. Int. J. Food Sci. Technol. 2011, 46, 170–178. [Google Scholar]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Romero, A.; Tous, J.; Caixach, J. The Activity of Healthy Olive Microbiota during Virgin Olive Oil Extraction Influences Oil Chemical Composition. J. Agric. Food Chem. 2011, 59, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Grossi, M.; Palagano, R.; Bendini, A.; Riccò, B.; Servili, M.; García-González, D.L.; Gallina Toschi, T. Design and in-house validation of a portable system for the determination of free acidity in virgin olive oil. Food Control 2019, 104, 208–216. [Google Scholar] [CrossRef]
- Iqdiam, B.M.; Abuagela, M.O.; Marshall, S.M.; Yagiz, Y.; Goodrich-Schneider, R.; Baker, G.L.; Welt, B.A.; Marshall, M.R. Combining high power ultrasound pre-treatment with malaxation oxygen control to improve quantity and quality of extra virgin olive oil. J. Food Eng. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Marquez, A.J.; Díaz, A.M.; Reguera, M.I.P. Using optical NIR sensor for on-line virgin olive oils characterization. Sens. Actuators B Chem. 2005, 107, 64–68. [Google Scholar] [CrossRef]
- Abu-Khalaf, N.; Hmidat, M. Visible/Near Infrared (VIS/NIR) spectroscopy as an optical sensor for evaluating olive oil quality. Comput. Electron. Agric. 2020, 173, 105445. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Jimenez, A.; Sanchez-Villasclaras, S.; Uceda, M.; Beltran, G. Modulation of bitterness and pungency in virgin olive oil from unripe “Picual” fruits. Eur. J. Lipid Sci. Technol. 2015, 117, 1463–1472. [Google Scholar] [CrossRef]
- Hernández, M.L.; Sicardo, M.D.; Belaj, A.; Martínez-Rivas, J.M. The Oleic/Linoleic Acid Ratio in Olive (Olea europaea L.) Fruit Mesocarp Is Mainly Controlled by OeFAD2-2 and OeFAD2-5 Genes Together With the Different Specificity of Extraplastidial Acyltransferase Enzymes. Front. Plant Sci. 2021, 12, 345. [Google Scholar] [CrossRef]
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic compounds and quality parameters of family farming versus protected designation of origin (PDO) extra-virgin olive oils. J. Food Compos. Anal. 2015, 43, 75–81. [Google Scholar] [CrossRef]
- Angelino, D.; Gennari, L.; Blasa, M.; Selvaggini, R.; Urbani, S.; Esposto, S.; Servili, M.; Ninfali, P. Chemical and Cellular Antioxidant Activity of Phytochemicals Purified from Olive Mill Waste Waters. J. Agric. Food Chem. 2011, 59, 2011–2018. [Google Scholar] [PubMed]
- Trombetta, D.; Smeriglio, A.; Marcoccia, D.; Giofrè, S.V.; Toscano, G.; Mazzotti, F.; Giovanazzi, A.; Lorenzetti, S. Analytical Evaluation and Antioxidant Properties of Some Secondary Metabolites in Northern Italian Mono- and Multi-Varietal Extra Virgin Olive Oils (EVOOs) from Early and Late Harvested Olives. Int. J. Mol. Sci. 2017, 18, 797. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-García, A.M.; Gómez-Rico, A.; Desamparados Salvador, M.; Fregapane, G. Effect of Preprocessing Olive Storage Conditions on Virgin Olive Oil Quality and Composition. J. Agric. Food Chem. 2010, 58, 4858–4865. [Google Scholar] [CrossRef] [PubMed]
- Pitozzi, V.; Jacomelli, M.; Catelan, D.; Servili, M.; Taticchi, A.; Biggeri, A.; Dolara, P.; Giovannelli, L. Long-Term Dietary Extra-Virgin Olive Oil Rich in Polyphenols Reverses Age-Related Dysfunctions in Motor Coordination and Contextual Memory in Mice: Role of Oxidative Stress. Rejuvenation Res. 2012, 15, 601–612. [Google Scholar] [CrossRef]
- Rubió, L.; Valls, R.-M.; Macià, A.; Pedret, A.; Giralt, M.; Romero, M.-P.; de la Torre, R.; Covas, M.-I.; Solà, R.; Motilva, M.-J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and healthText with EEA relevance. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS Assays to Assess the Antioxidant Profile of Untreated Oils. Food Anal. Methods 2015, 8, 1294–1302. [Google Scholar] [CrossRef]
- Borges, T.H.; Serna, A.; López, L.C.; Lara, L.; Nieto, R.; Seiquer, I. Composition and Antioxidant Properties of Spanish Extra Virgin Olive Oil Regarding Cultivar, Harvest Year and Crop Stage. Antioxidants 2019, 8, 217. [Google Scholar] [CrossRef]
- Liu, R.; Lu, M.; Zhang, T.; Zhang, Z.; Jin, Q.; Chang, M.; Wang, X. Evaluation of the Antioxidant Properties of Micronutrients in Different Vegetable Oils. Eur. J. Lipid Sci. Technol. 2020, 122, 1900079. [Google Scholar] [CrossRef]
- Habibi-Nodeh, F.; Farhoosh, R.; Sharif, A. Frying stability time of olive oils estimated from the oxidative stability index. Food Meas. 2019, 13, 1831–1838. [Google Scholar] [CrossRef]
- Heidarpour, M.; Farhoosh, R. A preliminary Rancimat-based kinetic approach of detecting olive oil adulteration. LWT 2018, 90, 77–82. [Google Scholar] [CrossRef]
- Pascale, R.; Bianco, G.; Cataldi, T.R.I.; Buchicchio, A.; Losito, I.; Altieri, G.; Genovese, F.; Tauriello, A.; Di Renzo, G.C.; Lafiosca, M.C. Investigation of the Effects of Virgin Olive Oil Cleaning Systems on the Secoiridoid Aglycone Content Using High Performance Liquid Chromatography-Mass Spectrometry. J. Am. Oil. Chem. Soc. 2018, 95, 665–671. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M.; Boskou, D. α-Tocopherol Content of Greek Virgin Olive Oils. J. Agric. Food Chem. 2000, 48, 1770–1775. [Google Scholar] [CrossRef]
- Bakre, S.M.; Gadmale, D.K.; Toche, R.B.; Gaikwad, V.B. Rapid determination of alpha tocopherol in olive oil adulterated with sunflower oil by reversed phase high-performance liquid chromatography. J. Food Sci. Technol. 2015, 52, 3093–3098. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Taticchi, A.; Urbani, S.; Di Maio, I.; Veneziani, G.; Selvaggini, R. New approaches to virgin olive oil quality, technology, and by-products valorization: New approaches to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2015, 117, 1882–1892. [Google Scholar] [CrossRef]
- Dugo, L.; Russo, M.; Cacciola, F.; Mandolfino, F.; Salafia, F.; Vilmercati, A.; Fanali, C.; Casale, M.; De Gara, L.; Dugo, P.; et al. Determination of the Phenol and Tocopherol Content in Italian High-Quality Extra-Virgin Olive Oils by Using LC-MS and Multivariate Data Analysis. Food Anal. Methods 2020, 13, 1027–1041. [Google Scholar] [CrossRef]
- Cayuela, J.A.; García, J.F. Sorting olive oil based on alpha-tocopherol and total tocopherol content using near-infra-red spectroscopy (NIRS) analysis. J. Food Eng. 2017, 202, 79–88. [Google Scholar] [CrossRef]
- Martakos, I.; Kostakis, M.; Dasenaki, M.; Pentogennis, M.; Thomaidis, N. Simultaneous Determination of Pigments, Tocopherols, and Squalene in Greek Olive Oils: A Study of the Influence of Cultivation and Oil-Production Parameters. Foods 2019, 9, 31. [Google Scholar] [CrossRef]
- Kosma, I.; Vavoura, M.; Kontakos, S.; Karabagias, I.; Kontominas, M.; Apostolos, K.; Badeka, A. Characterization and Classification of Extra Virgin Olive Oil from Five Less Well-Known Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2016, 93, 837–848. [Google Scholar] [CrossRef]
- Roca, M.; Gandul-Rojas, B.; Gallardo-Guerrero, L.; Mínguez-Mosquera, M.I. Pigment parameters determining spanish virgin olive oil authenticity: Stability during storage. J. Am. Oil Chem. Soc. 2003, 80, 1237–1240. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal degradation kinetics of lutein, β-carotene and β-cryptoxanthin in virgin olive oils. J. Food Compos. Anal. 2011, 24, 811–820. [Google Scholar] [CrossRef]
- Bailey, J.R. (Ed.) Lycopene: Food Sources, Potential Role in Human Health and Antioxidant Effects; Food Science and Technology; Nova Publishers: New York, NY, USA, 2015; ISBN 978-1-63117-927-3. [Google Scholar]
- Serani, A.; Piacenti, D. Kinetics of pheophytin-A photodecomposition in extra virgin olive oil. J. Am. Oil Chem. Soc. 1992, 69, 469–470. [Google Scholar] [CrossRef]
- Boskou, D.; Clodoveo, M.L. Products from Olive Tree; IntechOpen: London, UK, 2016; ISBN 978-953-51-2724-6. [Google Scholar]
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive Leaf Addition Increases Olive Oil Nutraceutical Properties. Molecules 2019, 24, 545. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Cai, Q.; Xu, B. A critical review on analytical techniques to detect adulteration of extra virgin olive oil. Trends Food Sci. Technol. 2019, 91, 391–408. [Google Scholar] [CrossRef]
- Philippidis, A.; Poulakis, E.; Papadaki, A.; Velegrakis, M. Comparative Study using Raman and Visible Spectroscopy of Cretan Extra Virgin Olive Oil Adulteration with Sunflower Oil. Anal. Lett. 2017, 50, 1182–1195. [Google Scholar] [CrossRef]
- De Lima, T.K.; Musso, M.; Bertoldo Menezes, D. Using Raman spectroscopy and an exponential equation approach to detect adulteration of olive oil with rapeseed and corn oil. Food Chem. 2020, 333, 127454. [Google Scholar] [CrossRef]
- Vasconcelos, M.; Coelho, L.; Barros, A.; de Almeida, J.M.M.M. Study of adulteration of extra virgin olive oil with peanut oil using FTIR spectroscopy and chemometrics. Cogent Food Agric. 2015, 1, 1018695. [Google Scholar] [CrossRef]
- Özdemir, D.; Öztürk, B. Near infrared spectroscopic determination of olive oil adulteration with sunflower and corn oil. J. Food Drug Anal. 2007, 15, 12. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhao, Q.; Ouyang, J.; Wu, Y. Rapid detection of authenticity and adulteration of walnut oil by FTIR and fluorescence spectroscopy: A comparative study. Food Chem. 2015, 181, 25–30. [Google Scholar] [CrossRef]
- Mendes, T.O.; da Rocha, R.A.; Porto, B.L.S.; de Oliveira, M.A.L.; dos Anjos, V.d.C.; Bell, M.J.V. Quantification of Extra-virgin Olive Oil Adulteration with Soybean Oil: A Comparative Study of NIR, MIR, and Raman Spectroscopy Associated with Chemometric Approaches. Food Anal. Methods 2015, 8, 2339–2346. [Google Scholar] [CrossRef]
- Salah, W.A.; Nofal, M. Review of some adulteration detection techniques of edible oils. J. Sci. Food Agric. 2021, 101, 811–819. [Google Scholar] [CrossRef] [PubMed]
- IOC Standards, Methods and Guides. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 27 October 2021).
- Valli, E.; Bendini, A.; Berardinelli, A.; Ragni, L.; Riccò, B.; Grossi, M.; Gallina Toschi, T. Rapid and innovative instrumental approaches for quality and authenticity of olive oils: Innovative approaches for quality of virgin olive oils. Eur. J. Lipid Sci. Technol. 2016, 118, 1601–1619. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef]
- Mabrouk, S. Characterization and Classification of Different Tunisian Geographical Olive Oils using Voltammetric Electronic Tongue. J. Food Process. Technol. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Dias, L.G.; Rodrigues, N.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Monovarietal extra-virgin olive oil classification: A fusion of human sensory attributes and an electronic tongue. Eur. Food Res. Technol. 2016, 242, 259–270. [Google Scholar] [CrossRef]
- Slim, S.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Oueslati, S.; Peres, A.M. Application of an electronic tongue for Tunisian olive oils’ classification according to olive cultivar or physicochemical parameters. Eur. Food Res. Technol. 2017, 243, 1459–1470. [Google Scholar] [CrossRef]
- Souayah, F.; Rodrigues, N.; Veloso, A.C.A.; Dias, L.G.; Pereira, J.A.; Oueslati, S.; Peres, A.M. Discrimination of Olive Oil by Cultivar, Geographical Origin and Quality Using Potentiometric Electronic Tongue Fingerprints. J. Am. Oil. Chem. Soc. 2017, 94, 1417–1429. [Google Scholar] [CrossRef]
- Veloso, A.C.A.; Silva, L.M.; Rodrigues, N.; Rebello, L.P.G.; Dias, L.G.; Pereira, J.A.; Peres, A.M. Perception of olive oils sensory defects using a potentiometric taste device. Talanta 2018, 176, 610–618. [Google Scholar] [CrossRef]
- Veloso, A.C.A.; Dias, L.G.; Rodrigues, N.; Pereira, J.A.; Peres, A.M. Sensory intensity assessment of olive oils using an electronic tongue. Talanta 2016, 146, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.; Ghasemi-Varnamkhasti, M.; Mirela Apetrei, I. Chapter 27—Olive Oil and Combined Electronic Nose and Tongue. In Electronic Noses and Tongues in Food Science; Rodríguez Méndez, M.L., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 277–289. ISBN 978-0-12-800243-8. [Google Scholar]
- Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Monitoring olive oils quality and oxidative resistance during storage using an electronic tongue. LWT 2016, 73, 683–692. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Jeleń, H.H. Detection of olive oil adulteration with rapeseed and sunflower oils using mos electronic nose and smpe-ms. J. Food Qual. 2010, 33, 21–41. [Google Scholar] [CrossRef]
- Santonico, M.; Grasso, S.; Genova, F.; Zompanti, A.; Parente, F.; Pennazza, G. Unmasking of Olive Oil Adulteration Via a Multi-Sensor Platform. Sensors 2015, 15, 21660–21672. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.M.; Apetrei, C. Detection of virgin olive oil adulteration using a voltammetric e-tongue. Comput. Electron. Agric. 2014, 108, 148–154. [Google Scholar] [CrossRef]
- Marx, Í.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Sensory classification of table olives using an electronic tongue: Analysis of aqueous pastes and brines. Talanta 2017, 162, 98–106. [Google Scholar] [CrossRef]
- Marx, Í.M.G.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Quantification of table olives’ acid, bitter and salty tastes using potentiometric electronic tongue fingerprints. LWT-Food Sci. Technol. 2017, 79, 394–401. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.L.; Apetrei, C.; De Saja, J.A. Evaluation of the polyphenolic content of extra virgin olive oils using an array of voltammetric sensors. Electrochim. Acta 2008, 53, 5867–5872. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.L.; Apetrei, C.; De Saja, J.A. Electronic tongues purposely designed for the organoleptic characterization of olive oils. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 525–532. [Google Scholar]
- Prata, R.; Pereira, J.A.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Casal, S.; Peres, A.M. Olive Oil Total Phenolic Contents and Sensory Sensations Trends during Oven and Microwave Heating Processes and Their Discrimination Using an Electronic Tongue. J. Food Qual. 2018, 2018, 7826428. [Google Scholar] [CrossRef]
- Borges, T.H.; Peres, A.M.; Dias, L.G.; Seiquer, I.; Pereira, J.A. Application of a potentiometric electronic tongue for assessing phenolic and volatile profiles of Arbequina extra virgin olive oils. LWT 2018, 93, 150–157. [Google Scholar] [CrossRef]
- Benedetti, S.; Cosio, M.S.; Scampicchio, M.; Mannino, S. Nanoemulsions for the Determination of the Antioxidant Capacity of Oils by an Electrochemical Method. Electroanalysis 2012, 24, 1356–1361. [Google Scholar] [CrossRef]
- Ouni, Y.; Flamini, G.; Zarrouk, M. The Chemical Properties and Volatile Compounds of Virgin Olive Oil from Oueslati Variety: Influence of Maturity Stages in Olives. J. Am. Oil Chem. Soc. 2016, 93, 1265–1273. [Google Scholar] [CrossRef]
- Goulas, V.; Orphanides, A.; Pelava, E.; Gekas, V. Impact of Thermal Processing Methods on Polyphenols and Antioxidant Activity of Olive Oil Polar Fraction: Stability of Olive Oil Phenolics during Heating. J. Food Process. Preserv. 2015, 39, 1919–1924. [Google Scholar] [CrossRef]
- Apetrei, C.; Rodríguez-Méndez, M.L.; De Saja, J.A. Modified carbon paste electrodes for discrimination of vegetable oils. Sens. Actuators B Chem. 2005, 111, 403–409. [Google Scholar] [CrossRef]
- Ávila, M.; Crevillén, A.G.; González, M.C.; Escarpa, A.; Hortigüela, L.V.; de Lorenzo Carretero, C.; Pérez Martín, R.A. Electroanalytical Approach to Evaluate Antioxidant Capacity in Honeys: Proposal of an Antioxidant Index. Electroanalysis 2006, 18, 1821–1826. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique—A Review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Voltamperometric Sensors and Biosensors Based on Carbon Nanomaterials Used for Detecting Caffeic Acid—A Review. Int. J. Mol. Sci. 2020, 21, 9275. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a Novel Electrochemical Biosensor Based on Carbon Nanofibers–Cobalt Phthalocyanine–Laccase for the Detection of p-Coumaric Acid in Phytoproducts. Int. J. Mol. Sci. 2021, 22, 9302. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a Novel Electrochemical Biosensor Based on Carbon Nanofibers–Gold Nanoparticles–Tyrosinase for the Detection of Ferulic Acid in Cosmetics. Sensors 2020, 20, 6724. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Bayat, S.; Mansoori Derakhshan, S.; Mansoori Derakhshan, N.; Shekari Khaniani, M.; Alivand, M.R. Downregulation of HDAC2 and HDAC3 via oleuropein as a potent prevention and therapeutic agent in MCF-7 breast cancer cells. J. Cell Biochem. 2019, 120, 9172–9180. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-Free Microwave-Assisted Extraction of Polyphenols from Olive Tree Leaves: Antioxidant and Antimicrobial Properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Fleuriet, A.; Macheix, J.J. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 1986, 34, 823–826. [Google Scholar] [CrossRef]
- Amiot, M.-J.; Fleuriet, A.; Macheix, J.-J. Accumulation of oleuropein derivatives during olive maturation. Phytochemistry 1989, 28, 67–69. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Alonso, S.; Fregapane, G.; Salvador, M.D. Evaluation of minor components, sensory characteristics and quality of virgin olive oil by near infrared (NIR) spectroscopy. Food Res. Int. 2013, 50, 250–258. [Google Scholar] [CrossRef]
- Vicario, G.; Francini, A.; Cifelli, M.; Domenici, V.; Sebastiani, L. Near UV-Vis and NMR Spectroscopic Methods for Rapid Screening of Antioxidant Molecules in Extra-Virgin Olive Oil. Antioxidants 2020, 9, 1245. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Grossi, M.; Lecce, G.D.; Toschi, T.G.; Ricco, B. Fast and Accurate Determination of Olive Oil Acidity by Electrochemical Impedance Spectroscopy. IEEE Sens. J. 2014, 14, 2947–2954. [Google Scholar] [CrossRef]
- Rodrigues, N.; Marx, Í.M.G.; Casal, S.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Application of an electronic tongue as a single-run tool for olive oils’ physicochemical and sensory simultaneous assessment. Talanta 2019, 197, 363–373. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Lvova, L. Chapter 15—Electronic Tongue Principles and Applications in the Food Industry. In Electronic Noses and Tongues in Food Science; Rodríguez Méndez, M.L., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 151–160. ISBN 978-0-12-800243-8. [Google Scholar]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-Based Food Diagnostic Technologies: A Review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Bougadi, E.T.; Kalogianni, D.P. Paper-based DNA biosensor for food authenticity testing. Food Chem. 2020, 322, 126758. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. The biocomposite screen-printed biosensor based on immobilization of tyrosinase onto the carboxyl functionalised carbon nanotube for assaying tyramine in fish products. J. Food Eng. 2015, 149, 1–8. [Google Scholar] [CrossRef]
- Da Silva, W.; Ghica, M.E.; Ajayi, R.F.; Iwuoha, E.I.; Brett, C.M.A. Tyrosinase based amperometric biosensor for determination of tyramine in fermented food and beverages with gold nanoparticle doped poly(8-anilino-1-naphthalene sulphonic acid) modified electrode. Food Chem. 2019, 282, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kochana, J.; Wapiennik, K.; Knihnicki, P.; Pollap, A.; Janus, P.; Oszajca, M.; Kuśtrowski, P. Mesoporous carbon-containing voltammetric biosensor for determination of tyramine in food products. Anal. Bioanal. Chem. 2016, 408, 5199–5210. [Google Scholar] [CrossRef]
- Wu, L.; Yan, H.; Wang, J.; Liu, G.; Xie, W. Tyrosinase Incorporated with Au-Pt@SiO2 Nanospheres for Electrochemical Detection of Bisphenol A. J. Electrochem. Soc. 2019, 166, B562. [Google Scholar] [CrossRef]
- Sánchez-Paniagua López, M.; López-Ruiz, B. Electrochemical biosensor based on ionic liquid polymeric microparticles. An analytical platform for catechol. Microchem. J. 2018, 138, 173–179. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Laccase and Tyrosinase Biosensors Used in the Determination of Hydroxycinnamic Acids. Int. J. Mol. Sci. 2021, 22, 4811. [Google Scholar] [CrossRef]
- Yildiz, H.B.; Castillo, J.; Guschin, D.A.; Toppare, L.; Schuhmann, W. Phenol biosensor based on electrochemically controlled integration of tyrosinase in a redox polymer. Microchim. Acta 2007, 159, 27–34. [Google Scholar] [CrossRef]
- Arduini, F.; Amine, A.; Majorani, C.; Di Giorgio, F.; De Felicis, D.; Cataldo, F.; Moscone, D.; Palleschi, G. High performance electrochemical sensor based on modified screen-printed electrodes with cost-effective dispersion of nanostructured carbon black. Electrochem. Commun. 2010, 12, 346–350. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Paschoal, C.W.A.; Arias, A.C. Printed and flexible biosensor for antioxidants using interdigitated ink-jetted electrodes and gravure-deposited active layer. Biosens. Bioelectron. 2015, 67, 553–559. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, M.; Mostafavi, A.; Torkzadeh-Mahani, M. Voltammetric behavior of uric acid on carbon paste electrode modified with salmon sperm dsDNA and its application as label-free electrochemical sensor. Biosens. Bioelectron. 2014, 54, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Rafique, B.; Khalid, A.M.; Akhtar, K.; Jabbar, A. Interaction of anticancer drug methotrexate with DNA analyzed by electrochemical and spectroscopic methods. Biosens. Bioelectron. 2013, 44, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.; Caminiti, R.; Ingo, G.M.; Sadun, C.; Tortolini, C.; Antonelli, M.L. Biologically friendly room temperature ionic liquids and nanomaterials for the development of innovative enzymatic biosensors. Talanta 2017, 175, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Heydari-Bafrooei, E.; Rezaei, B. Different interaction of codeine and morphine with DNA: A concept for simultaneous determination. Biosens. Bioelectron. 2013, 41, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Mirmoghtadaie, L.; Ensafi, A.A.; Kadivar, M.; Norouzi, P. Highly selective electrochemical biosensor for the determination of folic acid based on DNA modified-pencil graphite electrode using response surface methodology. Mater. Sci. Eng. C 2013, 33, 1753–1758. [Google Scholar] [CrossRef]
- Mohamadi, M.; Mostafavi, A.; Torkzadeh-Mahani, M. Electrochemical determination of biophenol oleuropein using a simple label-free DNA biosensor. Bioelectrochemistry 2015, 101, 52–57. [Google Scholar] [CrossRef]
- Mateos, R.; Cert, A.; Pérez-Camino, M.C.; García, J.M. Evaluation of virgin olive oil bitterness by quantification of secoiridoid derivatives. J. Am. Oil Chem. Soc. 2004, 81, 71–75. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.H.C.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-ligstroside Aglycon as a Key Contributor to Pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.L.H.C.; Hrncirik, K.; Bulukin, E.; Boucon, C.; Mascini, M. Biosensor Measurements of Polar Phenolics for the Assessment of the Bitterness and Pungency of Virgin Olive Oil. J. Agric. Food Chem. 2006, 54, 4371–4377. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Lucas, J.; Murray, L.; Ritz, S.; Staley, S.; Tamsut, B.; Facciotti, M.; Siegel, J.; Tagkopoulos, I.; Wang, S. Implications of an Enzymatic Biosensor for the Detection of Rancid Olive Oil. 2014. Available online: https://2014.igem.org/wiki/images/0/07/UC_Davis_iGEM_2014_Practical_Implications_for_the_Development_and_Deployment_of_Engineered_Biosensors_in_Olive_Oil_Production.pdf (accessed on 15 November 2021).
- The European Commission. Commission Implementing Regulation (EU) No 400/2014 of 22 April 2014 concerning a coordinated multiannual control programme of the Union for 2015, 2016 and 2017 to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues in and on food of plant and animal origin (Text with EEA relevance) (repealed). Off. J. Eur. Union 2014, 119, 44–56. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Forchielli, M.; Scognamiglio, V.; Nikolaevna, K.; Moscone, D. Organophosphorous Pesticide Detection in Olive Oil by Using a Miniaturized, Easy-to-Use, and Cost-Effective Biosensor Combined with QuEChERS for Sample Clean-Up. Sensors 2016, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- El-Moghazy, A.Y.; Soliman, E.A.; Ibrahim, H.Z.; Marty, J.-L.; Istamboulie, G.; Noguer, T. Biosensor based on electrospun blended chitosan-poly (vinyl alcohol) nanofibrous enzymatically sensitized membranes for pirimiphos-methyl detection in olive oil. Talanta 2016, 155, 258–264. [Google Scholar] [CrossRef]

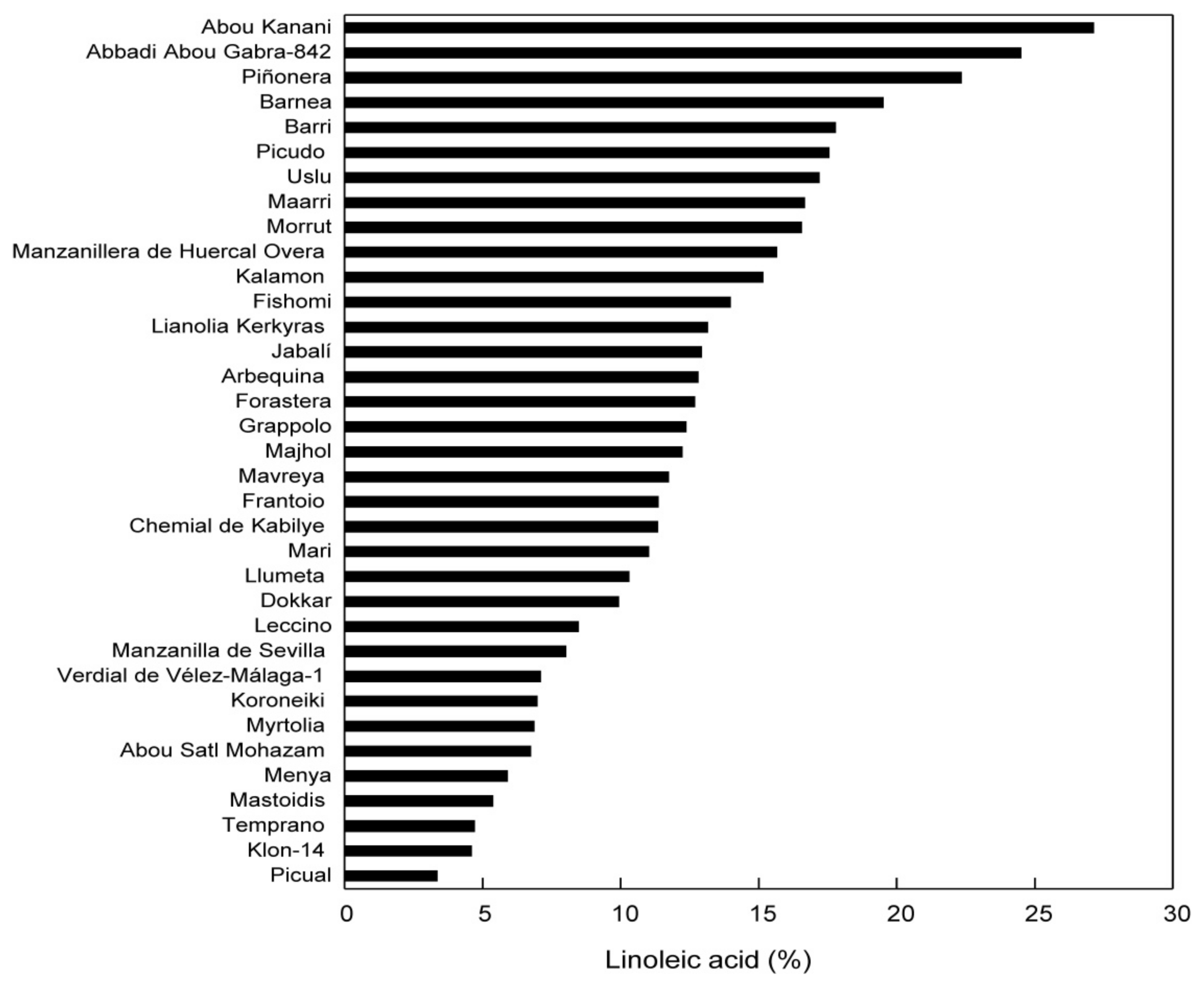
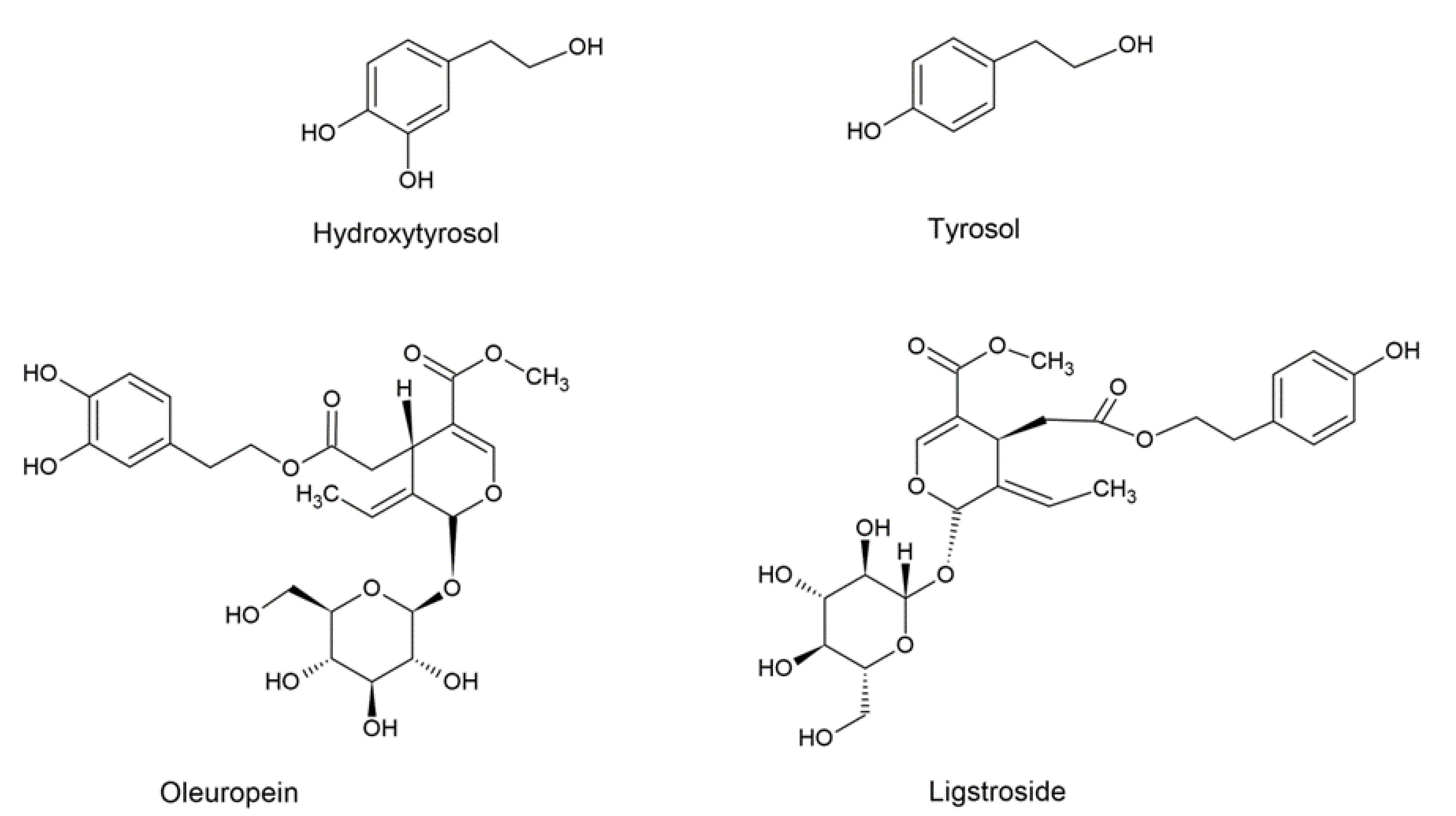

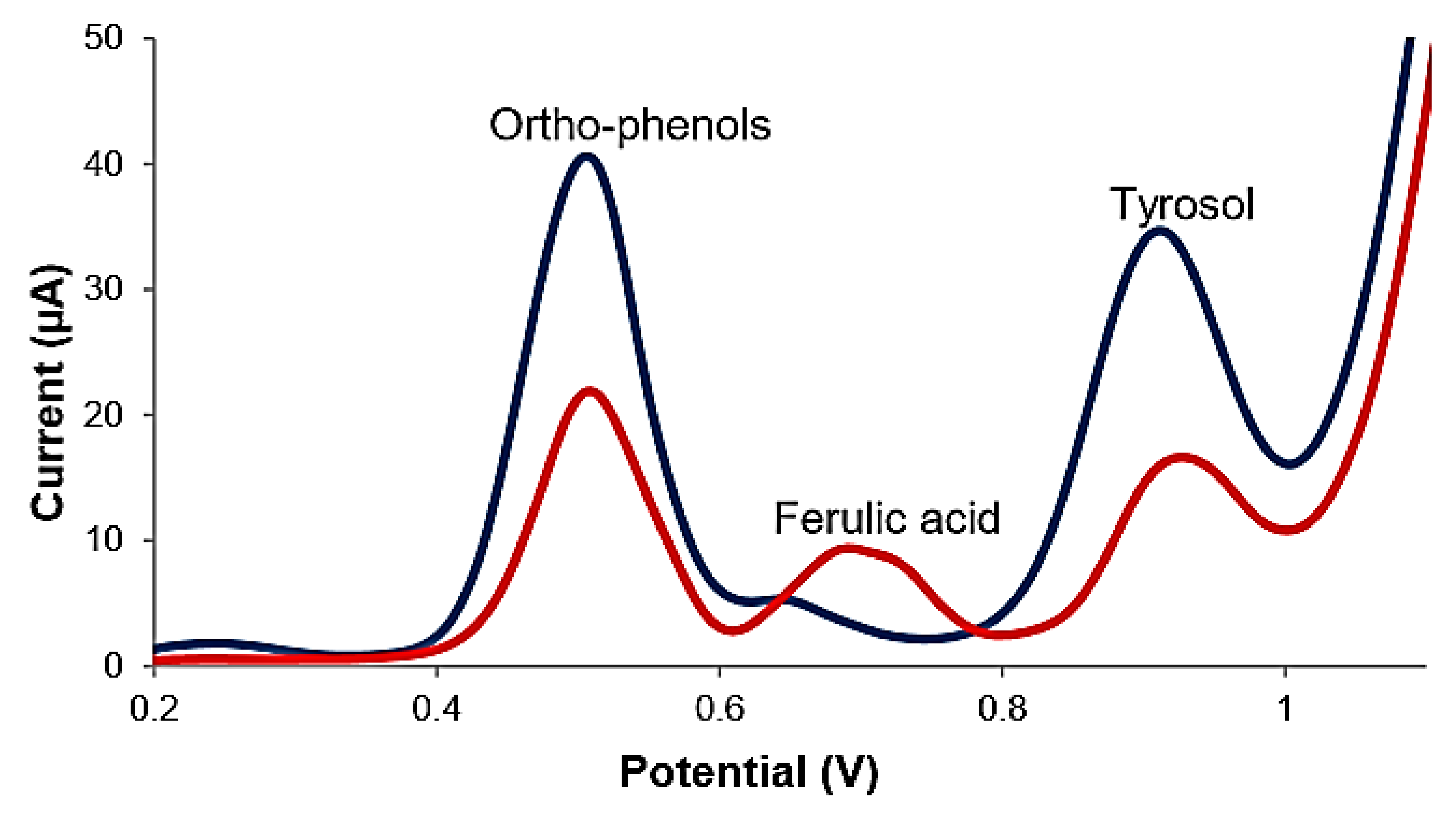


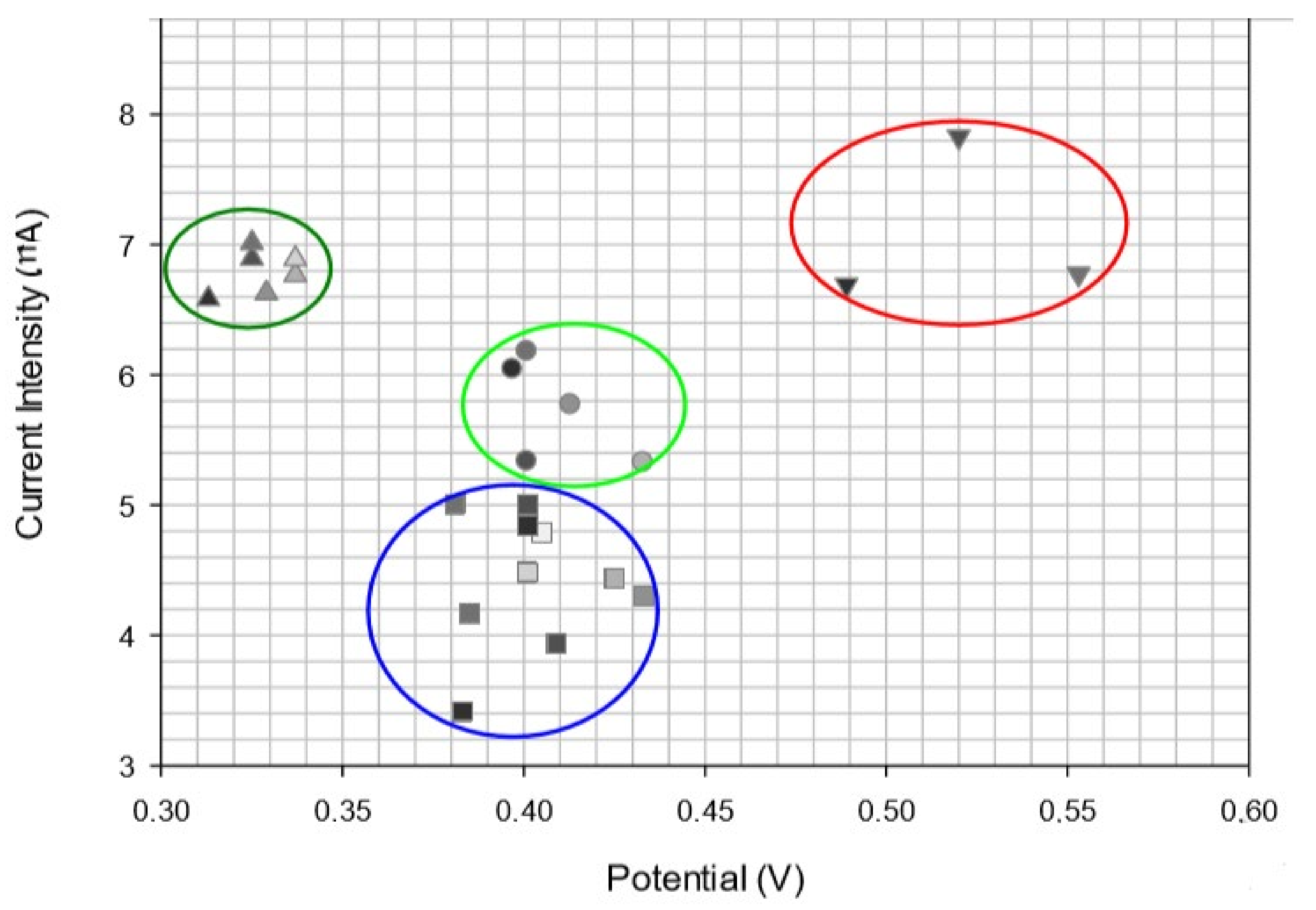

| (Bio)Sensor | Analyte | Detection Technique | LOD | Ref. |
|---|---|---|---|---|
| SPCE | caffeic acid | DPV | 0.022 mgL−1 | [32] |
| SPE | hydroxytyrosol | SWV | 0.4 μM | [44] |
| PtSPE | α-tocopherol | DPV | 0.365 μM | [45] |
| MWCNT/TiO2/RTIL/SPE | free fatty acids | CV | - | [46] |
| CB-MoS2 | oleuropein hydroxytyrosol | DPV | 0.11 μM 1.0 μM | [47] |
| CBNP | hydroxytyrosoltyrosol | DPV | 0.006 μM 0.020 μM | [48] |
| GCE | oleuropein | Amp | 1.58 μM | [49] |
| CBPE-Tyr | catechol | Amp | 6 nM | [50] |
| Tyr/CS/NQ-SAM/GE | phenol | ChronoAmp | 0.019 μM | [51] |
| SPE | oleuropein | DPV | 0.25 ppM | [28] |
| Tyrosinase-based biosensor | phenol | FIA | 4.0 ppM | [28] |
| PB-GC | peroxide | CV | 0.001 mequiv | [52] |
| GCA | nordihydroguaiaretic acid | CV DPV | - | [53] |
| Mo-MW-CNT-NH2/SPE | catechol | FIA | - | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bounegru, A.V.; Apetrei, C. Evaluation of Olive Oil Quality with Electrochemical Sensors and Biosensors: A Review. Int. J. Mol. Sci. 2021, 22, 12708. https://doi.org/10.3390/ijms222312708
Bounegru AV, Apetrei C. Evaluation of Olive Oil Quality with Electrochemical Sensors and Biosensors: A Review. International Journal of Molecular Sciences. 2021; 22(23):12708. https://doi.org/10.3390/ijms222312708
Chicago/Turabian StyleBounegru, Alexandra Virginia, and Constantin Apetrei. 2021. "Evaluation of Olive Oil Quality with Electrochemical Sensors and Biosensors: A Review" International Journal of Molecular Sciences 22, no. 23: 12708. https://doi.org/10.3390/ijms222312708
APA StyleBounegru, A. V., & Apetrei, C. (2021). Evaluation of Olive Oil Quality with Electrochemical Sensors and Biosensors: A Review. International Journal of Molecular Sciences, 22(23), 12708. https://doi.org/10.3390/ijms222312708







