Drosophila Trachea as a Novel Model of COPD
Abstract
:1. Urgent Need to Reveal Novel Mechanisms of COPD
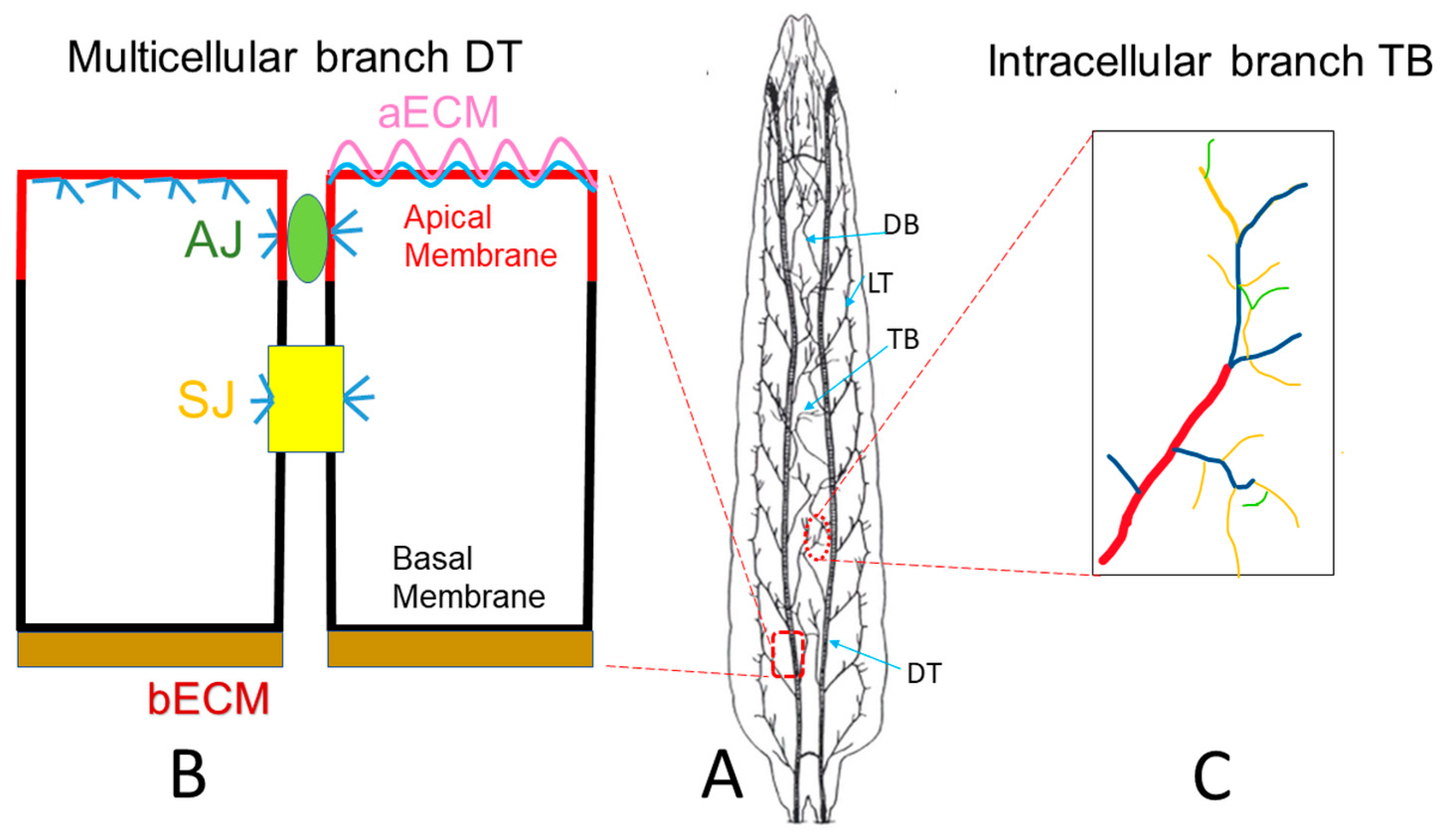
2. Drosophila Trachea as a Model to Reveal Underlying Mechanisms of Tube Morphogenesis
3. Drosophila Trachea as a COPD Model to Systematically Study the Development of COPD
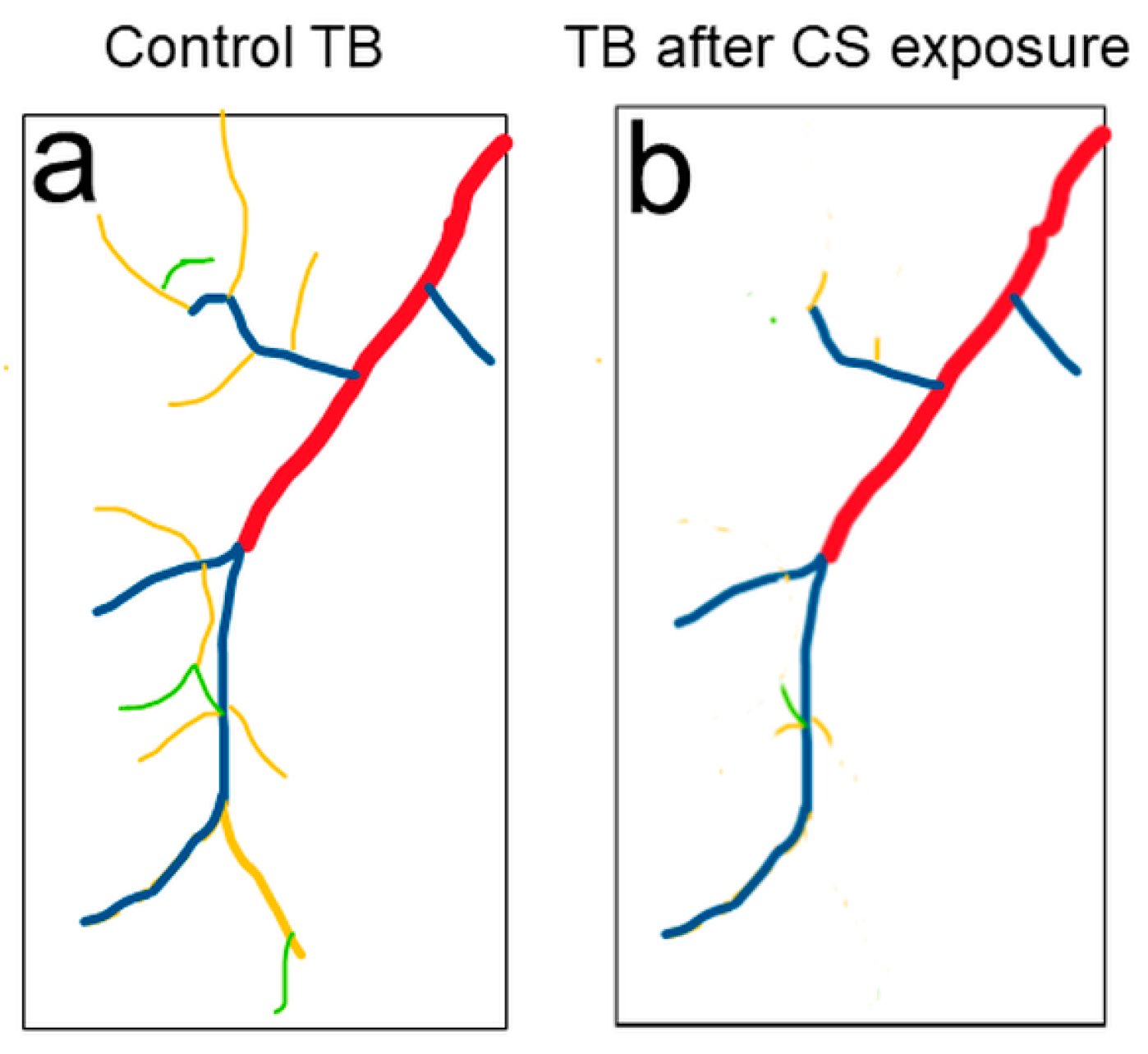
4. Drosophila Trachea as a Model to Reveal Novel Mechanisms of COPD
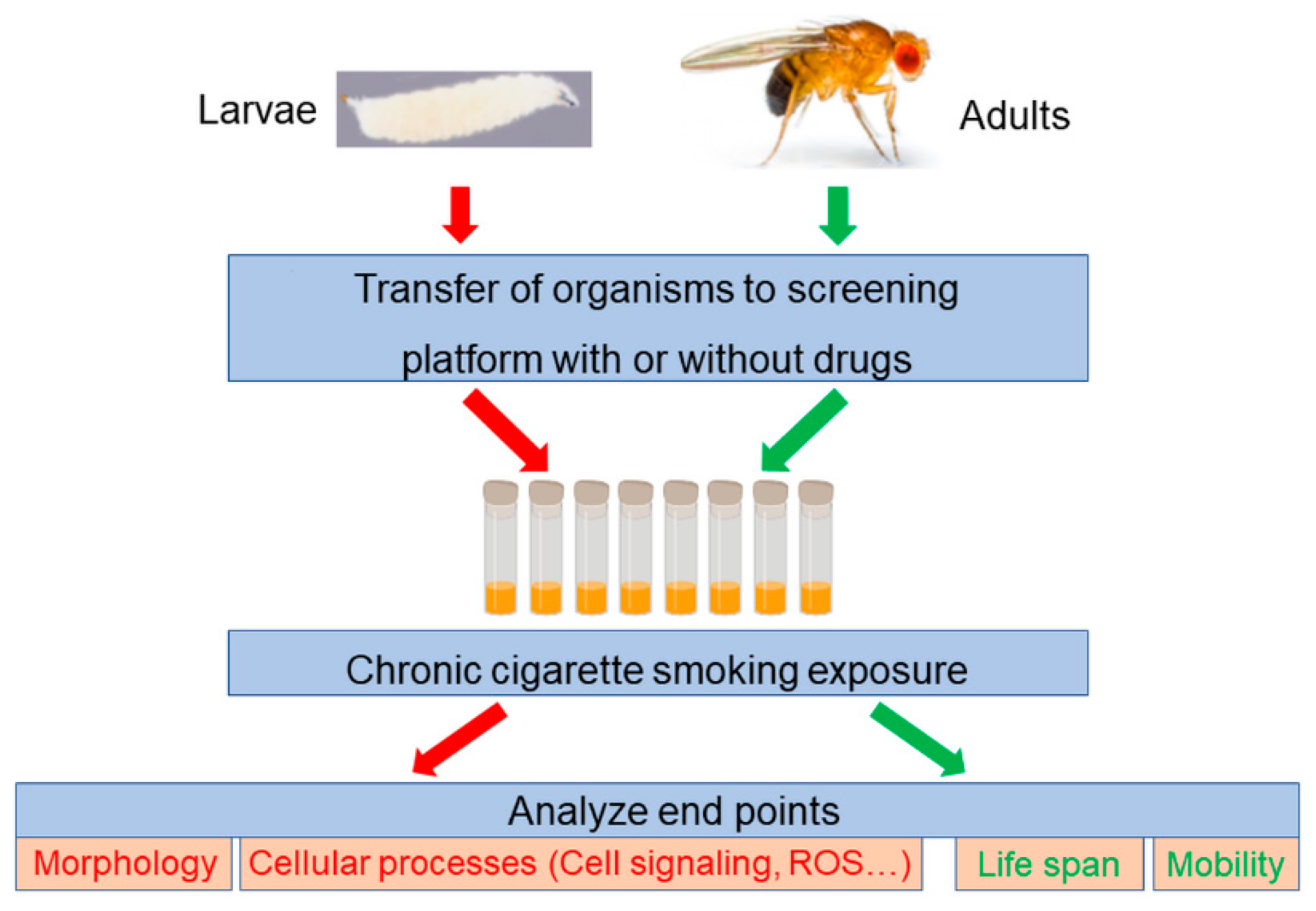
5. Drosophila Trachea as a Screening Platform to Identify Novel Treatments for COPD
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Viegi, G.; Maio, S.; Fasola, S.; Baldacci, S. Global Burden of Chronic Respiratory Diseases. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 171–177. [Google Scholar] [CrossRef]
- Terzikhan, N.; Verhamme, K.M.; Hofman, A.; Stricker, B.H.; Brusselle, G.G.; Lahousse, L. Prevalence and incidence of COPD in smokers and non-smokers: The Rotterdam Study. Eur. J. Epidemiol. 2016, 31, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Jørgensen, J.T.; Ljungman, P.; Pershagen, G.; Bellander, T.; Leander, K.; Magnusson, P.K.E.; Rizzuto, D.; Hvidtfeldt, U.A.; Raaschou-Nielsen, O.; et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environ. Int. 2021, 146, 106267. [Google Scholar] [CrossRef] [PubMed]
- Hendryx, M.; Luo, J.; Chojenta, C.; Byles, J.E. Air pollution exposures from multiple point sources and risk of incident chronic obstructive pulmonary disease (COPD) and asthma. Environ. Res. 2019, 179, 108783. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Li, F.; Ryffel, B.; Togbe, D.; Chung, K.F. Oxidative Stress in Ozone-Induced Chronic Lung Inflammation and Emphysema: A Facet of Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 1957. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Petrache, I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Investig. 2012, 122, 2749–2755. [Google Scholar] [CrossRef]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef]
- Kluchova, Z.; Petrasova, D.; Joppa, P.; Dorkova, Z.; Tkacova, R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiol. Res. 2007, 56, 51–56. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, 205. [Google Scholar] [CrossRef] [Green Version]
- Baarsma, H.A.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Gunther, A.; Bracke, K.R.; Dagouassat, M.; et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017, 214, 143–163. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; McGrath, S.; Neptune, E. The pathobiological mechanisms of emphysema models: What do they have in common? Pulm. Pharmacol. Ther. 2003, 16, 67–78. [Google Scholar] [CrossRef]
- Martinez, F.J.; Donohue, J.F.; Rennard, S.I. The future of chronic obstructive pulmonary disease treatment--difficulties of and barriers to drug development. Lancet 2011, 378, 1027–1037. [Google Scholar] [CrossRef]
- Michaudel, C.; Mackowiak, C.; Maillet, I.; Fauconnier, L.; Akdis, C.A.; Sokolowska, M.; Dreher, A.; Tan, H.T.; Quesniaux, V.F.; Ryffel, B.; et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J. Allergy Clin. Immunol. 2018, 142, 942–958. [Google Scholar] [CrossRef] [Green Version]
- Nishida, K.; Brune, K.A.; Putcha, N.; Mandke, P.; O’Neal, W.K.; Shade, D.; Srivastava, V.; Wang, M.; Lam, H.; An, S.S.; et al. Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L581–L591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wangler, M.F.; Yamamoto, S.; Chao, H.T.; Posey, J.E.; Westerfield, M.; Postlethwait, J.; Members of the Undiagnosed Diseases Network, (UDN); Hieter, P.; Boycott, K.M.; Campeau, P.M.; et al. Model Organisms Facilitate Rare Disease Diagnosis and Therapeutic Research. Genetics 2017, 207, 9–27. [Google Scholar] [CrossRef]
- Schneider, D. Using Drosophila as a model insect. Nat. Rev. Genet. 2000, 1, 218–226. [Google Scholar] [CrossRef]
- Ghabrial, A.; Luschnig, S.; Metzstein, M.M.; Krasnow, M.A. Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 2003, 19, 623–647. [Google Scholar] [CrossRef] [Green Version]
- Ohshiro, T.; Emori, Y.; Saigo, K. Ligand-dependent activation of breathless FGF receptor gene in Drosophila developing trachea. Mech. Dev. 2002, 114, 3–11. [Google Scholar] [CrossRef]
- Zuo, L.; Iordanou, E.; Chandran, R.R.; Jiang, L. Novel mechanisms of tube-size regulation revealed by the Drosophila trachea. Cell Tissue Res. 2013, 354, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Ruhle, H. Das larvale Tracheensystem von Drosophila melanogaster Meigen und seine Variabilita. Z. Wiss. Zool. 1932, 159–245. [Google Scholar]
- Devine, W.P.; Lubarsky, B.; Shaw, K.; Luschnig, S.; Messina, L.; Krasnow, M.A. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 17014–17019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonning, A.; Hemphala, J.; Tang, E.; Nannmark, U.; Samakovlis, C.; Uv, A. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell 2005, 9, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Behr, M.; Wingen, C.; Wolf, C.; Schuh, R.; Hoch, M. Wurst is essential for airway clearance and respiratory-tube size control. Nat. Cell Biol. 2007, 9, 847–853. [Google Scholar] [CrossRef]
- Tsarouhas, V.; Senti, K.A.; Jayaram, S.A.; Tiklova, K.; Hemphala, J.; Adler, J.; Samakovlis, C. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev. Cell 2007, 13, 214–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkau, G.; Wingen, C.; Jussen, L.C.; Radtke, T.; Behr, M. Obstructor-A is required for epithelial extracellular matrix dynamics, exoskeleton function, and tubulogenesis. J. Biol. Chem. 2012, 287, 21396–21405. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Hayashi, S. Shaping of biological tubes by mechanical interaction of cell and extracellular matrix. Curr. Opin. Genet. Dev. 2015, 32, 129–134. [Google Scholar] [CrossRef]
- Armbruster, K.; Luschnig, S. The Drosophila Sec7 domain guanine nucleotide exchange factor protein Gartenzwerg localizes at the cis-Golgi and is essential for epithelial tube expansion. J. Cell Sci. 2012, 125, 1318–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Meyer, H.; Ochoa-Espinosa, A.; Buchwald, U.; Onel, S.; Altenhein, B.; Heinisch, J.J.; Affolter, M.; Paululat, A. GBF1 (Gartenzwerg)-dependent secretion is required for Drosophila tubulogenesis. J. Cell Sci. 2012, 125, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Llimargas, M.; Strigini, M.; Katidou, M.; Karagogeos, D.; Casanova, J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development 2004, 131, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.M.; Schulte, J.; Hirschi, A.; Tepass, U.; Beitel, G.J. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 2004, 164, 313–323. [Google Scholar] [CrossRef]
- Wilkin, M.B.; Becker, M.N.; Mulvey, D.; Phan, I.; Chao, A.; Cooper, K.; Chung, H.J.; Campbell, I.D.; Baron, M.; MacIntyre, R. Drosophila dumpy is a gigantic extracellular protein required to maintain tension at epidermal-cuticle attachment sites. Curr. Biol. 2000, 10, 559–567. [Google Scholar] [CrossRef]
- Zhang, L.; Ward, R.E. uninflatable encodes a novel ectodermal apical surface protein required for tracheal inflation in Drosophila. Dev. Biol. 2009, 336, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Hannezo, E.; Dong, B.; Recho, P.; Joanny, J.F.; Hayashi, S. Cortical instability drives periodic supracellular actin pattern formation in epithelial tubes. Proc. Natl. Acad. Sci. USA 2015, 112, 8620–8625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matusek, T.; Djiane, A.; Jankovics, F.; Brunner, D.; Mlodzik, M.; Mihaly, J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development 2006, 133, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Araujo, S.J.; Cela, C.; Llimargas, M. Tramtrack regulates different morphogenetic events during Drosophila tracheal development. Development 2007, 134, 3665–3676. [Google Scholar] [CrossRef] [Green Version]
- Hemphala, J.; Uv, A.; Cantera, R.; Bray, S.; Samakovlis, C. Grainy head controls apical membrane growth and tube elongation in response to Branchless/FGF signalling. Development 2003, 130, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Gervais, L.; Casanova, J. In vivo coupling of cell elongation and lumen formation in a single cell. Curr. Biol. 2010, 20, 359–366. [Google Scholar] [CrossRef]
- Best, B.T. Single-cell branching morphogenesis in the Drosophila trachea. Dev. Biol. 2019, 451, 5–15. [Google Scholar] [CrossRef]
- Jones, T.A.; Metzstein, M.M. A novel function for the PAR complex in subcellular morphogenesis of tracheal terminal cells in Drosophila melanogaster. Genetics 2011, 189, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Ozturk-Colak, A.; Moussian, B.; Araujo, S.J. Drosophila chitinous aECM and its cellular interactions during tracheal development. Dev. Dyn. 2016, 245, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.A.; Nikolova, L.S.; Schjelderup, A.; Metzstein, M.M. Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Dev. Biol. 2014, 390, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Mathew, R.; Rios-Barrera, L.D.; Machado, P.; Schwab, Y.; Leptin, M. Transcytosis via the late endocytic pathway as a cell morphogenetic mechanism. EMBO J. 2020, 39, e105332. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp. Ther. Med. 2018, 16, 4553–4561. [Google Scholar] [CrossRef] [Green Version]
- Bacon, N.C.; Wappner, P.; O’Rourke, J.F.; Bartlett, S.M.; Shilo, B.; Pugh, C.W.; Ratcliffe, P.J. Regulation of the Drosophila bHLH-PAS protein Sima by hypoxia: Functional evidence for homology with mammalian HIF-1 alpha. Biochem. Biophys. Res. Commun. 1998, 249, 811–816. [Google Scholar] [CrossRef]
- Centanin, L.; Dekanty, A.; Romero, N.; Irisarri, M.; Gorr, T.A.; Wappner, P. Cell Autonomy of HIF Effects in Drosophila: Tracheal Cells Sense Hypoxia and Induce Terminal Branch Sprouting. Dev. Cell 2008, 14, 547–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, P.; Jung, J.; Wilms, G.M.; Kokott-Vuong, A.; Habib, S.; Schulz, J.B.; Voigt, A. Posthypoxic behavioral impairment and mortality of Drosophila melanogaster are associated with high temperatures, enhanced predeath activity and oxidative stress. Exp. Mol. Med. 2021, 53, 264–280. [Google Scholar] [CrossRef]
- Sato, M.; Kornberg, T.B. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev. Cell 2002, 3, 195–207. [Google Scholar] [CrossRef]
- Cruz, J.; Bota-Rabassedas, N.; Franch-Marro, X. FGF coordinates air sac development by activation of the EGF ligand Vein through the transcription factor PntP2. Sci. Rep. 2015, 5, 17806. [Google Scholar] [CrossRef] [Green Version]
- Cabernard, C.; Affolter, M. Distinct Roles for Two Receptor Tyrosine Kinases in Epithelial Branching Morphogenesis in Drosophila. Dev. Cell 2005, 9, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Chanut-Delalande, H.; Jung, A.C.; Baer, M.M.; Lin, L.; Payre, F.; Affolter, M. The Hrs/Stam Complex Acts as a Positive and Negative Regulator of RTK Signaling during Drosophila Development. PLoS ONE 2010, 5, e10245. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Brenneman, B.; Fields, C.; Srivastava, A. A Cathepsin-L is required for invasive behavior during Air Sac Primordium development inDrosophila melanogaster. FEBS Lett. 2015, 589, 3090–3097. [Google Scholar] [CrossRef] [Green Version]
- Llano, E.; Adam, G.; Pendás, A.M.; Quesada, V.; Sánchez, L.M.; Santamaría, I.; Noselli, S.; López-Otín, C. Structural and Enzymatic Characterization of Drosophila Dm2-MMP, a Membrane-bound Matrix Metalloproteinase with Tissue-specific Expression. J. Biol. Chem. 2002, 277, 23321–23329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, A.; Lin, L.; Kornberg, T.B. Regulation of Drosophila matrix metalloprotease Mmp2 is essential for wing imaginal disc:trachea association and air sac tubulogenesis. Dev. Biol. 2009, 335, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.Y.; Miranda, B.; Lebeche, D.; Hashimoto, G.; Cardoso, W.V. FGF-10 Is a Chemotactic Factor for Distal Epithelial Buds during Lung Development. Dev. Biol. 1998, 201, 125–134. [Google Scholar] [CrossRef]
- Abler, L.L.; Mansour, S.L.; Sun, X. Conditional gene inactivation reveals roles forFgf10andFgfr2in establishing a normal pattern of epithelial branching in the mouse lung. Dev. Dyn. 2009, 238, 1999–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandsma, C.A.; de Vries, M.; Costa, R.; Woldhuis, R.R.; Konigshoff, M.; Timens, W. Lung ageing and COPD: Is there a role for ageing in abnormal tissue repair? Eur. Respir. Rev. 2017, 26, 170073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yew-Booth, L.; Birrell, M.A.; Lau, M.S.; Baker, K.; Jones, V.; Kilty, I.; Belvisi, M.G. JAK-STAT pathway activation in COPD. Eur. Respir. J. 2015, 46, 843–845. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Betsuyaku, T.; Ito, Y.; Nagai, K.; Nasuhara, Y.; Kaga, K.; Kondo, S.; Nishimura, M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 2008, 39, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Roman-Rodriguez, M.; Singh, D.; Han, M.K.; Rodriguez-Roisin, R.; Ferguson, G.T. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir. Med. 2020, 166, 105938. [Google Scholar] [CrossRef]
- Santalla, M.; Pagola, L.; Gómez, I.; Balcazar, D.; Valverde, C.A.; Ferrero, P. Smoking flies: Testing the effect of tobacco cigarettes on heart function of Drosophila melanogaster. Biol. Open 2021, 10, bio055004. [Google Scholar] [CrossRef]
- Giannopoulos, A.; Giannakou, L.; Gourgouliani, N.; Lüpold, S.; Rouka, E.; Jagirdar, R.; Pitaraki, E.; Hatzoglou, C.; Gourgoulianis, K.; Blanckenhorn, W.; et al. Exposure of Drosophila melanogaster to cigarette smoke extract changes its sexual behavior. Eur. Respir. J. 2020, 56, 1326. [Google Scholar] [CrossRef]
- Morris, M.; Shaw, A.; Lambert, M.; Perry, H.H.; Lowenstein, E.; Valenzuela, D.; Velazquez-Ulloa, N.A. Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster. BMC Dev. Biol. 2018, 18, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Prange, R.; Thiedmann, M.; Bhandari, A.; Mishra, N.; Sinha, A.; Hasler, R.; Rosenstiel, P.; Uliczka, K.; Wagner, C.; Yildirim, A.O.; et al. A Drosophila model of cigarette smoke induced COPD identifies Nrf2 signaling as an expedient target for intervention. Aging 2018, 10, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5518. [Google Scholar] [CrossRef]
- Hoffmann, J.; Romey, R.; Fink, C.; Yong, L.; Roeder, T. Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging 2013, 5, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Wu, V.M.; Yu, M.H.; Paik, R.; Banerjee, S.; Liang, Z.; Paul, S.M.; Bhat, M.A.; Beitel, G.J. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development 2007, 134, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Kiss, M.; Kiss, A.A.; Radics, M.; Popovics, N.; Hermesz, E.; Csiszar, K.; Mink, M. Drosophila type IV collagen mutation associates with immune system activation and intestinal dysfunction. Matrix Biol. 2016, 49, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Ling, C.; Zheng, Y.; Yin, F.; Yu, J.; Huang, J.; Hong, Y.; Wu, S.; Pan, D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. USA 2010, 107, 10532–10537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, M.R.; Mattie, F.J.; Browder, K.C.; Radyk, M.D.; Crilly, S.E.; Bakerink, K.J.; Harper, S.L.; Speicher, D.W.; Thomas, G.H. Spectrin tetramer formation is not required for viable development in Drosophila. J. Biol. Chem. 2015, 290, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Kessenbrock, K.; Holak, T.A.; Sixt, M.; Jenne, D.; Werb, Z.; Riedl, J.; Wedlich-Soldner, R.; Neukirchen, D.; Bista, M.; Crevenna, A.H.; et al. Lifeact: A versatile marker to visualize F-actin. Nat. Methods 2008, 5, 605–607. [Google Scholar] [CrossRef]
- Chen, Y.J.; Huang, J.; Huang, L.; Austin, E.; Hong, Y. Phosphorylation potential of Drosophila E-Cadherin intracellular domain is essential for development and adherens junction biosynthetic dynamics regulation. Development 2017, 144, 1242–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghabrial, A.S.; Krasnow, M.A. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 2006, 441, 746–749. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Tang, J.; Yuan, J.; Wu, M.; Yao, C.; Hosoi, K.; Yu, S.; Zhao, X.; Han, Y.; et al. Dysfunction of pulmonary epithelial tight junction induced by silicon dioxide nanoparticles via the ROS/ERK pathway and protein degradation. Chemosphere 2020, 255, 126954. [Google Scholar] [CrossRef]
- Feng, S.; Zou, L.; Wang, H.; He, R.; Liu, K.; Zhu, H. RhoA/ROCK-2 Pathway Inhibition and Tight Junction Protein Upregulation by Catalpol Suppresses Lipopolysaccaride-Induced Disruption of Blood-Brain Barrier Permeability. Molecules 2018, 23, 2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, G.; Shen, J.; Tower, J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging 2012, 4, 768–789. [Google Scholar] [CrossRef] [Green Version]
- Dar, N.J.; Satti, N.K.; Dutt, P.; Hamid, A.; Ahmad, M. Attenuation of Glutamate-Induced Excitotoxicity by Withanolide-A in Neuron-Like Cells: Role for PI3K/Akt/MAPK Signaling Pathway. Mol. Neurobiol. 2018, 55, 2725–2739. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, J.; Diao, X.; Wang, S. Association between tumor necrosis factor-alpha and chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ther. Adv. Respir. Dis. 2019, 13, 1753466619866096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, E.; Yan, M.; Basler, K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 2002, 12, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Igaki, T.; Kanda, H.; Yamamoto-Goto, Y.; Kanuka, H.; Kuranaga, E.; Aigaki, T.; Miura, M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002, 21, 3009–3018. [Google Scholar] [CrossRef]
- Boulay, J.L.; O’Shea, J.J.; Paul, W.E. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity 2003, 19, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Hu, N.; Hombria, J.C. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr. Biol. 2001, 11, 1700–1705. [Google Scholar] [CrossRef]
- Chen, H.W.; Chen, X.; Oh, S.W.; Marinissen, M.J.; Gutkind, J.S.; Hou, S.X. mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 2002, 16, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Lange, P.; Vestbo, J. Importance of Early COPD in Young Adults for Development of Clinical COPD: Findings from the Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2021, 203, 1245–1256. [Google Scholar] [CrossRef]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H., Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.; North, R.A.; Nagarathinam, K.; Tanabe, M. Structures and General Transport Mechanisms by the Major Facilitator Superfamily (MFS). Chem. Rev. 2021, 121, 5289–5335. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Gallo, L.; Jadhav, A.; Hawkins, R.; Parker, C.G. The Druggability of Solute Carriers. J. Med. Chem. 2020, 63, 3834–3867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Sun, K.; Meng, Z.; Chen, L. The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. J. Mol. Cell Biol. 2019, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Davis, K.D.; Coffman, C.R. Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the Outsiders monocarboxylate transporter. Development 2008, 135, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Chang, D.; Lu, G.; Deng, X. Genetic polymorphism and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1385–1393. [Google Scholar] [CrossRef] [Green Version]
- Ragland, M.F.; Benway, C.J.; Lutz, S.M.; Bowler, R.P.; Hecker, J.; Hokanson, J.E.; Crapo, J.D.; Castaldi, P.J.; DeMeo, D.L.; Hersh, C.P.; et al. Genetic Advances in Chronic Obstructive Pulmonary Disease. Insights from COPDGene. Am. J. Respir. Crit. Care Med. 2019, 200, 677–690. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, D.; Yang, C.; Kasela, S.; Kim, W.; Ma, Y.; Shrine, N.; Batini, C.; Sofer, T.; Taliun, S.A.G.; et al. Whole genome sequence analysis of pulmonary function and COPD in 19,996 multi-ethnic participants. Nat. Commun. 2020, 11, 5182–5187. [Google Scholar] [CrossRef] [PubMed]
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K.; et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Flockhart, I.; Vinayagam, A.; Bergwitz, C.; Berger, B.; Perrimon, N.; Mohr, S.E. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinform. 2011, 12, 357. [Google Scholar] [CrossRef] [Green Version]
- Su, T.T. Drug screening in Drosophila; why, when, and when not? Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e346. [Google Scholar] [CrossRef] [PubMed]
- Bangi, E.; Murgia, C.; Teague, A.G.; Sansom, O.J.; Cagan, R.L. Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 2016, 7, 13615. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Marra, J.; LaMarca, N.M.; Young, S.D.; Weimer, L.H.; Levin, B.; McCabe, B. Lack of effect on ambulation of dalfampridine-ER (4-AP) treatment in adult SMA patients. Neuromuscul. Disord. 2020, 30, 693–700. [Google Scholar] [CrossRef]
- Shamon, S.D.; Perez, M.I.; Shamon, S.D. Blood pressure-lowering efficacy of reserpine for primary hypertension. Cochrane Libr. 2016, 2016, CD007655. [Google Scholar] [CrossRef] [PubMed]
- Lavara-Culebras, E.; Munoz-Soriano, V.; Gomez-Pastor, R.; Matallana, E.; Paricio, N. Effects of pharmacological agents on the lifespan phenotype of Drosophila DJ-1beta mutants. Gene 2010, 462, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Goven, D.; Boutten, A.; Lecon-Malas, V.; Marchal-Somme, J.; Amara, N.; Crestani, B.; Fournier, M.; Leseche, G.; Soler, P.; Boczkowski, J.; et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 2008, 63, 916–924. [Google Scholar] [CrossRef] [Green Version]
| COPD-Associated Gene | Human Function | Drosophila Homolog/Ortholog |
|---|---|---|
| ADAM19 | Cell matrix interactions | Meltrin |
| ADRB2 | Β-2-adrenergic receptor | Octβ2R |
| COL15A1 | Basement membrane adhesion to connective tissue | Mp |
| HTR4 | Serotonin receptor | Octβ1R |
| RAB4B | Vesicular trafficking | Rab4 |
| FGF18 | Cell proliferation, differentiation, and migration | bnl |
| SERP2 | Protect unfolded target proteins when under ER stress | CG32276 |
| RREB1 | Transcription factor that binds specifically to the RAS-responsive elements (RRE) of gene promoters | peb |
| ID4 | Gene expression regulator of numerous cellular processes | emc |
| TGFB2 | Tumor suppression, regulation of muscle and tissue development, wound healing, immune system function | dpp daw |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholl, A.; Ndoja, I.; Jiang, L. Drosophila Trachea as a Novel Model of COPD. Int. J. Mol. Sci. 2021, 22, 12730. https://doi.org/10.3390/ijms222312730
Scholl A, Ndoja I, Jiang L. Drosophila Trachea as a Novel Model of COPD. International Journal of Molecular Sciences. 2021; 22(23):12730. https://doi.org/10.3390/ijms222312730
Chicago/Turabian StyleScholl, Aaron, Istri Ndoja, and Lan Jiang. 2021. "Drosophila Trachea as a Novel Model of COPD" International Journal of Molecular Sciences 22, no. 23: 12730. https://doi.org/10.3390/ijms222312730
APA StyleScholl, A., Ndoja, I., & Jiang, L. (2021). Drosophila Trachea as a Novel Model of COPD. International Journal of Molecular Sciences, 22(23), 12730. https://doi.org/10.3390/ijms222312730






