Development of an Aged Full-Thickness Skin Model Using Flexible Skin-on-a-Chip Subjected to Mechanical Stimulus Reflecting the Circadian Rhythm
Abstract
:1. Introduction
2. Results and Discussion
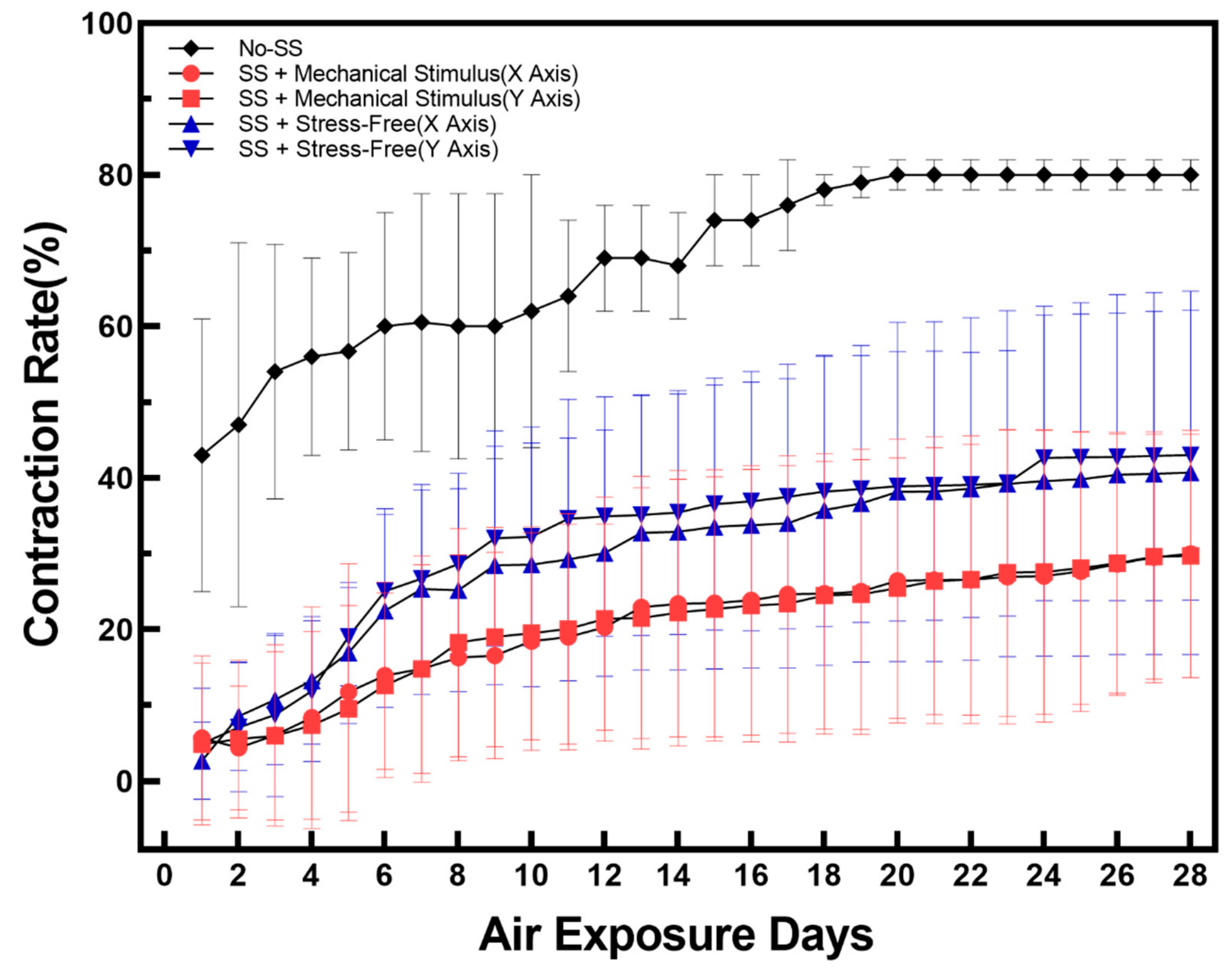
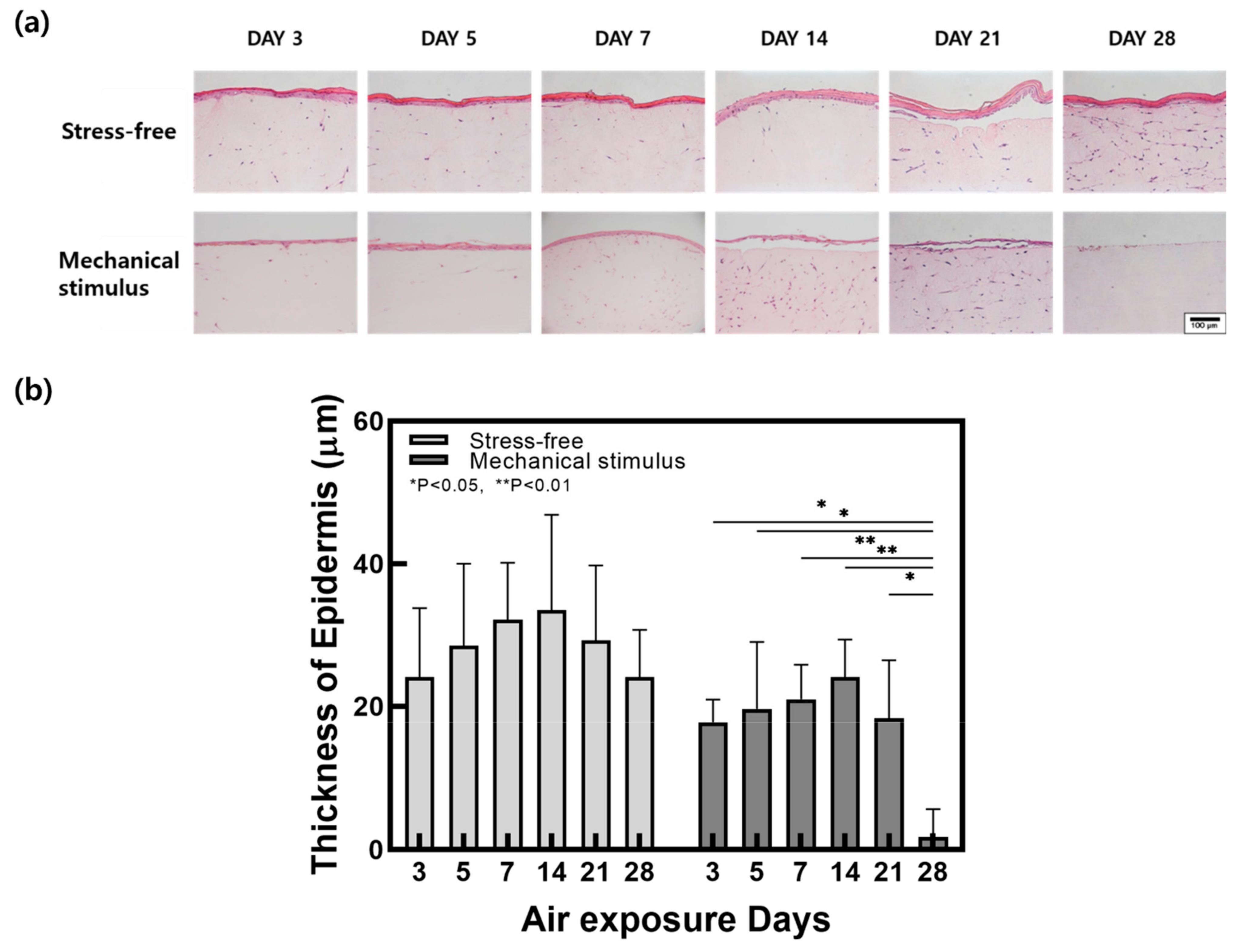
2.1. Contraction and Changes in Epidermal Thickness
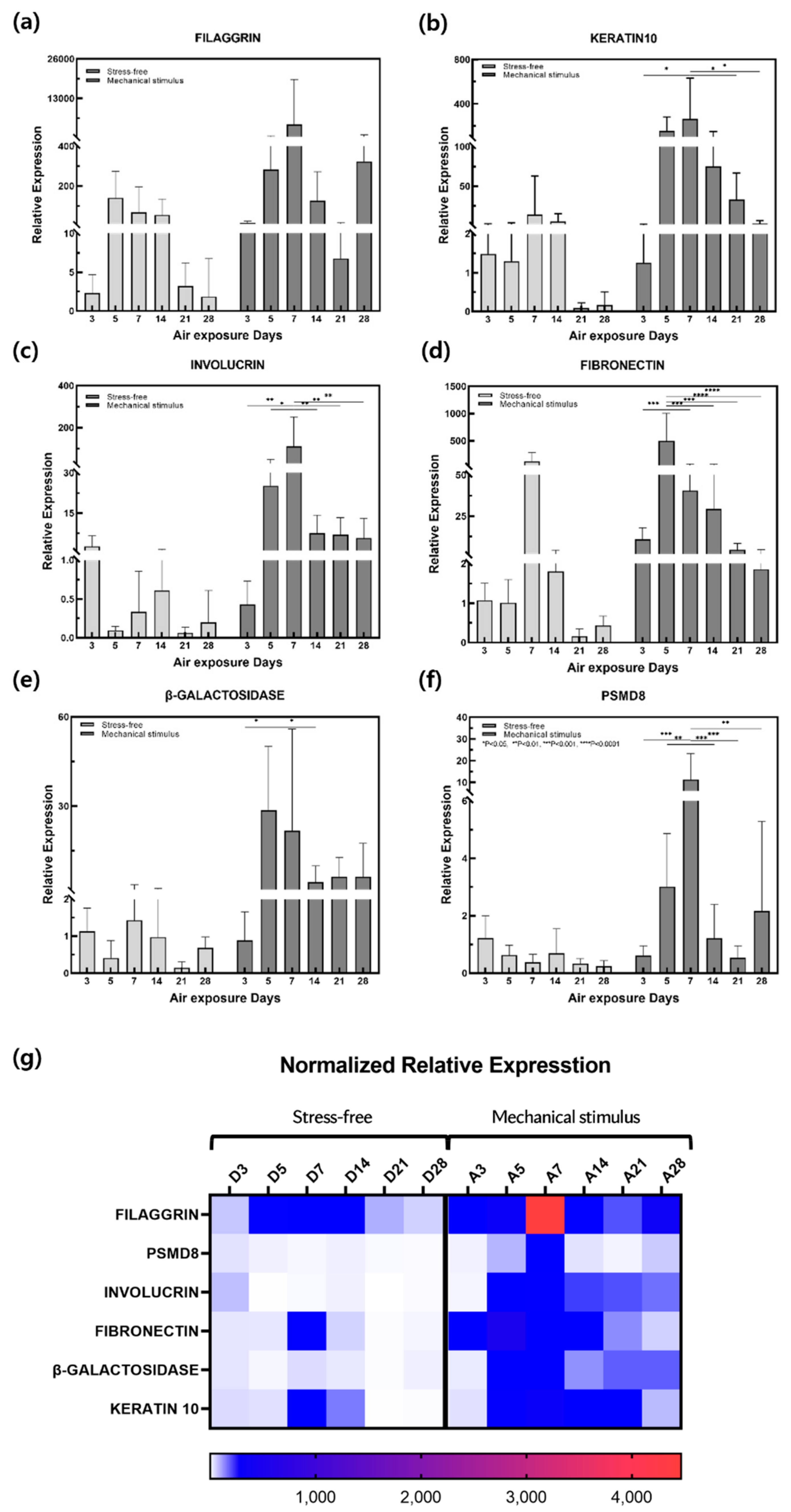
2.2. Gene Expression
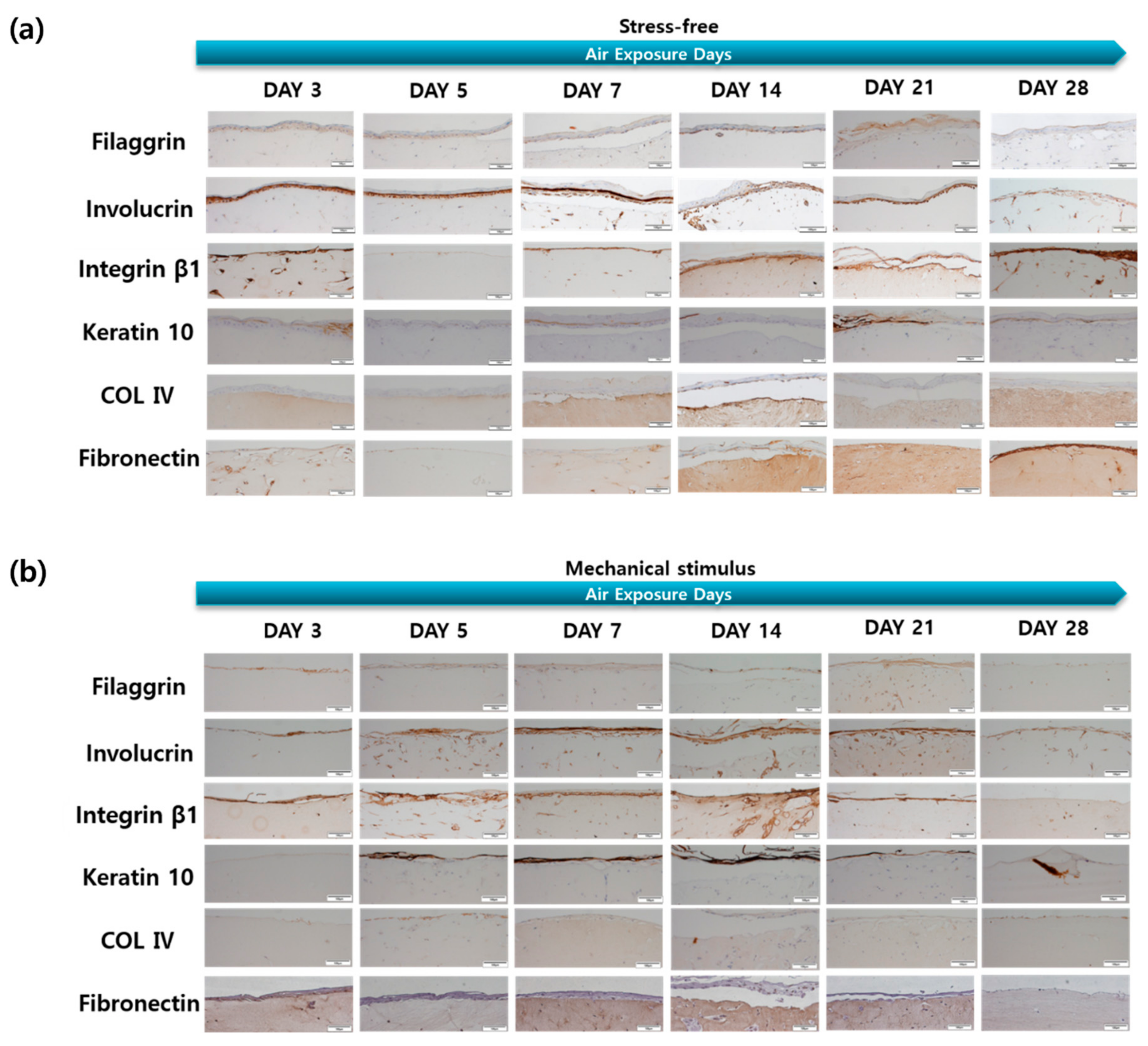
2.3. Protein Expression
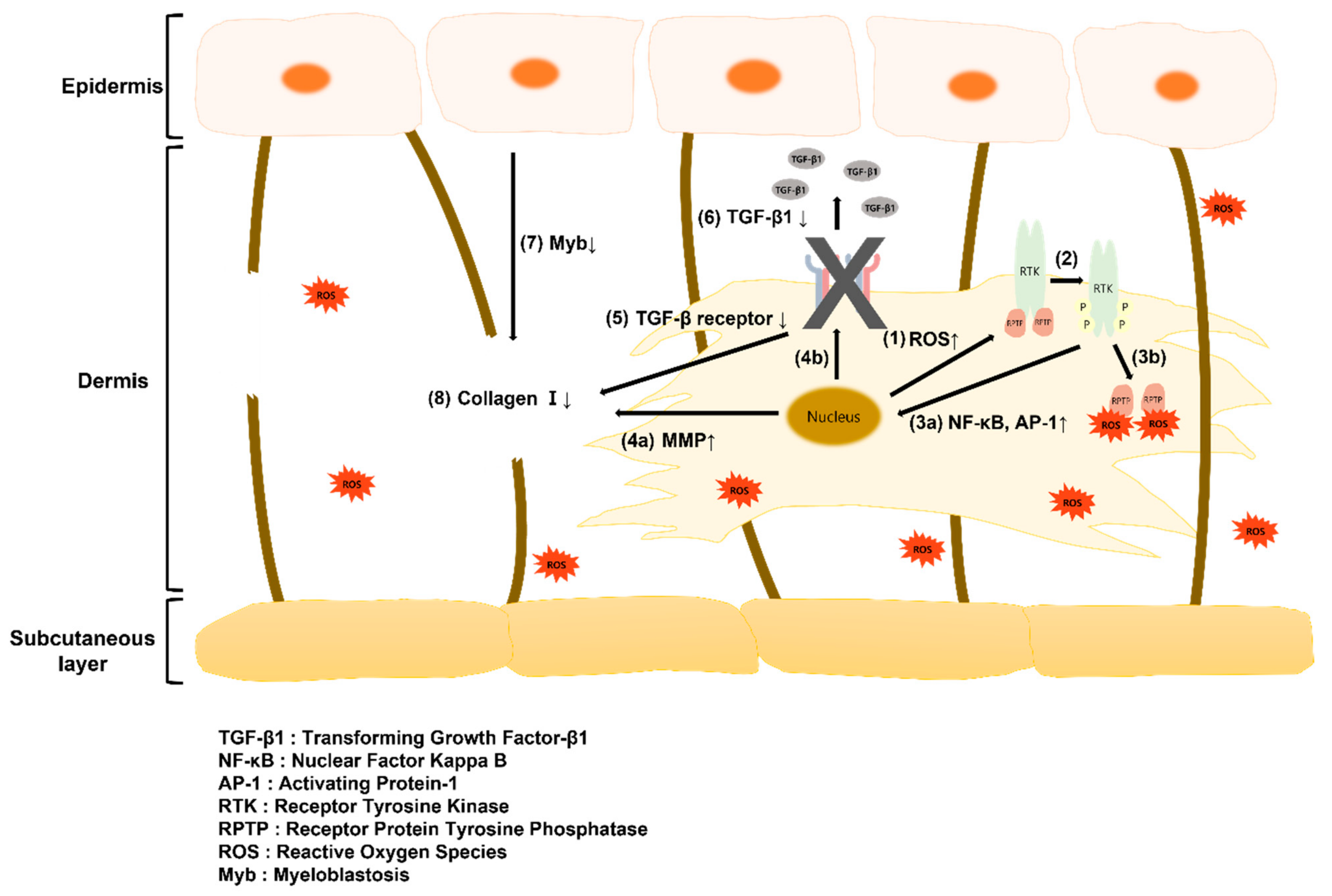
2.4. ROS Production and Cytokine Secretion
3. Materials and Methods
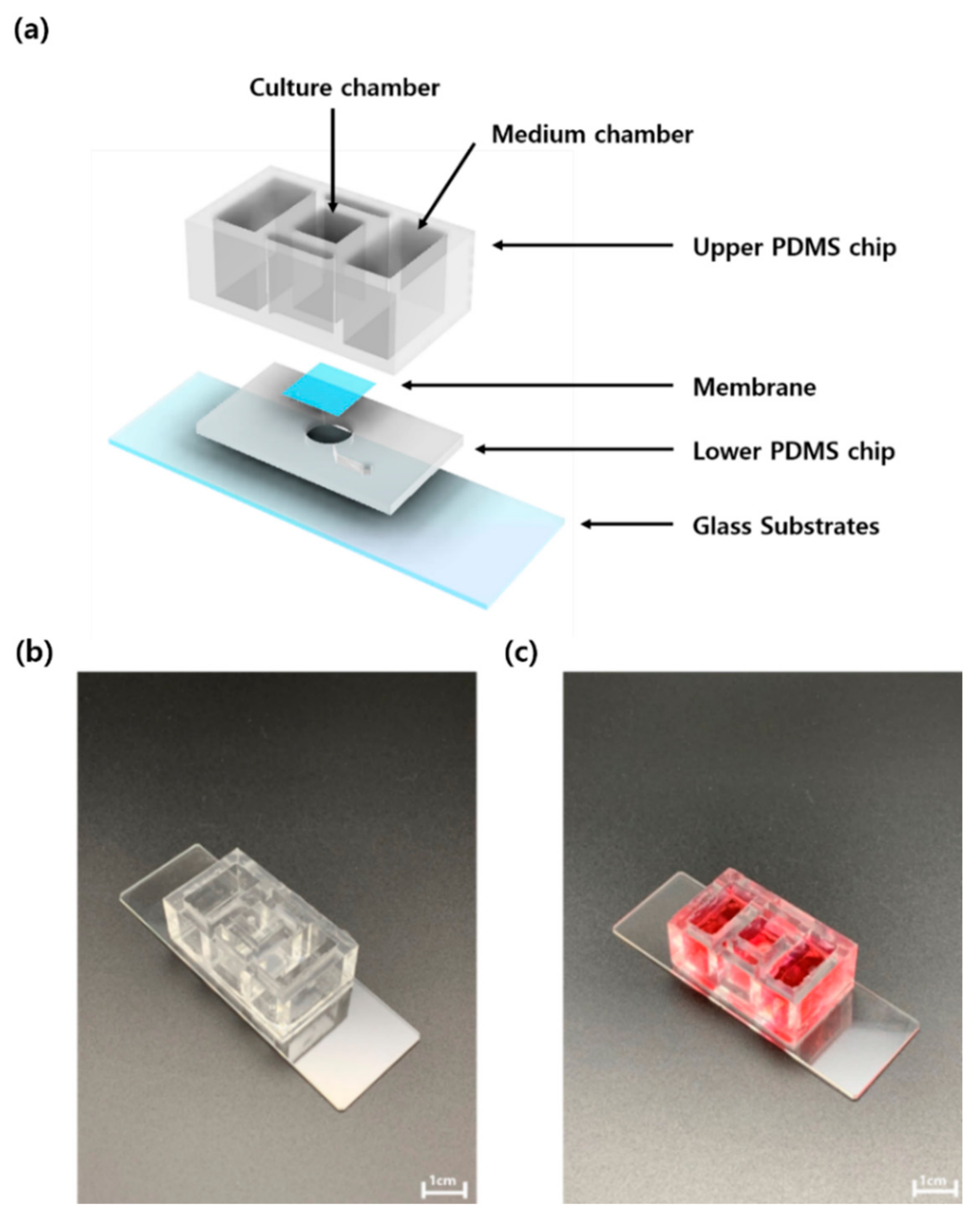
3.1. Flexible Skin-on-a-Chip (FSOC)
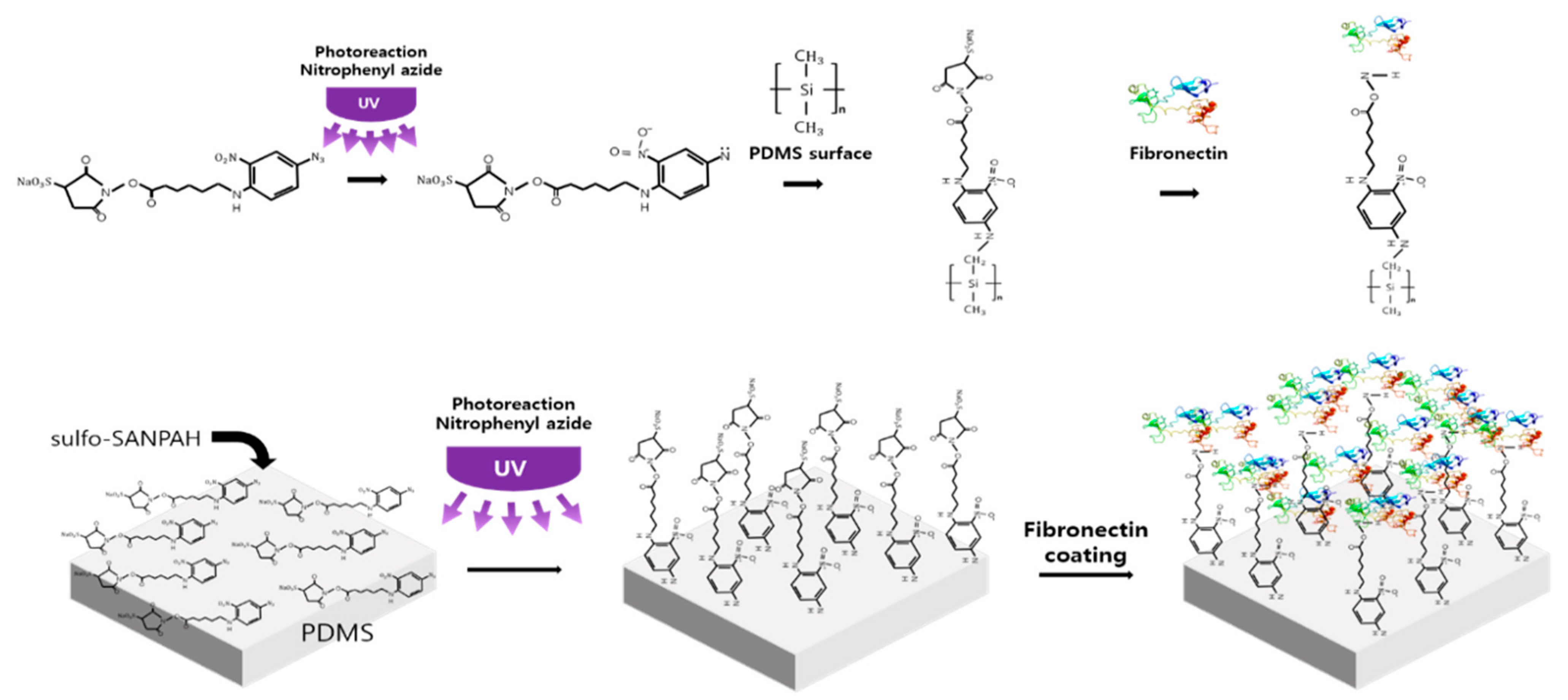
3.2. Crosslinker Processing
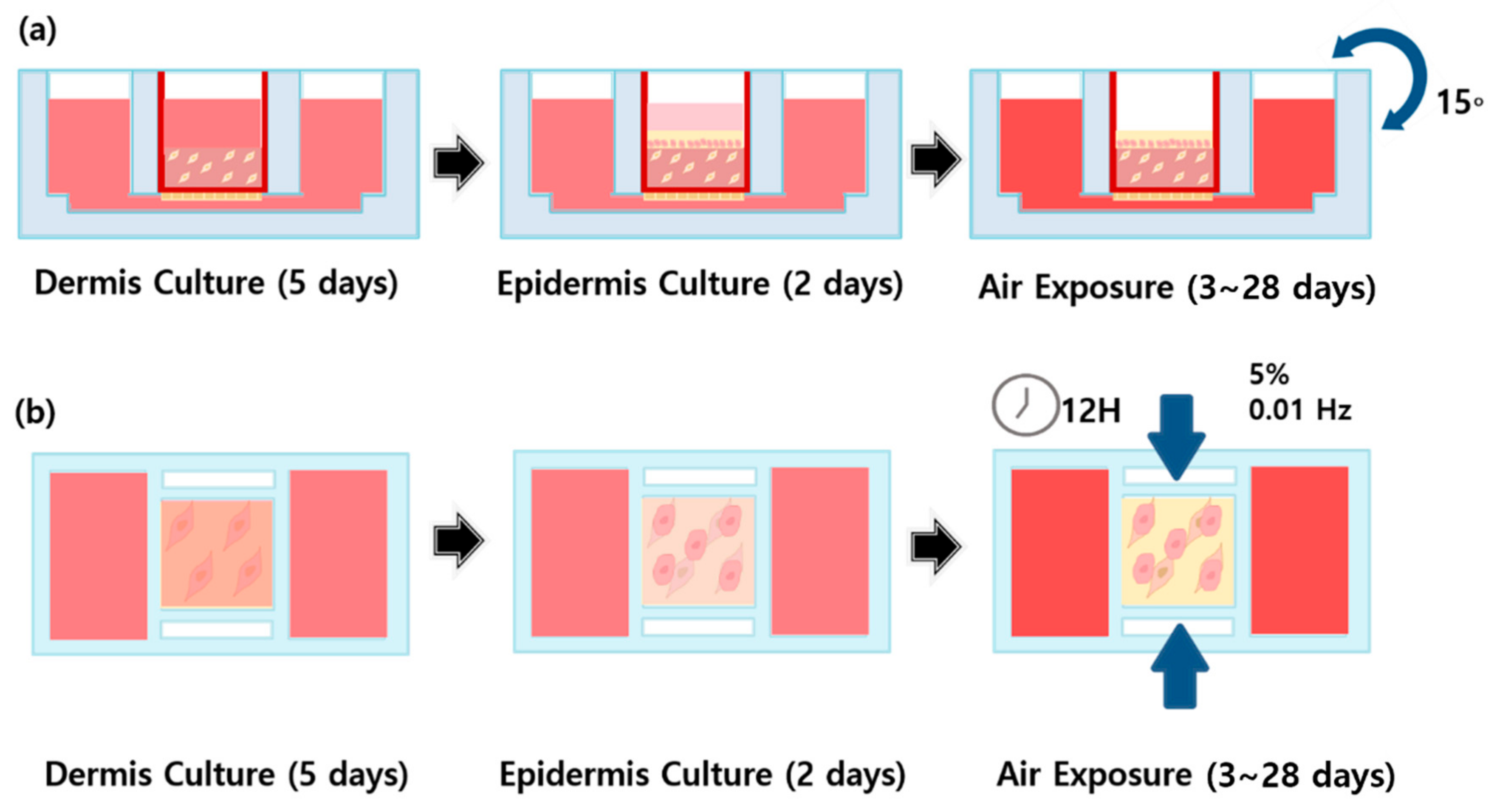
3.3. Cell Culture
3.4. Gravity-Flow System
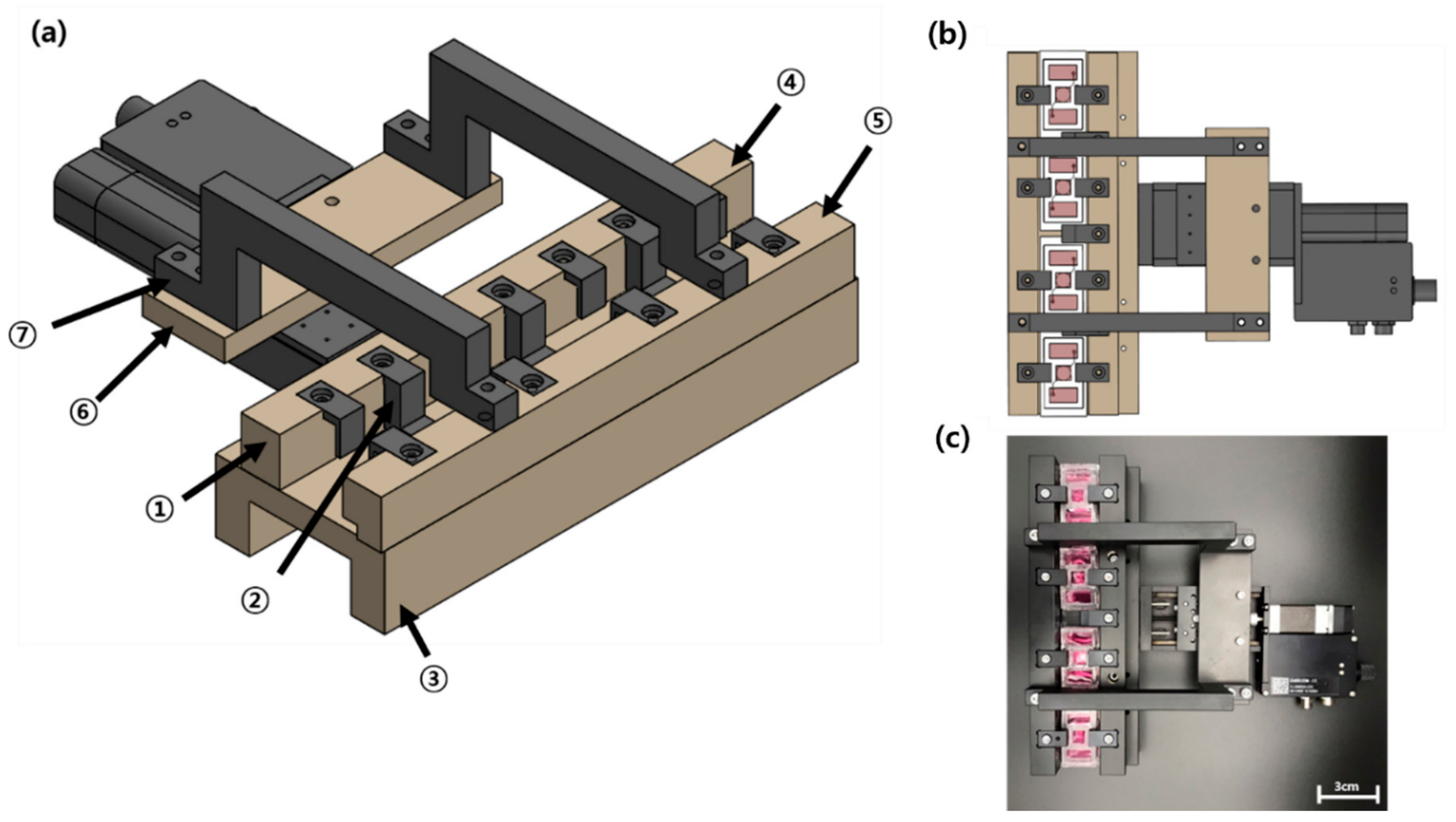
3.5. Mechanical Stimulus Actuating System (MSAS)
3.6. Hematoxylin and Eosin (H&E) Staining
3.7. Contraction Rate Analysis
3.8. Immunohistochemistry (IHC)
3.9. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
3.10. Culture Medium Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huertas, A.C.M.; Schmelzer, C.E.H.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-Level Insights into Aging Processes of Skin Elastin. Biochimie 2016, 128, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic Skin Aging: The Role of Oxidative Stress. Acta Derm. Alp Pannonica Adriat 2012, 21, 33–36. [Google Scholar]
- Gragnani, A.; Mac Cornick, S.; Chominski, V.; de Noronha, S.M.R.; de Noronha, S.A.A.C.; Ferreira, L.M. Review of Major Theories of Skin Aging. Adv. Aging Res. 2014, 3, 49375. [Google Scholar] [CrossRef] [Green Version]
- Rittié, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar Ultraviolet Irradiation Reduces Collagen in Photoaged Human Skin by Blocking Transforming Growth Factor-β Type II Receptor/Smad Signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E. Clinical Aspects and Molecular Diagnostics of Skin Aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Skin Aging and Photoaging: An Overview. J. Am. Acad. Dermatol. 1989, 21, 610–613. [Google Scholar] [CrossRef]
- Kazanci, A.; Kurus, M.; Atasever, A. Analyses of Changes on Skin by Aging. Ski. Res. Technol. 2017, 23, 48–60. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-Associated Beta-Galactosidase Is Lysosomal Beta-Galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gerland, L.-M.; Peyrol, S.; Lallemand, C.; Branche, R.; Magaud, J.-P.; Ffrench, M. Association of Increased Autophagic Inclusions Labeled for β-Galactosidase with Fibroblastic Aging. Exp. Gerontol. 2003, 38, 887–895. [Google Scholar] [CrossRef]
- Brunk, U.; Ericsson, J.L.E.; Ponten, J.; Westermark, B. Residual Bodies and “Aging” in Cultured Human Glia Cells: Effect of Entrance into Phase III and Prolonged Periods of Confluence. Exp. Cell Res. 1973, 79, 1–14. [Google Scholar] [CrossRef]
- Mishima, K.; Handa, J.T.; Aotaki-Keen, A.; Lutty, G.A.; Morse, L.S.; Hjelmeland, L.M. Senescence-Associated Beta-Galactosidase Histochemistry for the Primate Eye. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1590–1593. [Google Scholar]
- Melk, A.; Kittikowit, W.; Sandhu, I.; Halloran, K.M.; Grimm, P.; Schmidt, B.M.W.; Halloran, P.F. Cell Senescence in Rat Kidneys in Vivo Increases with Growth and Age despite Lack of Telomere Shortening. Kidney Int. 2003, 63, 2134–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I. Aging of Mice Is Associated with P16 (Ink4a)-and β-Galactosidase-Positive Macrophage Accumulation That Can Be Induced in Young Mice by Senescent Cells. Aging 2016, 8, 1294. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-K.; Klopp, R.G.; Weindruch, R.; Prolla, T.A. Gene Expression Profile of Aging and Its Retardation by Caloric Restriction. Science 1999, 285, 1390–1393. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.A.; Miyachi, K.J.; Cheng, B.; Sun, B.K. Aging-Associated Decline of Epidermal PSMD8 Contributes to Impaired Skin Function. J. Investig. Dermatol. 2018, 138, 976. [Google Scholar] [CrossRef] [Green Version]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK Protein in the Mammalian Circadian Mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Ko, C.H.; Lowrey, P.L.; Buhr, E.D.; Song, E.; Chang, S.; Yoo, O.J.; Yamazaki, S.; Lee, C.; Takahashi, J.S. A Noncanonical E-Box Enhancer Drives Mouse Period2 Circadian Oscillations in Vivo. Proc. Natl. Acad. Sci.USA 2005, 102, 2608–2613. [Google Scholar] [CrossRef] [Green Version]
- Sandu, C.; Dumas, M.; Malan, A.; Sambakhe, D.; Marteau, C.; Nizard, C.; Schnebert, S.; Perrier, E.; Challet, E.; Pévet, P. Human Skin Keratinocytes, Melanocytes, and Fibroblasts Contain Distinct Circadian Clock Machineries. Cell. Mol. Life Sci. 2012, 69, 3329–3339. [Google Scholar] [CrossRef]
- Refinetti, R. Comparison of Light, Food, and Temperature as Environmental Synchronizers of the Circadian Rhythm of Activity in Mice. J. Physiol. Sci. 2015, 65, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Kim, J.; Song, H.J.; Kim, K.; Choi, K.C.; Park, S.; Sung, G.Y. Development of Wrinkled Skin-on-a-Chip (WSOC) by Cyclic Uniaxial Stretching. J. Ind. Eng. Chem. 2018, 68, 238–245. [Google Scholar] [CrossRef]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Lee, T.; Sung, J.H.; Sung, G.Y. Development of 3D Skin-Equivalent in a Pump-Less Microfluidic Chip. J. Ind. Eng. Chem. 2018, 60, 355–359. [Google Scholar] [CrossRef]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.A.; Iván, K.; Erdő, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef]
- Sutterby, E.; Thurgood, P.; Baratchi, S.; Khoshmanesh, K.; Pirogova, E. Microfluidic Skin-on-a-Chip Models: Toward Biomimetic Artificial Skin. Small 2020, 16, e2002515. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.; Materne, E.-M.; Brincker, S.; Süßbier, U.; Frädrich, C.; Busek, M.; Sonntag, F.; Sakharov, D.A.; Trushkin, E.V.; Tonevitsky, A.G. A Dynamic Multi-Organ-Chip for Long-Term Cultivation and Substance Testing Proven by 3D Human Liver and Skin Tissue Co-Culture. Lab Chip 2013, 13, 3538–3547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Sito, L.; Mao, M.; He, J.; Zhang, Y.S.; Zhao, X. Current Advances in Skin-on-a-Chip Models for Drug Testing. Microphysiol. Syst. 2018, 2, 4. [Google Scholar] [CrossRef]
- Abaci, H.E.; Gledhill, K.; Guo, Z.; Christiano, A.M.; Shuler, M.L. Pumpless Microfluidic Platform for Drug Testing on Human Skin Equivalents. Lab Chip 2015, 15, 882–888. [Google Scholar] [CrossRef] [Green Version]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Sung, J.H.; Sung, G.Y. Fabrication of a Pumpless, Microfluidic Skin Chip from Different Collagen Sources. J. Ind. Eng. Chem. 2017, 56, 375–381. [Google Scholar] [CrossRef]
- Jeon, H.M.; Kim, K.; Choi, K.C.; Sung, G.Y. Side-Effect Test of Sorafenib Using 3-D Skin Equivalent Based on Microfluidic Skin-on-a-Chip. J. Ind. Eng. Chem. 2020, 82, 71–80. [Google Scholar] [CrossRef]
- Kim, K.; Jeon, H.M.; Choi, K.C.; Sung, G.Y. Testing the Effectiveness of Curcuma Longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2020, 21, 3898. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Sung, G.Y. Coenzyme Q10 Efficacy Test for Human Skin Equivalents Using a Pumpless Skin-On-A-Chip System. Int. J. Mol. Sci. 2020, 21, 8475. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jin, S.-P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H. Construction of 3D Multicellular Microfluidic Chip for an in Vitro Skin Model. Biomed. Microdevices 2017, 19, 22. [Google Scholar] [CrossRef]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Stretchable Culture Device of Skin-Equivalent with Improved Epidermis Thickness. In Proceedings of the 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; pp. 259–262. [Google Scholar]
- Kamble, H.; Barton, M.J.; Jun, M.; Park, S.; Nguyen, N.-T. Cell Stretching Devices as Research Tools: Engineering and Biological Considerations. Lab Chip 2016, 16, 3193–3203. [Google Scholar] [CrossRef] [Green Version]
- Kamotani, Y.; Bersano-Begey, T.; Kato, N.; Tung, Y.-C.; Huh, D.; Song, J.W.; Takayama, S. Individually Programmable Cell Stretching Microwell Arrays Actuated by a Braille Display. Biomaterials 2008, 29, 2646–2655. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-J.; Tsai, C.-J.; Tseng, F.-G.; Chen, T.-J.; Wang, T.-W. Micropatterned Stretching System for the Investigation of Mechanical Tension on Neural Stem Cells Behavior. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Mathieu, P.S.; Helmke, B.P. A Stretching Device for High-Resolution Live-Cell Imaging. Ann. Biomed. Eng. 2010, 38, 1728–1740. [Google Scholar] [CrossRef] [Green Version]
- Chipev, C.C.; Simon, M. Phenotypic Differences between Dermal Fibroblasts from Different Body Sites Determine Their Responses to Tension and TGFβ1. BMC Dermatol. 2002, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Sriram, G.; Bigliardi, P.L.; Bigliardi-Qi, M. Fibroblast Heterogeneity and Its Implications for Engineering Organotypic Skin Models in Vitro. Eur. J. Cell Biol. 2015, 94, 483–512. [Google Scholar] [CrossRef] [Green Version]
- Branchet, M.C.; Boisnic, S.; Frances, C.; Robert, A.M. Skin Thickness Changes in Normal Aging Skin. Gerontology 1990, 36, 28–35. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and Function of Skin, Hair and Nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Nemes, Z.; Steinert, P.M. Bricks and Mortar of the Epidermal Barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef]
- Müller, F.B.; Huber, M.; Kinaciyan, T.; Hausser, I.; Schaffrath, C.; Krieg, T.; Hohl, D.; Korge, B.P.; Arin, M.J. A Human Keratin 10 Knockout Causes Recessive Epidermolytic Hyperkeratosis. Hum. Mol. Genet. 2006, 15, 1133–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Cho, S.-Y.; Kang, J.; Lee, S.; Kim, H.-S.; Min, D.-J.; Son, E.; Cho, K.-H. Inhibition of 3-Phosphoinositide–Dependent Protein Kinase 1 (PDK1) Can Revert Cellular Senescence in Human Dermal Fibroblasts. Proc. Natl. Acad. Sci. USA 2020, 117, 31535–31546. [Google Scholar] [CrossRef] [PubMed]
- Elpek, G.Ö. Cellular and Molecular Mechanisms in the Pathogenesis of Liver Fibrosis: An Update. World J. Gastroenterol. WJG 2014, 20, 7260. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-Associated (Beta)-Galactosidase Reflects an Increase in Lysosomal Mass during Replicative Ageing of Human Endothelial Cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Sand, J.M.B.; Genovese, F.; Karsdal, M.A. Type IV Collagen. Biochem. Collagens Laminins Elastin Struct. Funct. Biomark. 2016, 1, 31–41. [Google Scholar] [CrossRef]
- Kwon, O.S.; Yoo, H.G.; Han, J.H.; Lee, S.R.; Chung, J.H.; Eun, H.C. Photoaging-Associated Changes in Epidermal Proliferative Cell Fractions in Vivo. Arch. Dermatol. Res. 2008, 300, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T. Oxidative Stress and Apoptosis: Impact on Cancer Therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Piccinini, G.; Golay, J.; Flora, A.; Songia, S.; Luchetti, M.; Gabrielli, A.; Introna, M. C-Myb, but Not B-Myb, Upregulates Type I Collagen Gene Expression in Human Fibroblasts. J. Investig. Dermatol. 1999, 112, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Mondrinos, M.J.; Alisafaei, F.; Alex, Y.Y.; Ahmadzadeh, H.; Lee, I.; Blundell, C.; Seo, J.; Osborn, M.; Jeon, T.-J.; Kim, S.M. Surface-Directed Engineering of Tissue Anisotropy in Microphysiological Models of Musculoskeletal Tissue. Sci. Adv. 2021, 7, eabe9446. [Google Scholar] [CrossRef]
- Huh, D.; Mondrinos, M.; Blundell, C.; Seo, J. Systems and Methods for Immobilizing Extracellular Matrix Material on Organ on Chip, Multilayer Microfluidics Microdevices, and Three-Dimensional Cell Culture Systems. U.S. Patent Application No. 20,180,223,251A1, 9 August 2018. [Google Scholar]
- Puccinelli, T.J.; Bertics, P.J.; Masters, K.S. Regulation of Keratinocyte Signaling and Function via Changes in Epidermal Growth Factor Presentation. Acta Biomater. 2010, 6, 3415–3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstam, L.I.; Vaughan, F.L.; Bernstein, I.A. Stratified Cornified Primary Cultures of Human Keratinocytes Grown on Microporous Membranes at the Air-Liquid Interface. J. Dermatol. Sci. 1990, 1, 173–181. [Google Scholar] [CrossRef]
- Bernstam, L.I.; Vaughan, F.L.; Bernstein, I.A. Keratinocytes Grown at the Air-Liquid Interface. Vitr. Cell. Dev. Biol. 1986, 22, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Rosdy, M.; Clauss, L.-C. Terminal Epidermal Differentiation of Human Keratinocytes Grown in Chemically Defined Medium on Inert Filter Substrates at the Air-Liquid Interface. J. Investig. Dermatol. 1990, 95, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Choi, N.; Sung, J.H. A Microfluidic Chip with Gravity-induced Unidirectional Flow for Perfusion Cell Culture. Biotechnol. Prog. 2019, 35, e2701. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, J.; Kim, H.; Sung, G.Y. Effect of α-Lipoic Acid on the Development of Human Skin Equivalents Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2021, 22, 2160. [Google Scholar] [CrossRef]


| Forward | Reverse | |
|---|---|---|
| GAPDH | 5′-CTCCTCTGACTTCAACAGCG-3′ | 5′-GCCAAATTCGTTGTCATACCAG-3′ |
| Filaggrin | 5′-GGAGTCACGTGGCAGTCCTCA-3′ | 5′-GGTGTCTAAACCCGGATTCAC-3′ |
| PSMD8 | 5′-ATGTACGAGCAACTCAAGGG-3′ | 5′-CTGTGGTTGGCAAGAAGTTG-3′ |
| Involucrin | 5′-CCGCAAATGAAACAGCCAACTCC-3′ | 5′-GGATTCCTCATGCTGTTCCCAG-3′ |
| Fibronectin | 5′-CTGAGGGCAGAAGAGACAAC-3′ | 5′-TCATGCTGCTTATCCCACTG-3′ |
| β-galactosidase | 5′-AATCAAGACCGAAGCAGTGG-3′ | 5′-GGCATCATAGTCGTAGCTGG-3′ |
| Keratin10 | 5′-CCGGAGATGGTGGCCTTCTCTCT-3′ | 5′-GGCCTGATGTGAGTTGCCATGCT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kim, J.; Jeon, H.M.; Kim, K.; Sung, G.Y. Development of an Aged Full-Thickness Skin Model Using Flexible Skin-on-a-Chip Subjected to Mechanical Stimulus Reflecting the Circadian Rhythm. Int. J. Mol. Sci. 2021, 22, 12788. https://doi.org/10.3390/ijms222312788
Jeong S, Kim J, Jeon HM, Kim K, Sung GY. Development of an Aged Full-Thickness Skin Model Using Flexible Skin-on-a-Chip Subjected to Mechanical Stimulus Reflecting the Circadian Rhythm. International Journal of Molecular Sciences. 2021; 22(23):12788. https://doi.org/10.3390/ijms222312788
Chicago/Turabian StyleJeong, Subin, Jisue Kim, Hye Mi Jeon, Kyunghee Kim, and Gun Yong Sung. 2021. "Development of an Aged Full-Thickness Skin Model Using Flexible Skin-on-a-Chip Subjected to Mechanical Stimulus Reflecting the Circadian Rhythm" International Journal of Molecular Sciences 22, no. 23: 12788. https://doi.org/10.3390/ijms222312788
APA StyleJeong, S., Kim, J., Jeon, H. M., Kim, K., & Sung, G. Y. (2021). Development of an Aged Full-Thickness Skin Model Using Flexible Skin-on-a-Chip Subjected to Mechanical Stimulus Reflecting the Circadian Rhythm. International Journal of Molecular Sciences, 22(23), 12788. https://doi.org/10.3390/ijms222312788







