Reductive Stress of Sulfur-Containing Amino Acids within Proteins and Implication of Tandem Protein–Lipid Damage
Abstract
:1. Reductive Stress
2. Transient Reductive Species Generated by γ-Radiolysis
 e−aq (0.27), HO• (0.28), H• (0.06), H+ (0.27), H2O2 (0.07)
e−aq (0.27), HO• (0.28), H• (0.06), H+ (0.27), H2O2 (0.07)
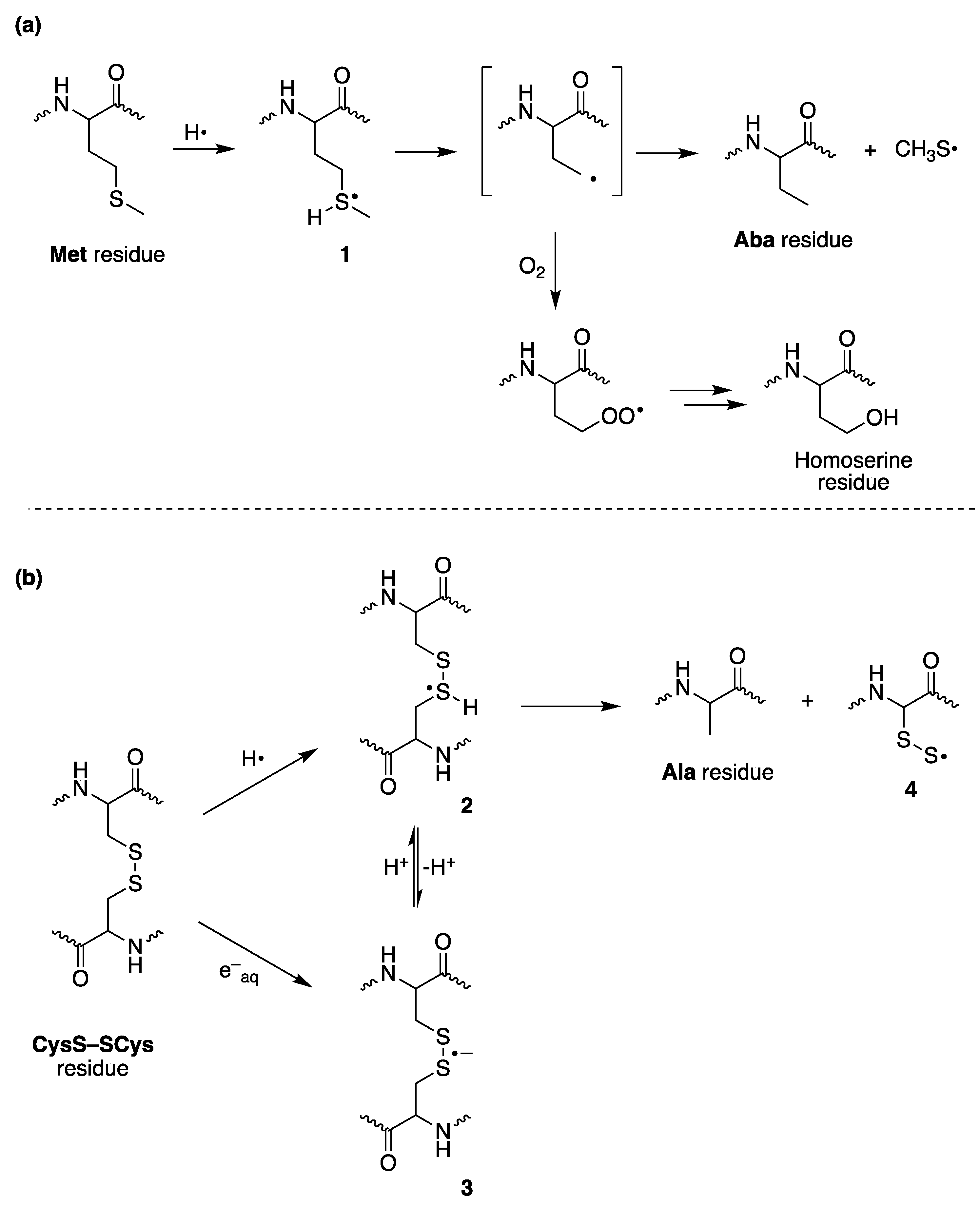
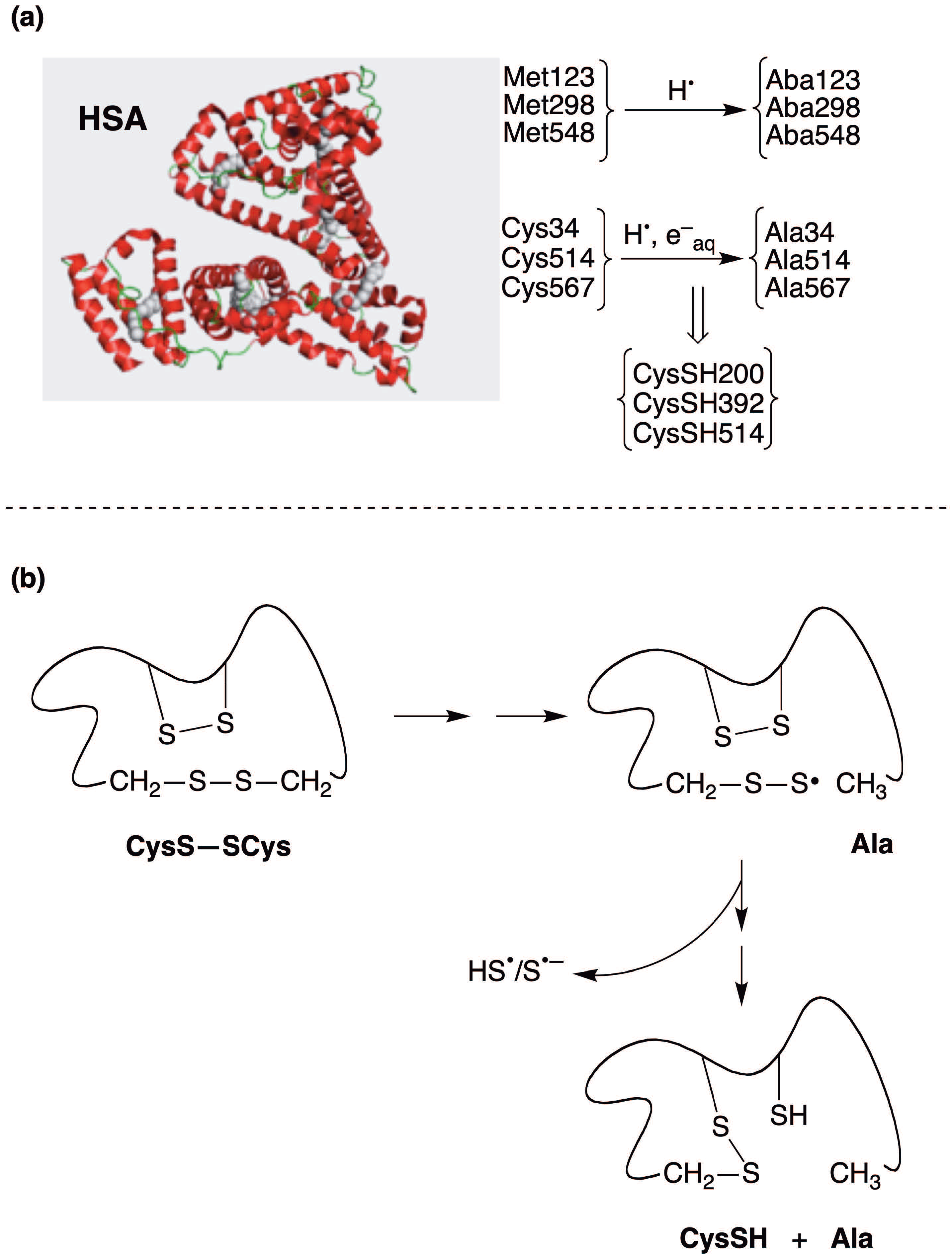
3. The Reductive Desulfurization of Methionine (Met) and Cystine (CysS-SCys) Residues in Peptides/Proteins
4. Biomimetic Radical Chemistry
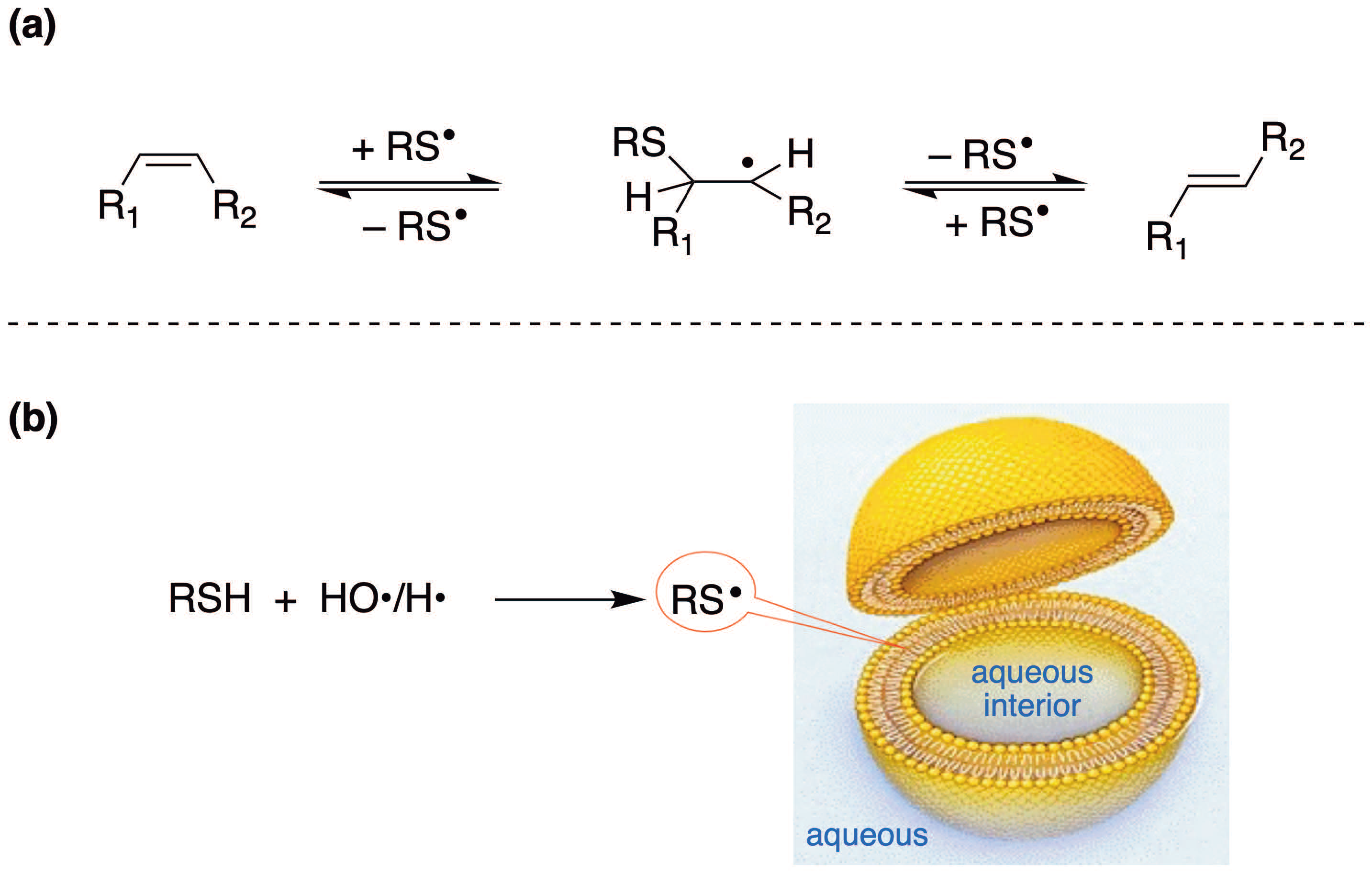
4.1. Cis–Trans Isomerization of Fatty Acid Residues in Liposomes by Sulfur-Centered Radicals
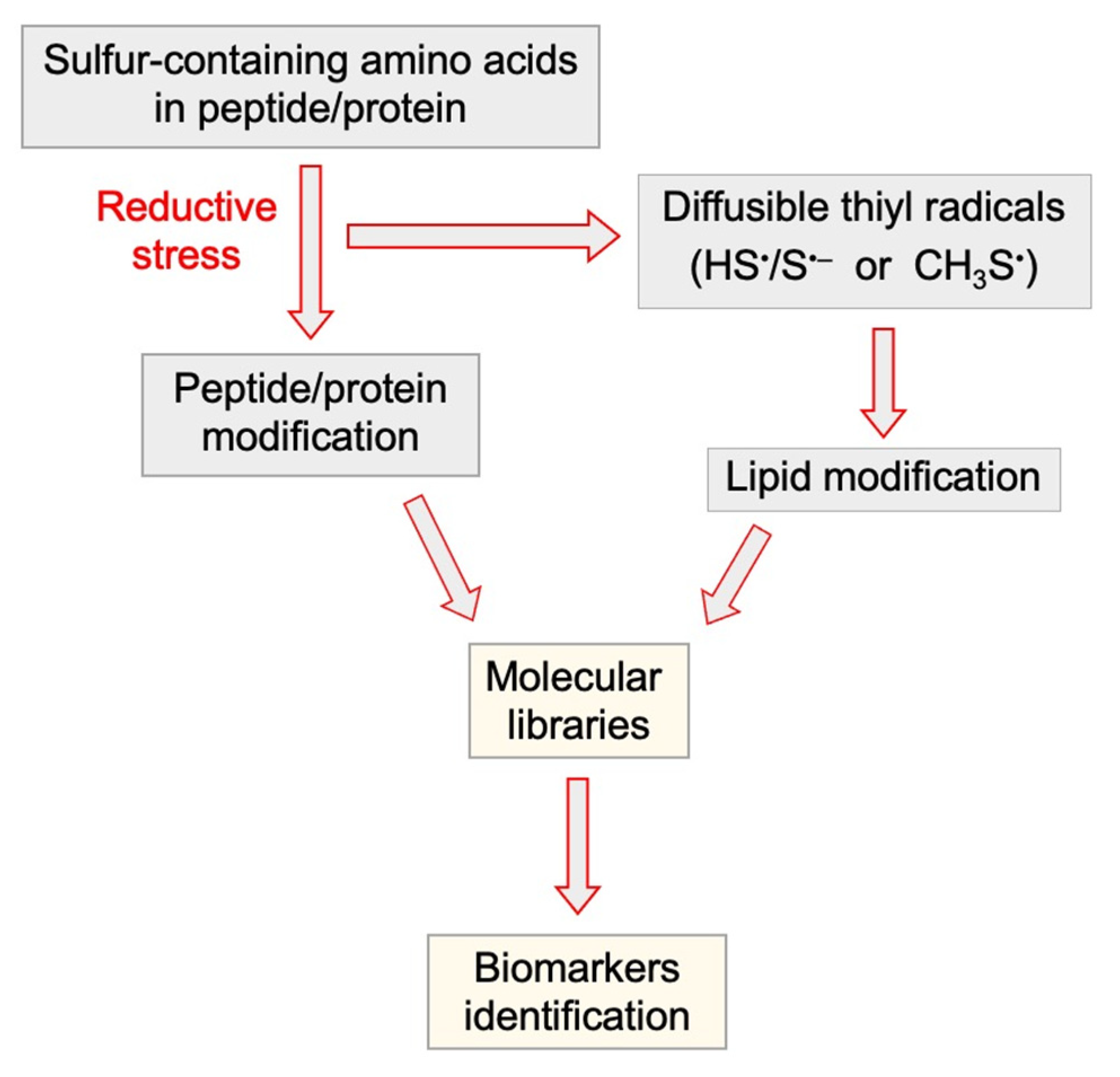
4.2. Tandem Protein–Lipid Damage and Build-Up of Molecular Libraries
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rajasekaran, N.S. Reductive stress—Neglected science. Antioxid. Redox Signal. 2020, 8114. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.J.; Hawkins, C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, B. Evidence in support of a concept of reductive stress. Br. J. Nutr. 2002, 87, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Loscalzo, J. Metabolic responses to reductive stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, N.S.; Connell, P.; Christians, E.S.; Yan, L.J.; Taylor, R.P.; Orosz, A.; Zhang, X.Q.; Stevenson, T.J.; Peshock, R.M.; Leopold, J.A.; et al. Human αB-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 2007, 130, 427–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavi, P.S.; Singh, S.; Kumar, A. Reductive stress: New insights in physiology and drug tolerance of mycobacterium. Antioxid. Redox Signal. 2020, 32, 1348–1366. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, R.S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Korge, P.; Calmettes, G.; Weiss, N.J. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Schuler, R.H. Rate constants for reaction of hydrogen atoms with compounds of biochemical interest. Radiat. Res. 1971, 47, 612–627. [Google Scholar] [CrossRef]
- Tung, T.L.; Kuntz, R.R. The reactions of hydrogen atoms in aqueous solutions: Thiols. Radiat. Res. 1973, 55, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stanley, J.P.; Griffith, M.G. The hydrogen atom and its reactions in solution. Science 1970, 169, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Getoff, N. Vitamin-induced intracellular electrons are the mechanism for their well-known beneficial effects: A review. Nutrition 2013, 29, 597–604. [Google Scholar] [CrossRef]
- Ferreri, C.; Manco, I.; Faraone-Mennella, M.R.; Torreggiani, A.; Tamba, M.; Chatgilialoglu, C. A biomimetic model of tandem radical damage involving sulfur-containing protein and unsaturated lipids. ChemBioChem 2004, 5, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Manco, I.; Faraone-Mennella, M.R.; Torreggiani, A.; Tamba, M.; Manara, S.; Chatgilialoglu, C. The reaction of hydrogen atoms with methionine residues: A model of reductive radical stress causing tandem protein-lipid damage. ChemBioChem 2006, 7, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.T.; Benny, A.; Nolan, M.D.; Swinand, G.; Scanlan, E.M. Cysteinyl radicals in chemical synthesis and in nature. Chem. Soc. Rev. 2021, 50, 10857–10894. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Mozziconacci, O.; Bobrowski, K.; Ferreri, C.; Chatgilialoglu, C. Reaction of hydrogen atom with Met-enkephalin and related peptides. Chem. Eur. J. 2007, 13, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Ferreri, C.; Postigo, A.; Chatgilialoglu, C. Radiation chemical studies of methionine in aqueous solution: Understanding the role of molecular oxygen. Chem. Res. Toxicol. 2010, 23, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Ferreri, C.; Zhang, T.; Permentier, H.; Bischoff, R.; Bobrowski, K.; Chatgilialoglu, C. Radiation chemical studies of Gly-Met-Gly in aqueous solution. Free Radic. Res. 2016, 50, S24–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadlcik, V.; Sicard-Roselli, C.; Houée-Levin, C.; Kodicek, M.; Ferreri, C.; Chatgilialoglu, C. Reductive modification of methionine residue in amyloid-β peptide. Angew. Chem. Int. Ed. 2006, 45, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.E.; Navon, G.; Stein, G. Action of atomic hydrogen on ribonuclease in aqueous solution. Nature 1967, 213, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Shapira, R.; Stein, G. Reactions of aromatic and sulfur amino acids in ribonuclease with hydrogen atoms in water solution. Science 1968, 162, 1489–1491. [Google Scholar] [CrossRef]
- Mee, L.K.; Adelstein, S.J. Inactivation of ribonuclease S-peptide by the primary aqueous radicals. Radiat. Res. 1974, 60, 422–431. [Google Scholar] [CrossRef]
- Torreggiani, A.; Tamba, M.; Manco, I.; Faraone-Mennella, M.R.; Ferreri, C.; Chatgilialoglu, C. Investigation of radical-based damage of RNase A in aqueous solutions and lipid vesicles. Biopolymers 2006, 81, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C.; Torreggiani, A.; Salzano, A.M.; Renzone, G.; Scaloni, A. The reductive desulfurization of Met and Cys residues in bovine RNAse A associated with the trans lipid formation in a mimetic model of biological membranes. J. Proteome Res. 2008, 7, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, A.; Tamba, M.; Manco, I.; Faraone-Mennella, M.R.; Ferreri, C.; Chatgilialoglu, C. Radiation damage of lysozyme in a biomimetic model: Some insights by Raman spectroscopy. J. Mol. Struct. 2005, 744-747, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Faucitano, A.; Buttafava, A.; Mariani, A.; Chatgilialoglu, C. The Influence of solid–state molecular organization on the reaction paths of thiyl radicals: EPR experimental evidence for radiation induced reactions in clathrates, polymer matrices and lysozyme. ChemPhysChem 2005, 6, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Skotnicki, K.; Ferreri, C.; Marciniak, B.; Bobrowski, K.; Chatgilialoglu, C. Biomimetic ketone reduction by disulfide radical anion. Molecules 2021, 26, 5429. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar]
- Bourdon, E.; Blache, D. The importance of proteins in defense against oxidation. Antioxid. Redox Signal. 2001, 3, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.M.; Renzone, G.; Scaloni, A.; Torreggiani, A.; Ferreri, C.; Chatgilialoglu, C. Human serum albumin modifications associated with reducing radical stress. Mol. BioSyst. 2011, 7, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Torreggiani, A.; Renzone, G.; Salzano, A.M.; Scaloni, A. Radiation-induced reductive modifications of sulfur-containing amino acids within peptides and proteins. J. Proteomics 2011, 74, 2264–2273. [Google Scholar] [CrossRef]
- Capdevila, M.; Domènech, J.; Pagani, A.; Tío, L.; Villarreal, L.; Atrian, S. Zn- and Cd-metallothionein recombinant species from the most diverse phyla may contain sulfide (S2−) ligands. Angew. Chem. Int. Ed. 2005, 44, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, A.; Domènech, J.; Ferreri, C.; Orihuela, R.; Atrian, S.; Capdevila, M.; Chatgilialoglu, C. Zinc and cadmium complexes of a plant metallothionein under radical stress: Desulfurisation reactions associated with the formation of trans lipids in model membranes. Chem. Eur. J. 2009, 15, 6015–6024. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, A.; Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Atrian, S.; Capdevila, M. Non-enzymatic modifications in metallothioneins connected to lipid membrane damages: Structural and biomimetic studies under reductive radical stress. J. Proteom. 2013, 92, 204–215. [Google Scholar] [CrossRef]
- Tomàs Giner, M.; Jiménez-Marti, E.; Bofill Arasa, R.; Tinti, A.; Di Foggia, M.; Chatgilialoglu, C.; Torreggiani, A. Analysis of the soybean metallothionein system under radical stress: Protein modification connected to lipid membrane damages. Metallomics 2018, 10, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C. Trans lipids: The free radical path. Acc. Chem. Res. 2005, 38, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Geometrical trans lipid isomers: A new target for lipidomics. ChemBioChem 2005, 6, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Lipid isomerization. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 3, pp. 1599–1622. [Google Scholar]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Cort, A.; Melchiore, M.; Chatgilialoglu, C.; Ferreri, C.; Sansone, A.; Ozben, T. Effects of bleomycin and antioxidants on the fatty acid profile of testicular cancer cell membranes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 434–441. [Google Scholar] [CrossRef]
- Zambonin, L.; Ferreri, C.; Cabrini, L.; Prata, C.; Chatgilialoglu, C.; Landi, L. Occurrence of trans fatty acids in rats fed a trans-free diet: A free radical-mediated formation? Free Radic. Biol. Med. 2006, 40, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert Rev. Mol. Diagn. 2012, 12, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Membrane Lipidomics for Personalized Health; Wiley: Chichester, UK, 2015. [Google Scholar]
- Ferreri, C.; Samadi, A.; Sassatelli, F.; Landi, L.; Chatgilialoglu, C. Regioselective cis-trans isomerization of arachidonic double bonds by thiyl radicals: The influence of phospholipid supramolecular organization. J. Am. Chem. Soc. 2004, 126, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Faraone Mennella, M.R.; Formisano, C.; Landi, L.; Chatgilialoglu, C. Arachidonate geometrical isomers generated by thiyl radicals: The relationship with trans lipids detected in biological samples. Free Radic. Biol. Med. 2002, 33, 1516–1526. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Altieri, A.; Fischer, H. The Kinetics of thiyl radical induced reactions of monounsaturated fatty acid esters. J. Am. Chem. Soc. 2002, 124, 12816–12823. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Bowry, V.W. Why not trans? Inhibited radical isomerization cycles and coupling chains of lipids and alkenes with alkane-thiols. J. Org. Chem. 2018, 83, 9178–9189. [Google Scholar] [CrossRef] [PubMed]
- Lykakis, I.N.; Ferreri, C.; Chatgilialoglu, C. Sulfhydryl radical (HS•/S•–): A contender for the isomerization of double bonds in membrane lipids. Angew. Chem. Int. Ed. 2007, 46, 1914–1916. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Kruger, N.; Lahiri, D.R.; Wang, D.; Vatele, J.M.; Balazy, M. Nitrogen dioxide induces cis-trans-isomerisation of arachidonic acid within cellular phospholipids. Detection of trans-arachidonic acids in vivo. J. Biol. Chem. 1999, 274, 16235–16241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balazy, M. Trans-arachidonic acids: New mediators of inflammation. J. Physiol. Pharmacol. 2000, 51, 597–607. [Google Scholar] [PubMed]
- Hung, W.-L.; Ho, C.-T.; Hwang, L.S. Inhibitory activity of natural occurring antioxidants on thiyl radical-induced trans-arachidonic acid formation. J. Agric. Food Chem. 2011, 59, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.-Y.; Hung, W.-L.; Ho, C.-T.; Cheng, I.H.; Hwang, L.S. Protective effects of sesamol and ferulic acid on the formation of endogenous trans-arachidonic acid in hAPP J20 mice. J. Funct. Foods 2015, 16, 378–385. [Google Scholar] [CrossRef]
- Hung, W.-L.; Hsu, B.-Y.; Tung, Y.-C.; Ho, C.-T.; Hwang, L.S. Inhibitory effects of antioxidant vitamins against thiyl radical-induced trans fatty acid formation in PC-12 cells. J. Funct. Foods 2016, 21, 212–222. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Ferreri, C.; Angelini, F.; Chatgilialoglu, C.; Dellonte, S.; Moschese, V.; Rossi, P.; Chini, L. Trans fatty acids and atopic eczema/dermatitis syndrome: The relationship with a free radical cis-trans isomerization of membrane lipids. Lipids 2005, 40, 661–667. [Google Scholar] [CrossRef]
- Sansone, A.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C. Hexadecenoic fatty acid isomers: A chemical biology approach for human plasma biomarker development. Chem. Res. Toxicol. 2013, 26, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Benerjee, R. Chemical biology of H2S signaling throughpersulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Kelly, S.S.; Xu, S.; Xian, M. The path to controlled delivery of reactive sulfur species. Acc. Chem. Res. 2021, 54, 3968–3978. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-P.R.; Griesser, M.; Pratt, D.A. Hydropersulfides: H-atom transfer agents par excellence. J. Am. Chem. Soc. 2017, 139, 6484–6493. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, A.; Tinti, A.; Jurasekova, Z.; Capdevila, M.; Saracino, M.; Foggia, M.D. Structural lesions of proteins connected to lipid membrane damages caused by radical stress: Assessment by biomimetic systems and Raman spectroscopy. Biomolecules 2019, 9, 794. [Google Scholar] [CrossRef] [Green Version]
- Hung, W.-L.; Hwang, L.S.; Shahidi, F.; Pan, M.H.; Wang, Y.; Ho, C.-T. Endogenous formation of trans fatty acids: Health implications and potential dietary intervention. J. Funct. Foods 2016, 25, 14–24. [Google Scholar] [CrossRef]
- Larocca, A.V.; Toniolo, G.; Tortorella, S.; Krokidis, M.G.; Menounou, G.; Chatgilialoglu, C.; Ferreri, C. The entrapment of somatostatin in a lipid formulation: Retarded release and free radical reactivity. Molecules 2019, 24, 3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prajapati, I.; Peters, B.-H.; Larson, N.R.; Wei, Y.; Choudhary, S.; Kalonia, C.; Hudak, S.; Esfandiary, R.; Middaugh, C.R.; Schöneich, C. Cis/trans isomerization of unsaturated fatty acids in polysorbate 80 during light exposure of a monoclonal antibody-containing formulation. J. Pharm. Sci. 2020, 109, 603–613. [Google Scholar] [CrossRef] [Green Version]

| Substrate | k(e−aq ) | k(H•) | k(HO•) |
|---|---|---|---|
| Methionine | 4.0 × 107 | 3.5 × 108 | 8.3 × 109 |
| Glutathione disulfide | 3.7 × 109 | 1.0 × 1010 | 9.6 × 109 |
| Met-enkephalin 1 | 3.8 × 1010 | ||
| Ribonuclease | 2.1 × 1010 | 1.5 × 1010 | 2.4 × 1010 |
| Lysozyme | 3.1 × 1010 | 4.9 × 1010 | |
| Serum albumin | 8.2 × 109 | 7.8 × 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatgilialoglu, C.; Ferreri, C. Reductive Stress of Sulfur-Containing Amino Acids within Proteins and Implication of Tandem Protein–Lipid Damage. Int. J. Mol. Sci. 2021, 22, 12863. https://doi.org/10.3390/ijms222312863
Chatgilialoglu C, Ferreri C. Reductive Stress of Sulfur-Containing Amino Acids within Proteins and Implication of Tandem Protein–Lipid Damage. International Journal of Molecular Sciences. 2021; 22(23):12863. https://doi.org/10.3390/ijms222312863
Chicago/Turabian StyleChatgilialoglu, Chryssostomos, and Carla Ferreri. 2021. "Reductive Stress of Sulfur-Containing Amino Acids within Proteins and Implication of Tandem Protein–Lipid Damage" International Journal of Molecular Sciences 22, no. 23: 12863. https://doi.org/10.3390/ijms222312863
APA StyleChatgilialoglu, C., & Ferreri, C. (2021). Reductive Stress of Sulfur-Containing Amino Acids within Proteins and Implication of Tandem Protein–Lipid Damage. International Journal of Molecular Sciences, 22(23), 12863. https://doi.org/10.3390/ijms222312863







