State-Targeting Stabilization of Adenosine A2A Receptor by Fusing a Custom-Made De Novo Designed α-Helical Protein
Abstract
:1. Introduction
2. Results
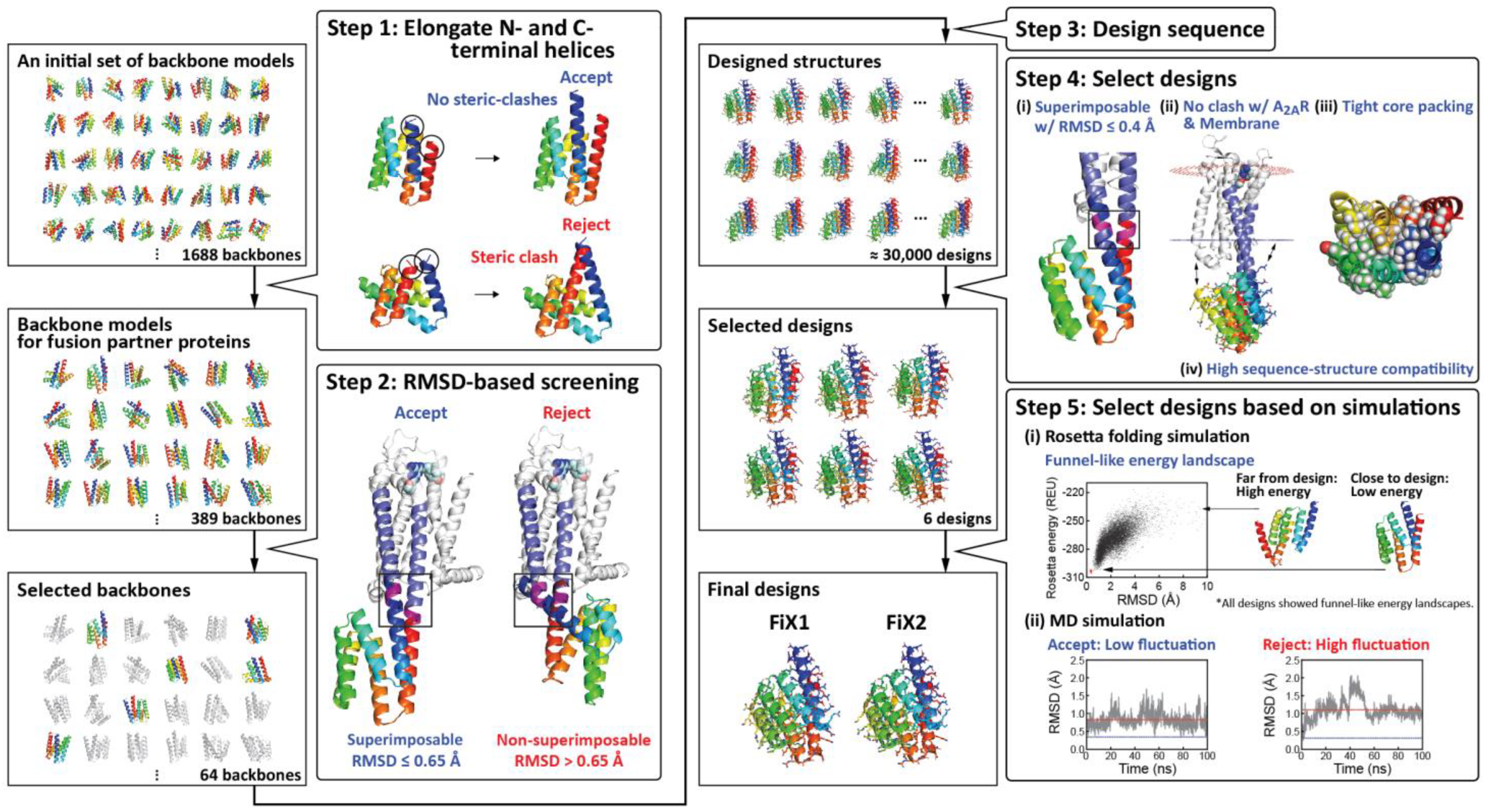
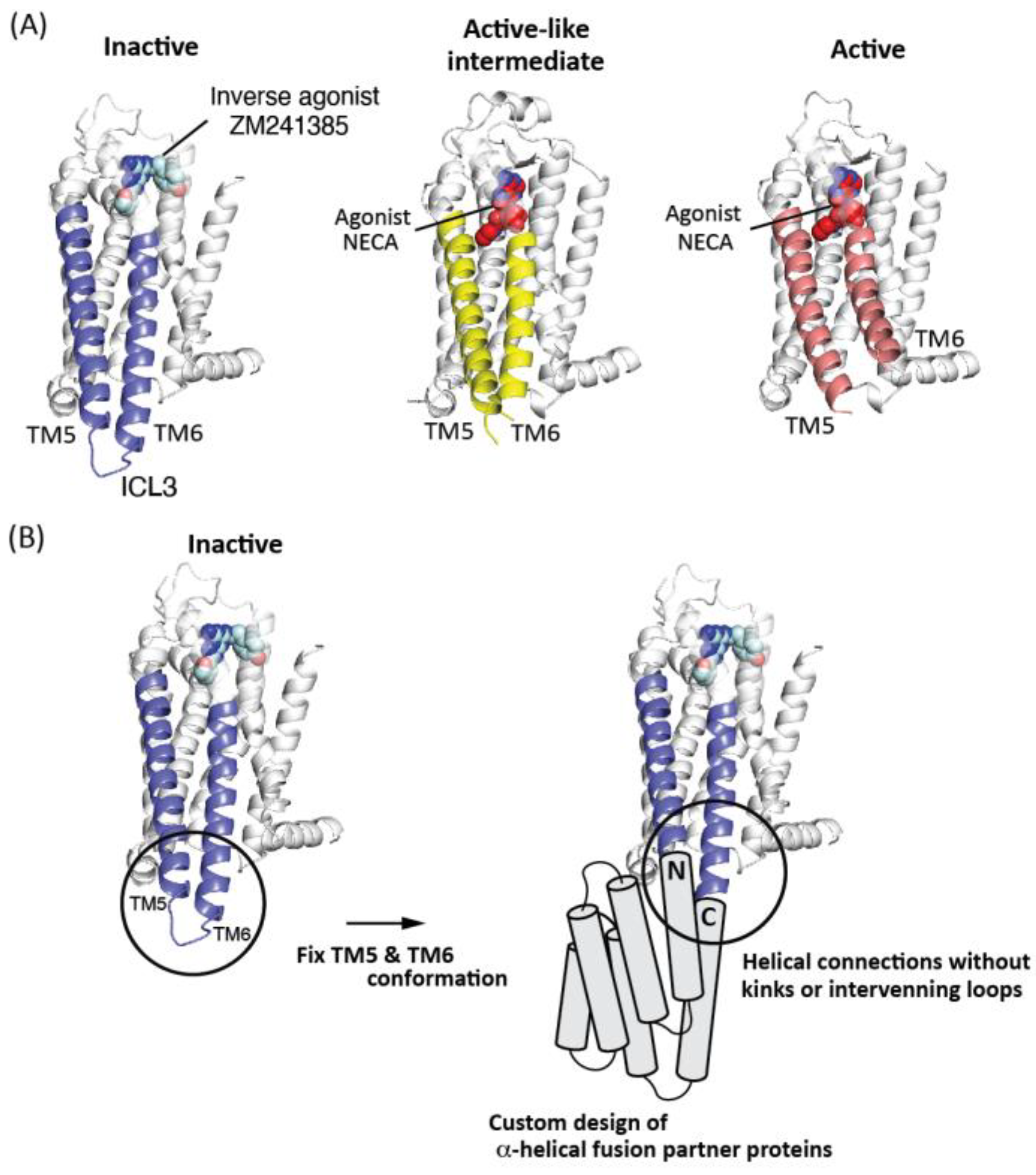
2.1. Computational Design of α-Helical Fusion Partner Proteins
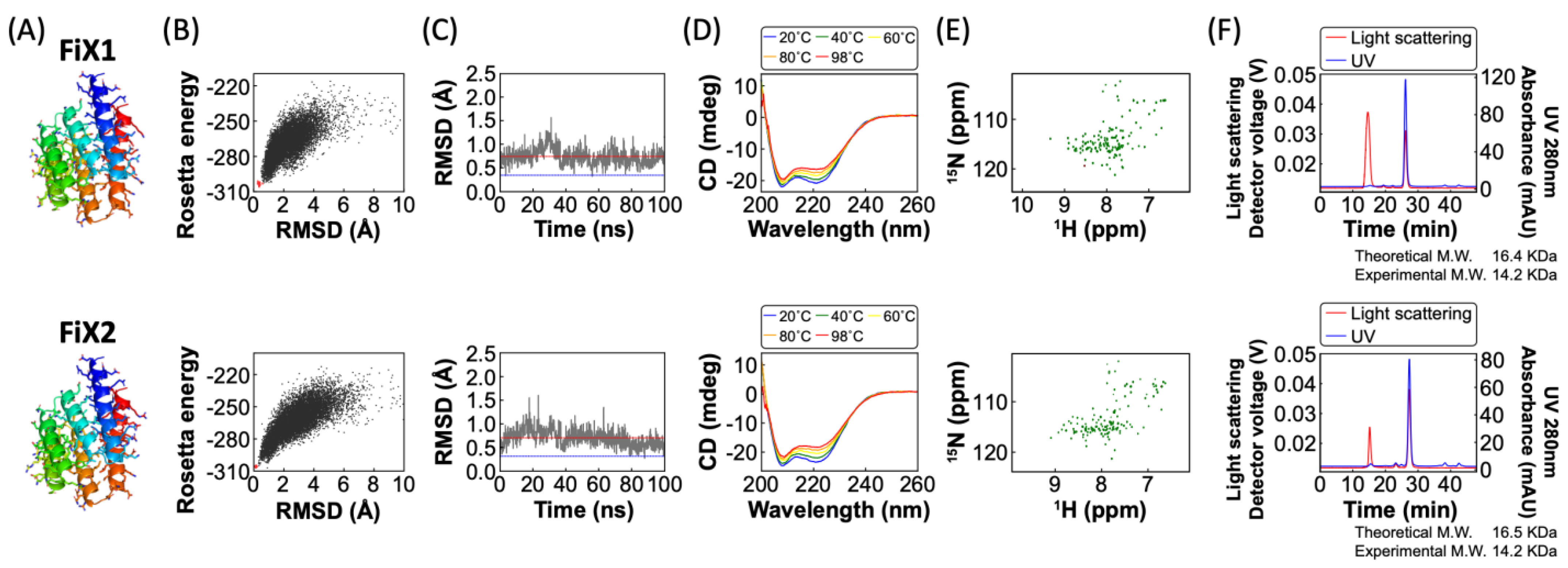
2.2. Experimental Characterization of FiX1 and FiX2
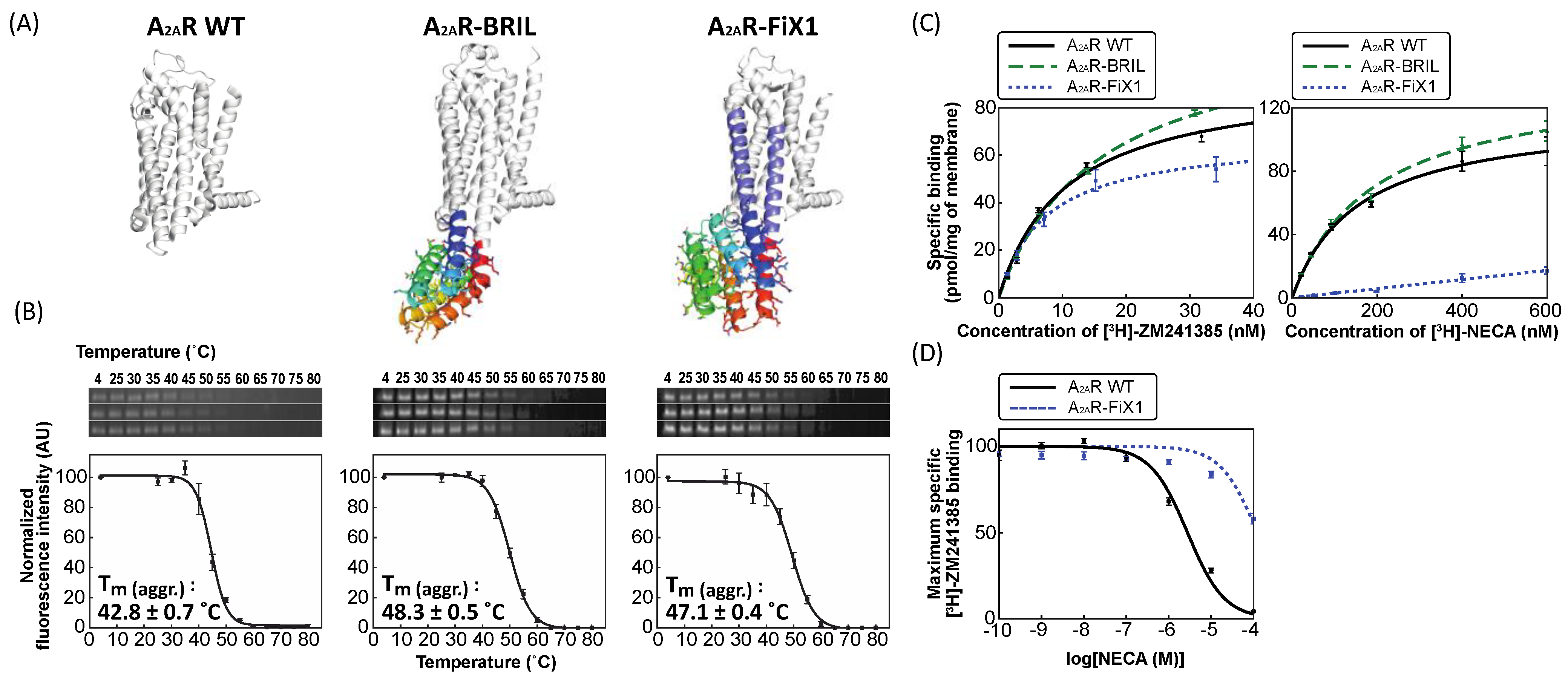
2.3. Experimental Characterization of A2AR Fused with FiX1 and FiX2
3. Discussion
4. Materials and Methods
4.1. Selection of Backbone Structure Models for Fusion Partners
4.2. Sequence Design for Further Backbone Selection
4.3. Sequence Design
4.4. Selection Criteria after Sequence Design
4.5. Rosetta Folding Simulation
4.6. Molecular Dynamics (MD) Simulation
4.7. A Manual Mutation Using Foldit
4.8. Experiments of De Novo Designed Fusion Partner Proteins: Protein Expression and Purification
4.9. Experiments of De Novo Designed Fusion Partner Proteins: Circular Dichroism (CD)
4.10. Experiments of De Novo Designed Fusion Partner Proteins: Size Exclusion Chromatography Combined with Multi-Angle Light Scattering (SEC-MALS)
4.11. Experiments of De Novo Designed Fusion Partner Proteins: 2D 1H–15N HSQC Measurement
4.12. Experiments of A2AR-Designed Fusion Partner Proteins: DNA Construction
4.13. Experiments of A2AR-Designed Fusion Partner Proteins: Solubilization Efficiency
4.14. Experiments of A2AR-Designed Fusion Partner Proteins: Clear-Native PAGE
4.15. Experiments of A2AR-Designed Fusion Partner Proteins: Radioligand Binding Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lagerström, M.C.; Schiöth, H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 339–357. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Weis, W.I.; Kobilka, B.K. The molecular basis of G protein–coupled receptor activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef]
- Magnani, F.; Shibata, Y.; Serrano-Vega, M.J.; Tate, C.G. Co-evolving stability and conformational homogeneity of the human adenosine A2a receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 10744–10749. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Vega, M.J.; Magnani, F.; Shibata, Y.; Tate, C.G. Conformational thermostabilization of the β1-adrenergic receptor in a detergent-resistant form. Proc. Natl. Acad. Sci. USA 2008, 105, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, C.A.; Dodevski, I.; Kenig, M.; Dudli, S.; Mohr, A.; Hermans, E.; Plückthun, A. Directed evolution of a G protein-coupled receptor for expression, stability, and binding selectivity. Proc. Natl. Acad. Sci. USA 2008, 105, 14808–14813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.Y.; Zhou, F.; Fryszczyn, B.G.; Barth, P. Naturally evolved G protein-coupled receptors adopt metastable conformations. Proc. Natl. Acad. Sci. USA 2012, 109, 13284–13289. [Google Scholar] [CrossRef] [Green Version]
- Popov, P.; Peng, Y.; Shen, L.; Stevens, R.C.; Cherezov, V.; Liu, Z.-J.; Katritch, V. Computational design of thermostabilizing point mutations for G protein-coupled receptors. eLife 2018, 7, e34729. [Google Scholar] [CrossRef] [PubMed]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.F.; Thian, F.S.; Kobilka, T.S.; Choi, H.-J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, D.M.; Cherezov, V.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Yao, X.J.; Weis, W.I.; Stevens, R.C.; et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 2007, 318, 1266–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, E.; Thompson, A.A.; Liu, W.; Roth, C.B.; Griffith, M.T.; Katritch, V.; Kunken, J.; Xu, F.; Cherezov, V.; Hanson, M.A.; et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 2012, 20, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chun, E.; Thompson, A.A.; Chubukov, P.; Xu, F.; Katritch, V.; Han, G.W.; Roth, C.B.; Heitman, L.H.; Ijzerman, A.P.; et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Zhu, Y.; Li, J.; Chen, Z.; Han, G.W.; Kufareva, I.; Li, T.; Ma, L.; Fenalti, G.; Li, J.; et al. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 2013, 341, 1387–1390. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Mobarec, J.C.; Kolb, P.; Rosenbaum, D.M. Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant. Nature 2015, 519, 247–250. [Google Scholar] [CrossRef]
- Jo, M.; Jung, S.T. Engineering therapeutic antibodies targeting G-protein–coupled receptors. Exp. Mol. Med. 2016, 48, e207. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 2021, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Tatsumi-Koga, R.; Liu, G.; Xiao, R.; Acton, T.B.; Montelione, G.T.; Baker, D. Principles for designing ideal protein structures. Nature 2012, 491, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.-S.; Boyken, S.E.; Baker, D. The coming of age of de novo protein design. Nature 2016, 537, 320–327. [Google Scholar] [CrossRef]
- Koga, R.; Koga, N. Consistency principle for protein design. Biophys. Phys. 2019, 16, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, K.; Kobayashi, N.; Sugiki, T.; Nagashima, T.; Fujiwara, T.; Suzuki, K.; Kobayashi, N.; Murata, T.; Kosugi, T.; Koga, R.; et al. Design of complicated all-α protein structures. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jaakola, V.P.; Griffith, M.T.; Hanson, M.A.; Cherezov, V.; Chien, E.Y.; Lane, J.R.; Ijzerman, A.P.; Stevens, R.C. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 2008, 322, 1211–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doré, A.S.; Robertson, N.; Errey, J.C.; Ng, I.; Hollenstein, K.; Tehan, B.; Hurrell, E.; Bennett, K.; Congreve, M.; Magnani, F.; et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 2011, 19, 1283–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Lebon, G.; Warne, T.; Edwards, P.C.; Bennett, K.; Langmead, C.J.; Leslie, A.G.W.; Tate, C.G. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 2011, 474, 521–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, B.; Nehmé, R.; Warne, T.; Leslie, A.G.W.; Tate, C.G. Structure of the adenosine A2A receptor bound to an engineered G protein. Nature 2016, 536, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, B.; Dantas, G.; Ireton, G.C.; Varani, G.; Stoddard, B.L.; Baker, D. Design of a novel globular protein fold with atomic-level accuracy. Science 2003, 302, 1364–1368. [Google Scholar] [CrossRef] [Green Version]
- Sheffler, W.; Baker, D. RosettaHoles2: A volumetric packing measure for protein structure refinement and validation. Protein Sci. 2010, 19, 1991–1995. [Google Scholar] [CrossRef] [Green Version]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef] [PubMed]
- Kleffner, R.; Flatten, J.; Leaver-Fay, A.; Baker, D.; Siegel, J.B.; Khatib, F.; Cooper, S. Foldit Standalone: A video game-derived protein structure manipulation interface using Rosetta. Bioinformatics 2017, 33, 2765–2767. [Google Scholar] [CrossRef]
- Shiroishi, M.; Tsujimoto, H.; Makyio, H.; Asada, H.; Yurugi-Kobayashi, T.; Shimamura, T.; Murata, T.; Nomura, N.; Haga, T.; Iwata, S.; et al. Platform for the rapid construction and evaluation of GPCRs for crystallography in Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Takamuku, Y.; Asakawa, T.; Inai, M.; Hino, T.; Iwata, S.; Kan, T.; Murata, T. An efficient screening method for purifying and crystallizing membrane proteins using modified clear-native PAGE. Anal. Biochem. 2018, 548, 7–14. [Google Scholar] [CrossRef]
- Ye, L.; Van Eps, N.; Zimmer, M.; Ernst, O.P.; Scott Prosser, R. Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature 2016, 533, 265–268. [Google Scholar] [CrossRef]
- Huang, S.K.; Pandey, A.; Tran, D.P.; Villanueva, N.L.; Kitao, A.; Sunahara, R.K.; Sljoka, A.; Prosser, R.S. Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling. Cell 2021, 184, 1884–1894. e14. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Takei, J.; Knowlton, J.R.; Andrykovitch, M.; Pei, W.; Kajava, A.V.; Steinbach, P.J.; Ji, X.; Bai, Y. Redesign of a four-helix bundle protein by phage display coupled with proteolysis and structural characterization by NMR and X-ray crystallography. J. Mol. Biol. 2002, 323, 253–262. [Google Scholar] [CrossRef]
- Mitternacht, S. FreeSASA: An open source C library for solvent accessible surface area calculations. F1000Research 2016, 5, 189. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-S.; Ban, Y.-E.A.; Richter, F.; Andre, I.; Vernon, R.; Schief, W.R.; Baker, D. RosettaRemodel: A Generalized Framework for Flexible Backbone Protein Design. PLoS ONE 2011, 6, e24109. [Google Scholar] [CrossRef] [Green Version]
- O’Meara, M.J.; Leaver-Fay, A.; Tyka, M.D.; Stein, A.; Houlihan, K.; DiMaio, F.; Bradley, P.; Kortemme, T.; Baker, D.; Snoeyink, J.; et al. Combined covalent-electrostatic model of hydrogen bonding improves structure prediction with rosetta. J. Chem. Theory Comput. 2015, 11, 609–622. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.R.; Koga, N.; Tatsumi-Koga, R.; Liu, G.; Clouser, A.F.; Montelione, G.T.; Baker, D. Control over overall shape and size in de novo designed proteins. Proc. Natl. Acad. Sci. USA 2015, 112, E5478–E5485. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, J.A.; Deupi, X.; Olivella, M.; Haaksma, E.E.J.; Pardo, L. Serine and threonine residues bend α-helices in the χ1=g− conformation. Biophys. J. 2000, 79, 2754–2760. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.G.; Bartlett, G.J.; Crump, M.P.; Sessions, R.B.; Linden, N.; Faul, C.F.J.; Woolfson, D.N. Local and macroscopic electrostatic interactions in single α-helices. Nat. Chem. Biol. 2015, 11, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham III, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. Amber 16; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins Struct. Funct. Bioinform. 2006, 65, 712–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hino, T.; Arakawa, T.; Iwanari, H.; Yurugi-Kobayashi, T.; Ikeda-Suno, C.; Nakada-Nakura, Y.; Kusano-Arai, O.; Weyand, S.; Shimamura, T.; Nomura, N.; et al. G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature 2012, 482, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newstead, S.; Kim, H.; von Heijne, G.; Iwata, S.; Drew, D. High-throughput fluorescent-based optimization of eukaryotic membrane protein overexpression and purification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 13936–13941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Construct | Solubilization Efficiency (%) |
|---|---|
| A2AR WT | 24 ± 10 |
| A2AR–BRIL | 57 ± 19 |
| A2AR–FiX1 | 59 ± 21 |
| A2AR–FiX2 | 42 ± 17 |
| Construct | Kd (nM) | |
|---|---|---|
| ZM241385 | NECA | |
| A2AR WT | 10.5 ± 0.3 | 162 ± 44 |
| A2AR–BRIL | 15.7 ± 0.6 | 191 ± 22 |
| A2AR–FiX1 | 7.3 ± 0.5 | N. D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsumoto, M.; Sugaya, K.; Kazama, K.; Nakano, R.; Kosugi, T.; Murata, T.; Koga, N. State-Targeting Stabilization of Adenosine A2A Receptor by Fusing a Custom-Made De Novo Designed α-Helical Protein. Int. J. Mol. Sci. 2021, 22, 12906. https://doi.org/10.3390/ijms222312906
Mitsumoto M, Sugaya K, Kazama K, Nakano R, Kosugi T, Murata T, Koga N. State-Targeting Stabilization of Adenosine A2A Receptor by Fusing a Custom-Made De Novo Designed α-Helical Protein. International Journal of Molecular Sciences. 2021; 22(23):12906. https://doi.org/10.3390/ijms222312906
Chicago/Turabian StyleMitsumoto, Masaya, Kanna Sugaya, Kazuki Kazama, Ryosuke Nakano, Takahiro Kosugi, Takeshi Murata, and Nobuyasu Koga. 2021. "State-Targeting Stabilization of Adenosine A2A Receptor by Fusing a Custom-Made De Novo Designed α-Helical Protein" International Journal of Molecular Sciences 22, no. 23: 12906. https://doi.org/10.3390/ijms222312906
APA StyleMitsumoto, M., Sugaya, K., Kazama, K., Nakano, R., Kosugi, T., Murata, T., & Koga, N. (2021). State-Targeting Stabilization of Adenosine A2A Receptor by Fusing a Custom-Made De Novo Designed α-Helical Protein. International Journal of Molecular Sciences, 22(23), 12906. https://doi.org/10.3390/ijms222312906






