Acetone Ingestion Mimics a Fasting State to Improve Glucose Tolerance in a Mouse Model of Gestational Hyperglycemia
Abstract
:1. Introduction
2. Results
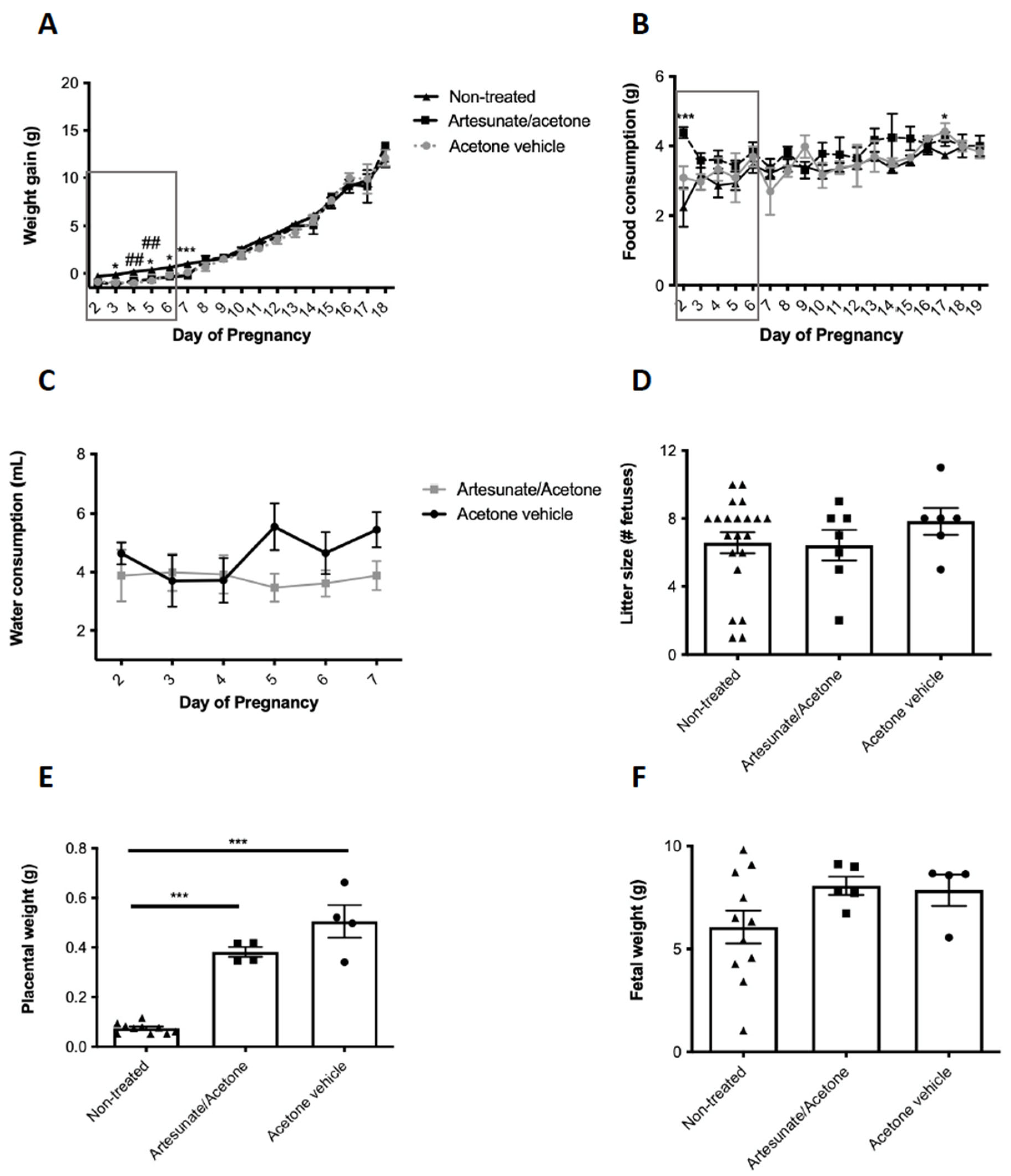
2.1. Artesunate Treatment in Early Gestation and Pregnancy Outcomes
2.2. Both Artesunate- and Acetone Vehicle-Treated Animals Have Improved Glucose Tolerance vs. Non-Treated Females
2.3. Acetone Treatment Alters Pancreas Histology during and after Pregnancy
2.4. Acetone Treatment Causes Hyperglucagonemia during and after Pregnancy
2.5. Acetone Treatment Increases Islet Bihormonal Cell Number
2.6. Acetone Treatment Increases Placental Apelinergic Gene Expression
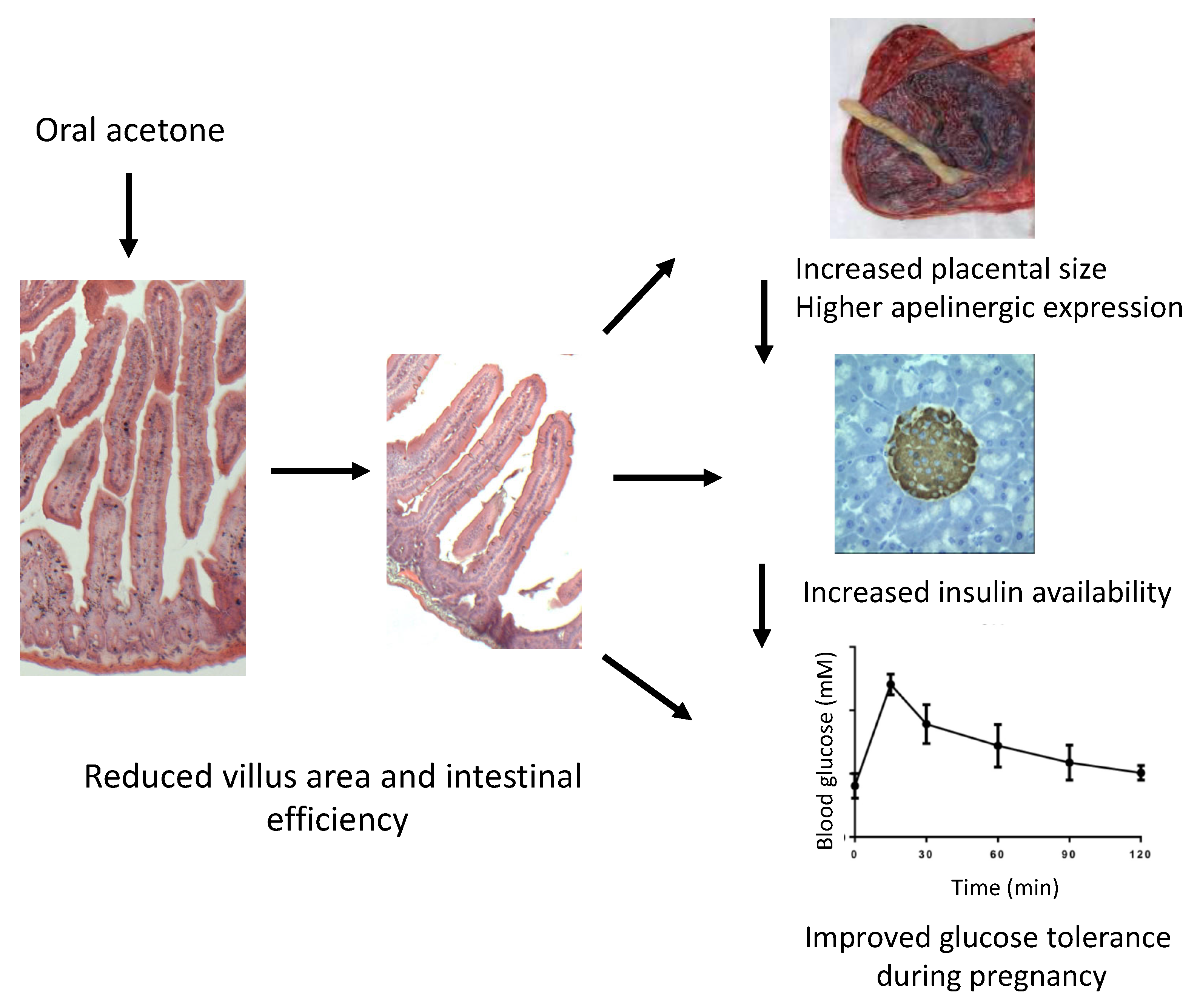
2.7. Acetone Treatment Alters Gut Morphology
3. Discussion
4. Materials and Methods
4.1. Animals, Treatment, and Sample Collection
4.2. Intra-Peritoneal Glucose Tolerance Test
4.3. Immunohistochemistry and Tissue Morphometry
4.4. Serum ELISA Assays
4.5. Quantitative Polymerase Chain Reaction
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachdeva, M.M.; Stoffers, D.A. Minireview: Meeting the Demand for Insulin: Molecular Mechanisms of Adaptive Postnatal ß-Cell Mass Expansion. Mol. Endocrinol. 2009, 23, 747–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vargas, L.; Addison, S.S.; Nistala, R.; Kurukulasuriya, D.; Sowers, J.R. Gestational Diabetes and the Offspring: Implications in the Development of the Cardiorenal Metabolic Syndrome in Offspring. Cardiorenal Med. 2012, 2, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Sharma, A.J.; Callaghan, W.M. Gestational diabetes and childhood obesity. Curr. Opin. Obstet. Gynecol. 2012, 24, 376–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitanchez, D. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J. Diabetes 2015, 6, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Heida, K.Y.; Franx, A.; van Rijn, B.B.; Eijkemans, M.J.; Boer, J.M.; Verschuren, M.W.; Oudijk, M.A.; Bots, M.L.; van der Schouw, Y.T. Earlier Age of Onset of Chronic Hypertension and Type 2 Diabetes Mellitus After a Hypertensive Disorder of Pregnancy or Gestational Diabetes Mellitus. Hypertension 2015, 66, 1116–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlapinski, S.K.; Botros, A.A.; Donegan, S.; King, R.T.; Retta, G.; Strutt, B.J.; Hill, D.J. Altered pancreas remodeling following glucose intolerance in pregnancy in mice. J. Endocrinol. 2020, 245, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, F.A.; Aerts, L.; Gepts, W. Morphological Changes in the Endocrine Pancreas in Pregnant Rats with Experimental Diabetes. J. Endocrinol. 1979, 80, 175–179. [Google Scholar] [CrossRef]
- Parsons, J.A.; Brelje, T.C.; Sorenson, R.L. Adaptation of islets of Langerhans to pregnancy: Increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 1992, 130, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, R.L.; Brelje, T.C. Adaptation of Islets of Langerhans to Pregnancy: β-Cell Growth, Enhanced Insulin Secretion and the Role of Lactogenic Hormones. Horm. Metab. Res. 1997, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Cao-Minh, L.; Galasso, R.; Rizza, R.A.; Corradin, A.; Cobelli, C.; Butler, P.C. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010, 53, 2167–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beamish, C.A.; Zhang, L.; Szlapinski, S.K.; Strutt, B.J.; Hill, D.J. An increase in immature β-cells lacking Glut2 precedes the expansion of β-cell mass in the pregnant mouse. PLoS ONE 2017, 12, e0182256. [Google Scholar] [CrossRef] [Green Version]
- Szlapinski, S.K.; King, R.T.; Retta, G.; Yeo, E.; Strutt, B.J.; Hill, D.J. A mouse model of gestational glucose intolerance through exposure to a low protein diet during fetal and neonatal development. J. Physiol. 2019, 597, 4237–4250. [Google Scholar] [CrossRef]
- Quesada-Candela, C.; Tudurí, E.; Marroquí, L.; Alonso-Magdalena, P.; Quesada, I.; Nadal, Á. Morphological and functional adaptations of pancreatic alpha-cells during late pregnancy in the mouse. Metabolism 2019, 102, 153963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieck, S.; Kaestner, K.H. Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab. 2010, 21, 151–158. [Google Scholar] [CrossRef] [Green Version]
- A Van Assche, F.; Aerts, L.; De Prins, F. A Morphological Study of the Endocrine Pancreas in Human Pregnancy. BJOG Int. J. Obstet. Gynaecol. 1978, 85, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Thorel, F.; Népote, V.; Avril, I.; Kohno, K.; Desgraz, R.; Chera, S.; Herrera, P.L. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 2010, 464, 1149–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Robertson, M.A.; Hesselson, D.; Stainier, D.Y.R.; Anderson, R.M. glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development 2015, 142, 1407–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Casteels, T.; Frogne, T.; Ingvorsen, C.; Honore, C.; Courtney, M.; Huber, K.V.; Schmitner, N.; Kimmel, R.A.; Romanov, R.A.; et al. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell 2017, 168, 86–100.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Shi, X.; Liu, J.; Shao, F.; Huang, G.; Zhou, Z.; Zheng, P. Artesunate prevents type 1 diabetes in NOD mice mainly by inducing protective IL-4—Producing T cells and regulatory T cells. FASEB J. 2019, 33, 8241–8248. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, W.; Xin, Y.; Bai, J.; Peng, H.; Fu, L.; Liu, J.; Li, L.; Ma, Y.; Jiang, H. Antidiabetic and Antiobesity Effects of Artemether in db/db Mice. BioMed Res. Int. 2018, 2018, 8639523. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, T.; Lee, S.; Noordeloos, E.; Donaldson, C.J.; Adams, M.W.; Noguchi, G.M.; Mawla, A.; Huising, M.O. Artemether Does Not Turn α Cells into β Cells. Cell Metab. 2017, 27, 218–225.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmanov, A.A.; Reed, D.; Beauchamp, G.K.; Tordoff, M. Food Intake, Water Intake, and Drinking Spout Side Preference of 28 Mouse Strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef]
- Ackermann, A.M.; Moss, N.G.; Kaestner, K.H. GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Cell Metab. 2018, 28, 787–792.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coddens, A.; Diswall, M.; Ångström, J.; Breimer, M.E.; Goddeeris, B.; Cox, E.; Teneberg, S. Recognition of Blood Group ABH Type 1 Determinants by the FedF Adhesin of F18-fimbriated Escherichia coli. J. Biol. Chem. 2009, 284, 9713–9726. [Google Scholar] [CrossRef] [Green Version]
- Jennemann, R.; Kaden, S.; Sandhoff, R.; Nordström, V.; Wang, S.; Volz, M.; Robine, S.; Amen, N.; Rothermel, U.; Wiegandt, H.; et al. Glycosphingolipids Are Essential for Intestinal Endocytic Function. J. Biol. Chem. 2012, 287, 32598–32616. [Google Scholar] [CrossRef] [Green Version]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Plaa, G.L.; Hewitt, W.R.; Du Souich, P.; Caillé, G.; Lock, S. Isopropanol and acetone potentiation of carbon tetrachloride-induced hepatotoxic1ty: Single versus repetitive pretreatments in rats. J. Toxicol. Environ. Health Part A 1982, 9, 235–250. [Google Scholar] [CrossRef]
- Scholl, H.R.; Iba, M.M. Pharmacokinetics of and CYP1A induction by pyridine and acetone in the rat: Interactions and effects of route of exposure. Xenobiotica 1997, 27, 265–277. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51 (Suppl. S3), S434–S442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.-W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-Mimicking Diet Promotes Ngn3-Driven β-Cell Regeneration to Reverse Diabetes. Cell 2017, 168, 775–788.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlapinski, S.K.; Bennett, J.; Strutt, B.J.; Hill, D.J. Increased alpha and beta cell mass during mouse pregnancy is not dependent on transdifferentiation. Exp. Biol. Med. 2020, 246, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Mouzon, S.H.-D. The Human Placenta in Gestational Diabetes Mellitus: The insulin and cytokine network. Diabetes Care 2007, 30 (Suppl. S2), S120–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayeur, S.; Wattez, J.-S.; Lukaszewski, M.-A.; Lecoutre, S.; Butruille, L.; Drougard, A.; Eberlé, D.; Bastide, B.; Laborie, C.; Storme, L.; et al. Apelin Controls Fetal and Neonatal Glucose Homeostasis and Is Altered by Maternal Undernutrition. Diabetes 2015, 65, 554–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castan-Laurell, I.; Dray, C.; Attané, C.; Duparc, T.; Knauf, C.; Valet, P. Apelin, diabetes, and obesity. Endocrine 2011, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Ali Babar, M.; et al. Climate Change Impact and Adaptation for Wheat Protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Snoeck, A.; Remacle, C.; Reusens, B.; Hoet, J.J. Effect of a Low Protein Diet during Pregnancy on the Fetal Rat Endocrine Pancreas. Neonatology 1990, 57, 107–118. [Google Scholar] [CrossRef]
- Beamish, C.A.; Strutt, B.J.; Arany, E.J.; Hill, D.J. Insulin-positive, Glut2-low cells present within mouse pancreas exhibit lineage plasticity and are enriched within extra-islet endocrine cell clusters. Islets 2016, 8, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Cigliola, V.; Ghila, L.; Thorel, F.; Van Gurp, L.; Baronnier, D.; Oropeza, D.; Gupta, S.; Miyatsuka, T.; Kaneto, H.; Magnuson, M.A.; et al. Pancreatic islet-autonomous insulin and smoothened-mediated signalling modulate identity changes of glucagon+ α-cells. Nature 2018, 20, 1267–1277. [Google Scholar] [CrossRef]
- Bru-Tari, E.; Cobo-Vuilleumier, N.; Magdalena, P.A.; dos Santos, R.S.; Marroqui, L.; Nadal, A.; Gauthier, B.; Quesada, I. Pancreatic alpha-cell mass in the early-onset and advanced stage of a mouse model of experimental autoimmune diabetes. Sci. Rep. 2019, 9, 9515. [Google Scholar] [CrossRef] [Green Version]
- Piran, R.; Lee, S.-H.; Li, C.-R.; Charbono, A.; Bradley, L.M.; Levine, F. Pharmacological induction of pancreatic islet cell transdifferentiation: Relevance to type I diabetes. Cell Death Dis. 2014, 5, e1357. [Google Scholar] [CrossRef] [Green Version]
- Chamson-Reig, A.; Thyssen, S.M.; Arany, E.; Hill, D.J. Altered pancreatic morphology in the offspring of pregnant rats given reduced dietary protein is time and gender specific. J. Endocrinol. 2006, 191, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlapinski, S.; Strutt, B.; Deane, M.; Arany, E.; Bennett, J.; Hill, D.J. Acetone Ingestion Mimics a Fasting State to Improve Glucose Tolerance in a Mouse Model of Gestational Hyperglycemia. Int. J. Mol. Sci. 2021, 22, 12914. https://doi.org/10.3390/ijms222312914
Szlapinski S, Strutt B, Deane M, Arany E, Bennett J, Hill DJ. Acetone Ingestion Mimics a Fasting State to Improve Glucose Tolerance in a Mouse Model of Gestational Hyperglycemia. International Journal of Molecular Sciences. 2021; 22(23):12914. https://doi.org/10.3390/ijms222312914
Chicago/Turabian StyleSzlapinski, Sandra, Brenda Strutt, Madeline Deane, Edith Arany, Jamie Bennett, and David J. Hill. 2021. "Acetone Ingestion Mimics a Fasting State to Improve Glucose Tolerance in a Mouse Model of Gestational Hyperglycemia" International Journal of Molecular Sciences 22, no. 23: 12914. https://doi.org/10.3390/ijms222312914
APA StyleSzlapinski, S., Strutt, B., Deane, M., Arany, E., Bennett, J., & Hill, D. J. (2021). Acetone Ingestion Mimics a Fasting State to Improve Glucose Tolerance in a Mouse Model of Gestational Hyperglycemia. International Journal of Molecular Sciences, 22(23), 12914. https://doi.org/10.3390/ijms222312914





