Comparison of Aging Resistance and Antimicrobial Properties of Ethylene–Norbornene Copolymer and Poly(Lactic Acid) Impregnated with Phytochemicals Embodied in Thyme (Thymus vulgaris) and Clove (Syzygium aromaticum)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Plant Extracts
2.2. Analysis of the Impregnation Process and Its Impact on Polymer Samples’ Properties
2.3. Characterization of the Aging Process
2.4. Antimicrobial Activity
3. Conclusions
4. Future Perspectives and Research Ideas
- investigation of the release kinetics of impregnated phytochemicals into food-grade products;
- tests on the properties of actual food products packed into impregnated films;
- combination of extraction and impregnation processes to create a simple one-pot method;
- trials of surface activation before the impregnation process, e.g., plasma, corona treatment;
- optimization of conditions of solvent-based impregnations (e.g., concentration, temperature, time) and comparison of the results with the effects of processes relying on supercritical CO2;
- further analysis of the aging processes regarding the application of plant-originated substances as color aging indicators.
5. Materials and Methods
5.1. Materials
5.2. Preparation of Polymer Composite Samples
5.3. Extraction of Phytochemicals and Impregnation Process
5.4. Solar Aging Process
5.5. Methods
5.5.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
5.5.2. Static Mechanical Analysis
5.5.3. Surface Free Energy (SFE)
5.5.4. Thermogravimetric Analysis (TGA)
5.5.5. Color Change
5.5.6. Hardness Tests
5.5.7. Dynamic Mechanical Analysis (DMA)
5.5.8. Oxidation Induction Time (OIT) Experiment
5.5.9. Antimicrobial Experiment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas, A.; Torres, A.; Galotto, J.M.; Guarda, A.; Julio, R. Supercritical impregnation for food applications: A review of the effect of the operational variables on the active compound loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of cellulose acetate for controlled release of thymol. Carbohydr. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D.A. Flax mucilage and barley beta-glucan aerogels obtained using supercritical carbon dioxide: Application as flax lignan carriers. Innov. Food Sci. Emerg. Technol. 2015, 28, 40–46. [Google Scholar] [CrossRef]
- Almeida, A.P.; Rodríguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplicio, A.L.; Delgadilho, I.; Da Costa, B.S.; Da Costa, B.L.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. Technol. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Kwon, S.J.; Chang, Y.; Han, J. Oregano essential oil-based natural antimicrobial packaging film to inactivate Salmonella enterica and yeasts/molds in the atmosphere surrounding cherry tomatoes. Food Microbiol. 2017, 65, 114–121. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martín, Á.; Cocero, M.J. Alginate aerogels dried by supercritical CO2 as herbal delivery carrier. Malays. J. Anal. Sci. 2018, 22, 522–531. [Google Scholar] [CrossRef]
- Pantić, M.; Knez, Ž.; Novak, Z. Supercritical impregnation as a feasible technique for entrapment of fat-soluble vitamins into alginate aerogels. J. Non-Cryst. Solids 2016, 432, 519–526. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A.; Kremensas, A. Nutmeg filler as a natural compound for the production of polyurethane composite foams with antibacterial and anti-aging properties. Polym. Test. 2020, 86, 106479. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, B.D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Antimicrobial and improved barrier properties of natural phenolic compound-coated polymeric films for active packaging applications. J. Coat. Technol. Res. 2019, 16, 147–157. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Pyla, R.; Kim, T.J.; Silva, J.L.; Jung, Y.S. Enhanced antimicrobial activity of starch-based film impregnated with thermally processed tannic acid, a strong antioxidant. Int. J. Food Microbiol. 2010, 137, 154–160. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Kosmela, P.; Kuzmin, A.; Piasecki, A.; Kulawik, A.; Chmielnicki, B. By-products from food industry as a promising alternative for the conventional fillers for wood–polymer composites. Polymers 2021, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Sowińska, A. Influence of fillers and ionic liquids on the crosslinking and performance of natural rubber biocomposites. Polymers 2021, 13, 1656. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential application of peppermint (Mentha piperita L.), german chamomile (Matricaria chamomilla L.) and yarrow (Achillea millefolium L.) as active fillers in natural rubber biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. [Google Scholar] [CrossRef]
- Klajn, K.; Gozdek, T.; Bieliński, D.M.; Siciński, M.; Zarzecka-Napierała, M.; Pędzich, Z. Sbr vulcanizates filled with modified ground tire rubber. Materials 2021, 14, 3991. [Google Scholar] [CrossRef]
- Urreaga, M.J.; González-Sánchez, C.; Martínez-Aguirre, A.; Fonseca-Valero, C.; Acosta, J.; De La Orden, M.U. Sustainable eco-composites obtained from agricultural and urban waste plastic blends and residual cellulose fibers. J. Clean. Prod. 2015, 108, 377–384. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Effect of recycling on rheological and mechanical properties of poly(lactic acid)/polystyrene polymer blend. J. Mater. Sci. 2011, 46, 3013–3019. [Google Scholar] [CrossRef]
- de Avila, D.R.; Kerche, F.E.; Gatto, D.A.; Esteves, M.W.L.; Petzhold, C.L.; Amico, C.S. Surface response and photodegradation performance of bio-based polyurethane-forest derivatives foam composites. Polym. Test. 2019, 80, 106102. [Google Scholar] [CrossRef]
- Kuciel, S.; Jakubowska, P.; Kuźniar, P. A study on the mechanical properties and the influence of water uptake and temperature on biocomposites based on polyethylene from renewable sources. Compos. Part B Eng. 2014, 64, 72–77. [Google Scholar] [CrossRef]
- Jaśkiewicz, A.; Budryn, G.; Nowak, A.; Efenberger-Szmechtyk, M. Novel biodegradable starch film for food packaging with antimicrobial chicory root extract and phytic acid as a cross-linking agent. Foods 2020, 9, 1696. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Zdanowicz, M.; Macieja, S.; Kowalczyk, K.; Bartkowiak, A. Development and characterization of bioactive poly(Butylene-succinate) films modified with quercetin for food packaging applications. Polymers 2021, 13, 1798. [Google Scholar] [CrossRef]
- Grylewicz, A.; Spychaj, T.; Zdanowicz, M. Thermoplastic starch/wood biocomposites processed with deep eutectic solvents. Compos. Part A Appl. Sci. Manuf. 2019, 121, 517–524. [Google Scholar] [CrossRef]
- Ludwiczak, J.; Frackowiak, S.; Leluk, K. Study of Thermal, Mechanical and Barrier Properties of Biodegradable PLA/PBAT Films with Highly Oriented MMT. Materials 2021, 14, 7189. [Google Scholar] [CrossRef]
- Kerche, E.F.; Bock, D.N.; de Avila, D.R.; Magalhães, W.L.E.; Amico, S.C. Micro fibrillated cellulose reinforced bio-based rigid high-density polyurethane foams. Cellulose 2021, 28, 4313–4326. [Google Scholar] [CrossRef]
- Csikós, Á.; Faludi, G.; Domján, A.; Renner, K.; Móczó, J.; Pukánszky, B. Modification of interfacial adhesion with a functionalized polymer in PLA/wood composites. Eur. Polym. J. 2015, 68, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Kai, D.; Zhang, K.; Jiang, L.; Wong, H.Z.; Li, Z.; Zhang, Z.; Loh, X.J. Sustainable and Antioxidant Lignin-Polyester Copolymers and Nanofibers for Potential Healthcare Applications. ACS Sustain. Chem. Eng. 2017, 5, 6016–6025. [Google Scholar] [CrossRef]
- Malkapuram, R.; Kumar, V.; Singh, N.Y. Recent development in natural fiber reinforced polypropylene composites. J. Reinf. Plast. Compos. 2009, 28, 1169–1189. [Google Scholar] [CrossRef]
- Członka, S.; Strakowska, A.; Strzelec, K.; Kairyte, A.; Kremensas, A. Bio-based polyurethane composite foams with improved mechanical, thermal, and antibacterial properties. Materials 2020, 13, 1108. [Google Scholar] [CrossRef] [Green Version]
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Polyurethane composites reinforced with walnut shell filler treated with perlite, montmorillonite and halloysite. Int. J. Mol. Sci. 2021, 22, 7304. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowinska, A.; Kucharsk, J. Organic zinc salts as pro-ecological activators for sulfur vulcanization of styrene-butadiene rubber. Polymers 2019, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Maslowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Common nettle (Urtica dioica L.) as an active filler of natural rubber biocomposites. Materials 2021, 14, 1616. [Google Scholar] [CrossRef]
- Szadkowski, B.; Kuśmierek, M.; Rybiński, P.; Zukowski, W.; Marzec, A. Application of earth pigments in cycloolefin copolymer: Protection against combustion and accelerated aging in the full sunlight spectrum. Materials 2020, 13, 3381. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites–current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Progress report on natural fiber reinforced composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Matykiewicz, D.; Skórczewska, K.; Lewandowski, K.; Andrzejewski, J.; Piasecki, A. Development of polylactide composites with improved thermomechanical properties by simultaneous use of basalt powder and a nucleating agent. Polym. Compos. 2020, 41, 2947–2957. [Google Scholar] [CrossRef]
- Aniśko, J.; Barczewski, M. Polylactide: From Synthesis and Modification to Final Properties. Adv. Sci. Technol. Res. J. 2021, 15, 9–29. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Skórczewska, K.; Kloziński, A. Improving the toughness and thermal resistance of polyoxymethylene/poly(lactic acid) blends: Evaluation of structure-properties correlation for reactive processing. Polymers 2020, 12, 307. [Google Scholar] [CrossRef] [Green Version]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Kloziński, A. Accelerated weathering of polylactide-based composites filled with linseed cake: The influence of time and oil content within the filler. Polymers 2019, 11, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zhang, D.; Li, J. Antibacterial Nanoparticles with Universal Adhesion Function Based on Dopamine and Eugenol. J. Bioresour. Bioprod. 2019, 4, 177–182. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Ye, Z.; Chizaram, E.P.; Jiang, J.; Liu, T.; Sun, F.; Zhang, S. Mold resistance of bamboo after laccase-catalyzed attachment of thymol and proposed mechanism of attachment. RSC Adv. 2020, 10, 7764–7770. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. In Vitro 2015, 29, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Shah, M.A.; Dar, B.N.; Wani, A.A.; Ganai, S.A.; Nishad, J. Supercritical Impregnation of Active Components into Polymers for Food Packaging Applications. Food Bioprocess Technol. 2017, 10, 1749–1754. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, 2477. [Google Scholar] [CrossRef]
- Bermejo, V.D.; Angelov, I.; Vicente, G.; Stateva, R.P.; García-Risco, R.M.; Reglero, G.; Ibañez, E.; Fornari, T. Extraction of thymol from different varieties of thyme plants using green solvents. J. Sci. Food Agric. 2015, 95, 2901–2907. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of Green Chemistry in Process Research and Development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, soxhlet, UAE and RSLDE techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; da Costa, W.A.; Pereira, D.S.; Botelho, J.R.S.; de Alencar, M.T.O.; de Aguiar, A.E.H.; da Silva, S.H.M.; da Silva, S.F.A.P.; de Carvalho, R.N. Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J. Supercrit. Fluids 2016, 118, 185–193. [Google Scholar] [CrossRef]
- Deng, J.; Yang, B.; Chen, C.; Liang, J. Renewable eugenol-based polymeric oil-absorbent microspheres: Preparation and oil absorption ability. ACS Sustain. Chem. Eng. 2015, 3, 599–605. [Google Scholar] [CrossRef]
- Vorobyova, V.; Chygyrynets’, O.; Skiba, M.; Overchenko, T. Experimental and theoretical investigations of anti-corrosive properties of thymol. Chem. Chem. Technol. 2019, 13, 261–268. [Google Scholar] [CrossRef]
- Franco, P.; Incarnato, L.; Marco, I. De Supercritical CO2 impregnation of α -tocopherol into PET/PP fi lms for active packaging applications. J. CO2 Util. 2019, 34, 266–273. [Google Scholar] [CrossRef]
- Fávaro, S.L.; Rubira, A.F.; Muniz, E.C.; Radovanovic, E. Surface modification of HDPE, PP, and PET films with KMnO4/HCl solutions. Polym. Degrad. Stab. 2007, 92, 1219–1226. [Google Scholar] [CrossRef]
- Khafagy, R.M.; Badr, Y.A. In situ FTIR spectroscopic study of the recently detected low-temperature-induced structural changes in isotactic polypropylene. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2829–2842. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Kazemi-Pasarvi, S.; Ebrahimi, G.N.; Shahrampour, D.; Arab-Bafrani, Z. Reducing cytotoxicity of poly (lactic acid)-based/zinc oxide nanocomposites while boosting their antibacterial activities by thymol for biomedical applications. Int. J. Biol. Macromol. 2020, 164, 4556–4565. [Google Scholar] [CrossRef]
- Paragkumar, N.T.; Edith, D.; Six, J.L. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A. Bio-based packaging materials containing substances derived from coffee and tea plants. Materials 2020, 13, 5719. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Effect of impregnation of biodegradable polyesters with polyphenols from cistus linnaeus and Juglans regia Linnaeus walnut green husk. Polymers 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masek, A.; Plota, A. Influence of a Natural Plant Antioxidant on the Ageing Process of Ethylene-norbornene Copolymer (Topas). Int. J. Mol. Sci. 2021, 22, 4018. [Google Scholar] [CrossRef]
- Kakanuru, P.; Pochiraju, K. Moisture Ingress and Degradation of Additively Manufactured PLA, ABS and PLA/SiC Composite Parts. Addit. Manuf. 2020, 36, 101529. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Dong, I.Y.; Shin, Y.; Hwan, C.K.; Hak, Y.K.; Yong, S.C.; Won, H.P.; Ji, H.Y. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Inagaki, T.; Siesler, H.W.; Mitsui, K.; Tsuchikawa, S. Difference of the crystal structure of cellulose in wood after hydrothermal and aging degradation: A NIR spectroscopy and XRD study. Biomacromolecules 2010, 11, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Ornaghi, H.L., Jr.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B 2019, 176, 107336. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Ferreira, S.R.S.; Carciofi, B.A.M. High pressure carbon dioxide for impregnation of clove essential oil in LLDPE films. Innov. Food Sci. Emerg. Technol. 2017, 41, 206–215. [Google Scholar] [CrossRef]
- Encinas, N.; Pantoja, M.; Abenojar, J.; Martínez, M.A. Control of wettability of polymers by surface roughness modification. J. Adhes. Sci. Technol. 2010, 24, 1869–1883. [Google Scholar] [CrossRef] [Green Version]

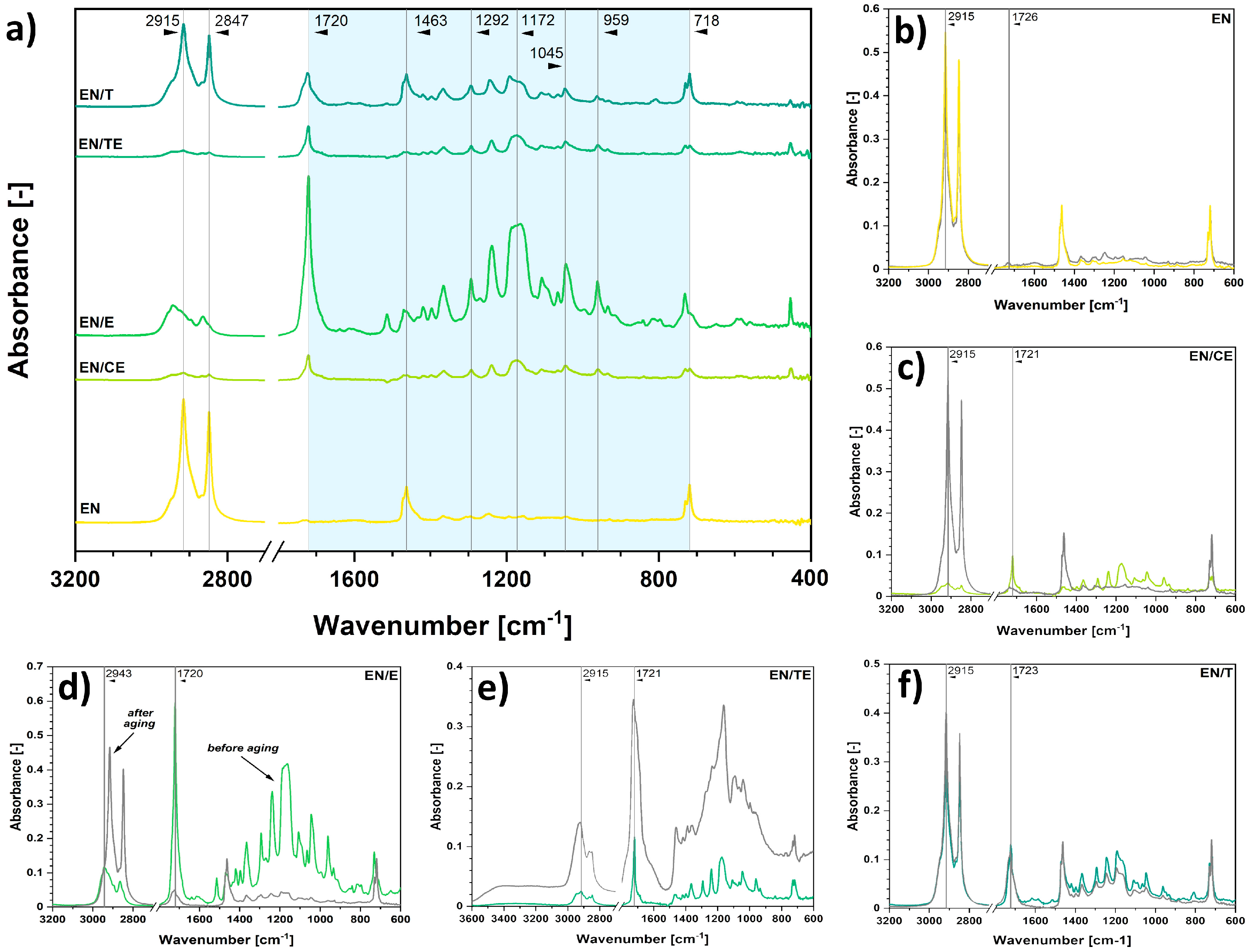
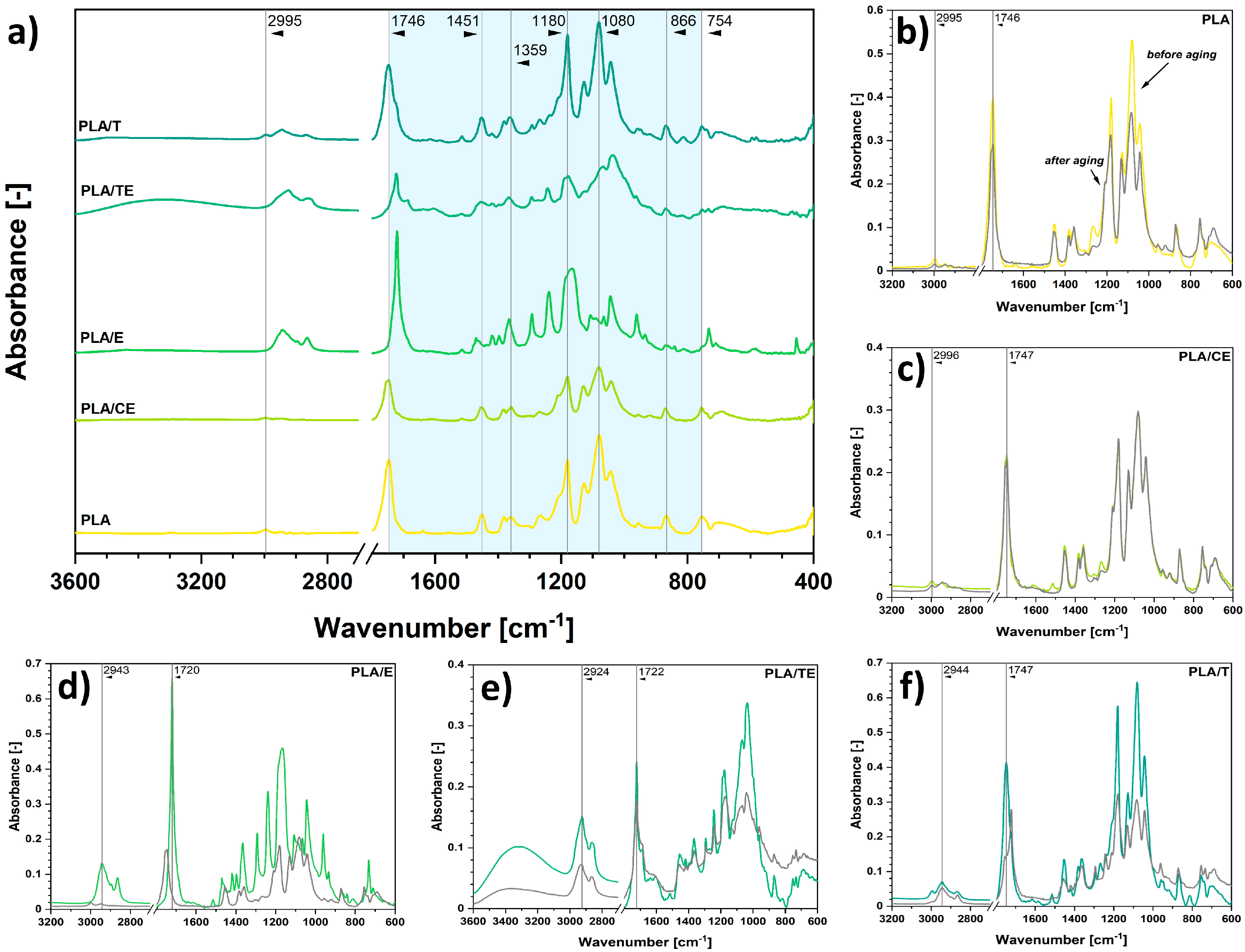

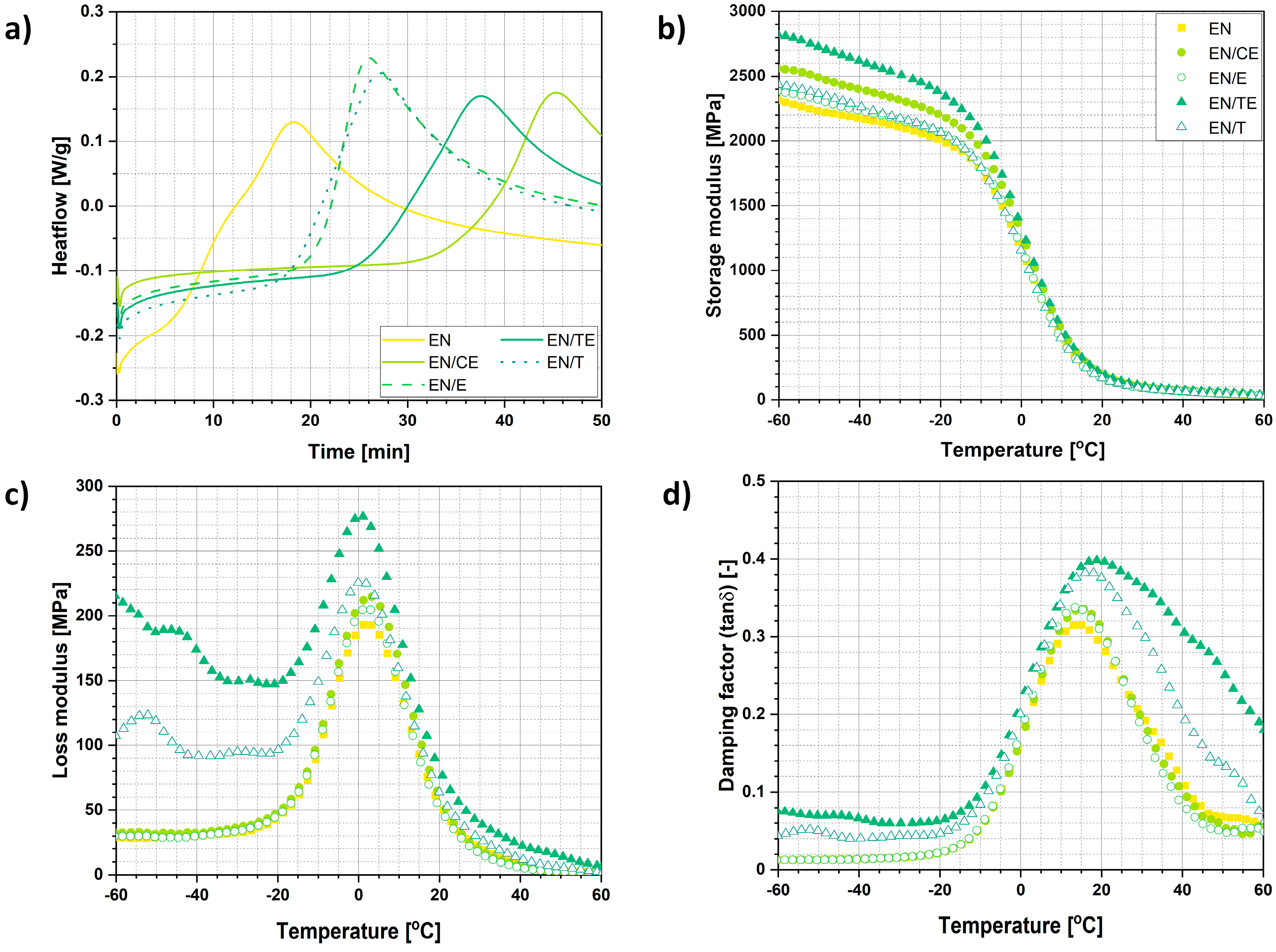

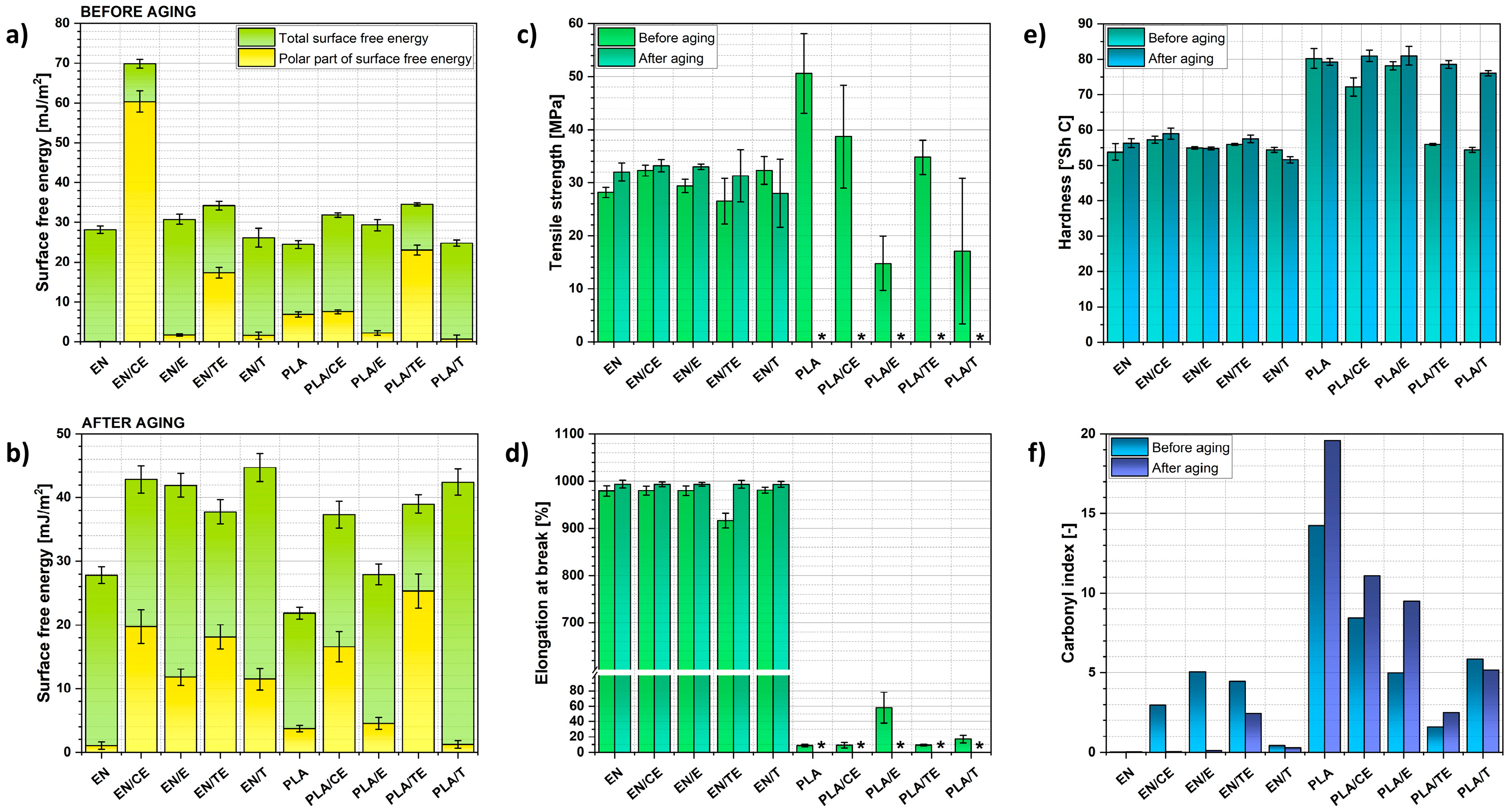
| Sample | Δmimpregnated (wt.%) |
|---|---|
| EN/CE | 0.61 |
| EN/E | 0.74 |
| EN/TE | 1.76 |
| EN/T | 1.51 |
| PLA/CE | 2.01 |
| PLA/E | 3.06 |
| PLA/TE | 7.88 |
| PLA/T | 4.84 |
| Sample | Thermal Decomposition (TGA) | Oxidation Peak Parameters (DSC) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T05 (°C) | T10 (°C) | T15 (°C) | T20 (°C) | T50 (°C) | T90 (°C) | Onset (min) | Maximum (min) | Endset (min) | |
| EN | 449.2 | 456.7 | 461.7 | 464.2 | 475.8 | 487.5 | 6.14 | 18.08 | 31.5 |
| EN/CE | 450.8 | 457.5 | 461.7 | 465.0 | 474.2 | 487.5 | 39.02 | 44.65 | 49.83 |
| EN/E | 449.1 | 456.7 | 461.7 | 464.7 | 473.5 | 487.5 | 21.09 | 25.9 | 37.66 |
| EN/TE | 449.2 | 455.8 | 460.8 | 464.2 | 473.3 | 485.8 | 27.73 | 37.18 | 47.05 |
| EN/T | 447.5 | 456.7 | 460.8 | 464.2 | 473.5 | 486.7 | 18.41 | 27.08 | 38.58 |
| PLA | 330.0 | 338.3 | 343.3 | 347.5 | 360.8 | 375.8 | ------ | ------ | ------ |
| PLA/CE | 304.2 | 315.0 | 322.5 | 327.5 | 345.8 | 363.3 | ------ | ------ | ------ |
| PLA/E | 179.2 | 298.3 | 312.5 | 320.0 | 342.5 | 362.5 | ------ | ------ | ------ |
| PLA/TE | 296.7 | 311.7 | 320.0 | 325.0 | 345.0 | 363.3 | ------ | ------ | ------ |
| PLA/T | 296.7 | 3125.0 | 321.7 | 326.7 | 346.7 | 365.0 | ------ | ------ | ------ |
| Sample | Storage Modulus Changes in Temperature | Tmax. E″ (°C) | Tmax. tanδ (Tg) (°C) | |||||
|---|---|---|---|---|---|---|---|---|
| E′-60 (MPa) | E′-40 (MPa) | E′-20 (MPa) | E′0 (MPa) | E′20 (MPa) | E′40 (MPa) | |||
| EN | 2314.7 | 2174.4 | 2009.4 | 1157.4 | 187.2 | 66.4 | 2.5 | 14.3 |
| EN/CE | 2560.0 | 2402.4 | 2203.4 | 1261.3 | 181.9 | 68.8 | 3.0 | 15.3 |
| EN/E | 2381.9 | 2235.3 | 2057.9 | 1166.4 | 167.1 | 63.2 | 1.8 | 13.8 |
| EN/TE | 2437.2 | 2269.1 | 2072.3 | 1124.2 | 164.2 | 60.1 | 0.8 | 17.2 |
| EN/T | 2830.5 | 2618.5 | 2393.0 | 1303.5 | 198.4 | 75.3 | 0.3 | 19.6 |
| Wavenumber Shifts between the Peaks during Aging (cm−1) | ||||
|---|---|---|---|---|
| EN | EN/CE | EN/E | EN/TE | EN/T |
| 2915 → 2915 (-) | 2915 → 2915 (-) | 2934 → 2915 (−19) | 2915 → 2922 (+7) | 2915 → 2915 (-) |
| 2847 → 2847 (-) | 2847 → 2847 (-) | 2864 → 2847 (−17) | 2847 → 2866 (+19) | 2847 → 2847 (-) |
| 1720 → 1735 (+15) | 1720 → 1724 (−4) | 1721 → 1727 (+6) | 1723 → 1724 (+1) | |
| 1514 → X | ||||
| 1463 → 1462 (+1) | 1461 → 1462 (+1) | 1470 → 1462 (−8) | 1461 → 1460 (−1) | 1463 → 1462 (-) |
| 1419 → X | 1419 → X | 1419 → X | ||
| 1387 → X | 1396 → X | 1397 → 1388 (−9) | 1366 → 1366 (-) | |
| 1364 → 1366 (+2) | 1365 → 1365 (-) | 1364 → 1358 (−6) | ||
| 1292 → X | 1293 → 1294 (+1) | 1292 → X | 1293 → 1294 (+1) | |
| 1239 → X | 1238 → 1244 (+6) | 1238 → X | 1243 → 1245 (+2) | |
| 1172 → 1154 (−18) | 1162 → 1193 (+31) | 1173 → 1161 (−12) | 1191 → 1193 (+2) | |
| 1108 → X | 1107 → X | 1107 → 1090 (−17) | 1108 → 1107 (−1) | |
| 1065 → X | 1065 → X | 1065 → X | ||
| 1045 → X | 1044 → 1046 (+2) | 1054 → 1040 (−14) | 1046 → 1047 (+1) | |
| 959 → X | 960 → X | 960 → 997 (+37) | 962 → X | |
| 934 → 928 (−6) | 933 → X | 934 → X | ||
| 838 → X | 816 → X | 840 → X | 807 → X | |
| 729 → 729 (-) | 731 → 729 (−2) | 729 → 729 (-) | 729 → 729 (-) | |
| 718 → 718 (-) | 718 → 718 (-) | 718 → X | 718 → 718 (-) | 718 → 718 (-) |
| PLA | PLA/CE | PLA/E | PLA/TE | PLA/T |
| 2995 → 2995 (-) | 2996 → X | X → 2996 | 2994 → X | |
| 2945 → 2946 (+1) | 2944 → 2944 (-) | 2943 → 2947 (+4) | 2944 → 2945 (+1) | |
| 2917 → X | 2924 → 2927 (+3) | |||
| 2864 → X | 2863 → 2864 (+1) | 2867 → 2865 (−2) | ||
| 1746 → 1746 (-) | 1746 → 1746 (-) | 1720 → 1747 (+27) | 1722 → 1722 (-) | 1747 → 1722 (−25) |
| 1451 → 1454 (+3) | 1454 → 1455 (+1) | 1470 → 1455 (−15) | 1455 → 1456 (+1) | 1452 → 1455 (+3) |
| 1381 → 1382 (+1) | 1382 → 1382 (-) | 1419 → X | 1419 → 1418 (−1) | 1419 → 1419 (-) |
| 1359 → 1358 (−1) | 1358 → 1358 (-) | 1397 → 1382 (−15) | ||
| X → 1304 | X → 1303 | 1365 → 1358 (−7) | 1366 → 1365 (−1) | 1363 → 1365 (+2) |
| 1266 → X | 1266 → 1210 (−58) | 1293 → 1303 (+10) | 1293 → 1293 (-) | 1292 → 1293 (+1) |
| 1180 → 1182 (+2) | 1180 → 1180 (-) | 1238 → 1266 (+28) | 1177 → 1174 (−3) | 1267 → 1242 (−25) |
| 1127 → 1131 (+4) | 1129 → 1130 (−1) | 1166 → 1180 (+14) | X → 1131 (-) | 1180 → 1178 (−2) |
| 1107 → 1129 (+22) | 1127 → 1130 (+3) | |||
| 1080 → 1084 (+4) | 1080 → 1081 (+1) | 1065 → 1081 (+16) | 1066→ 1066 (-) | 1080 → 1082 (+2) |
| 1042 → 1042 (-) | 1042 → 1042 (-) | 1043 → 1042 (−1) | 1035 → 1039 (+4) | 1043 → 1042 (−1) |
| 955 → 956 (+1) | X → 956 | 961 → 956 (−5) | 962 → 961 (−1) | |
| X → 920 | X → 920 | X → 918 | 957 → 961 (+4) | |
| 866 → 871 (+5) | 870 → 870 (-) | 868 → 871 (+3) | X → 869 | 868 → 870 (+2) |
| 754 → 754 (-) | 754 → 754 (-) | 732 → 755 (+23) | X → 753 | 754 → 754 (-) |
| 700 → 691 (−9) | 690 → 692 (+2) | X → 689 | 688 → 686 (−2) | 695 → 688 (−7) |
| Sample | Amount of Microorganisms (UFC/cm2) | D (-) | |
|---|---|---|---|
| t = 0 h | t = 24 h | ||
| EN | 2.7 × 104 | 4.1 × 105 | −1.18 |
| EN/CE | 3.0 × 100 | 3.95 | |
| EN/E | 7.3 × 105 | −1.43 | |
| EN/TE | 5.5 × 105 | −1.31 | |
| EN/T | 3.1 × 105 | −1.06 | |
| PLA | 4.9 × 104 | 3.5 × 105 | −0.85 |
| PLA/CE | 2.5 × 105 | −0.71 | |
| PLA/E | 1.6 × 105 | −0.60 | |
| PLA/TE | 1.7 × 106 | −1.54 | |
| PLA/T | 3.5 × 105 | −0.85 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masek, A.; Cichosz, S.; Piotrowska, M. Comparison of Aging Resistance and Antimicrobial Properties of Ethylene–Norbornene Copolymer and Poly(Lactic Acid) Impregnated with Phytochemicals Embodied in Thyme (Thymus vulgaris) and Clove (Syzygium aromaticum). Int. J. Mol. Sci. 2021, 22, 13025. https://doi.org/10.3390/ijms222313025
Masek A, Cichosz S, Piotrowska M. Comparison of Aging Resistance and Antimicrobial Properties of Ethylene–Norbornene Copolymer and Poly(Lactic Acid) Impregnated with Phytochemicals Embodied in Thyme (Thymus vulgaris) and Clove (Syzygium aromaticum). International Journal of Molecular Sciences. 2021; 22(23):13025. https://doi.org/10.3390/ijms222313025
Chicago/Turabian StyleMasek, Anna, Stefan Cichosz, and Małgorzata Piotrowska. 2021. "Comparison of Aging Resistance and Antimicrobial Properties of Ethylene–Norbornene Copolymer and Poly(Lactic Acid) Impregnated with Phytochemicals Embodied in Thyme (Thymus vulgaris) and Clove (Syzygium aromaticum)" International Journal of Molecular Sciences 22, no. 23: 13025. https://doi.org/10.3390/ijms222313025






