Silencing an E3 Ubiquitin Ligase Gene OsJMJ715 Enhances the Resistance of Rice to a Piercing-Sucking Herbivore by Activating ABA and JA Signaling Pathways
Abstract
:1. Introduction
2. Results
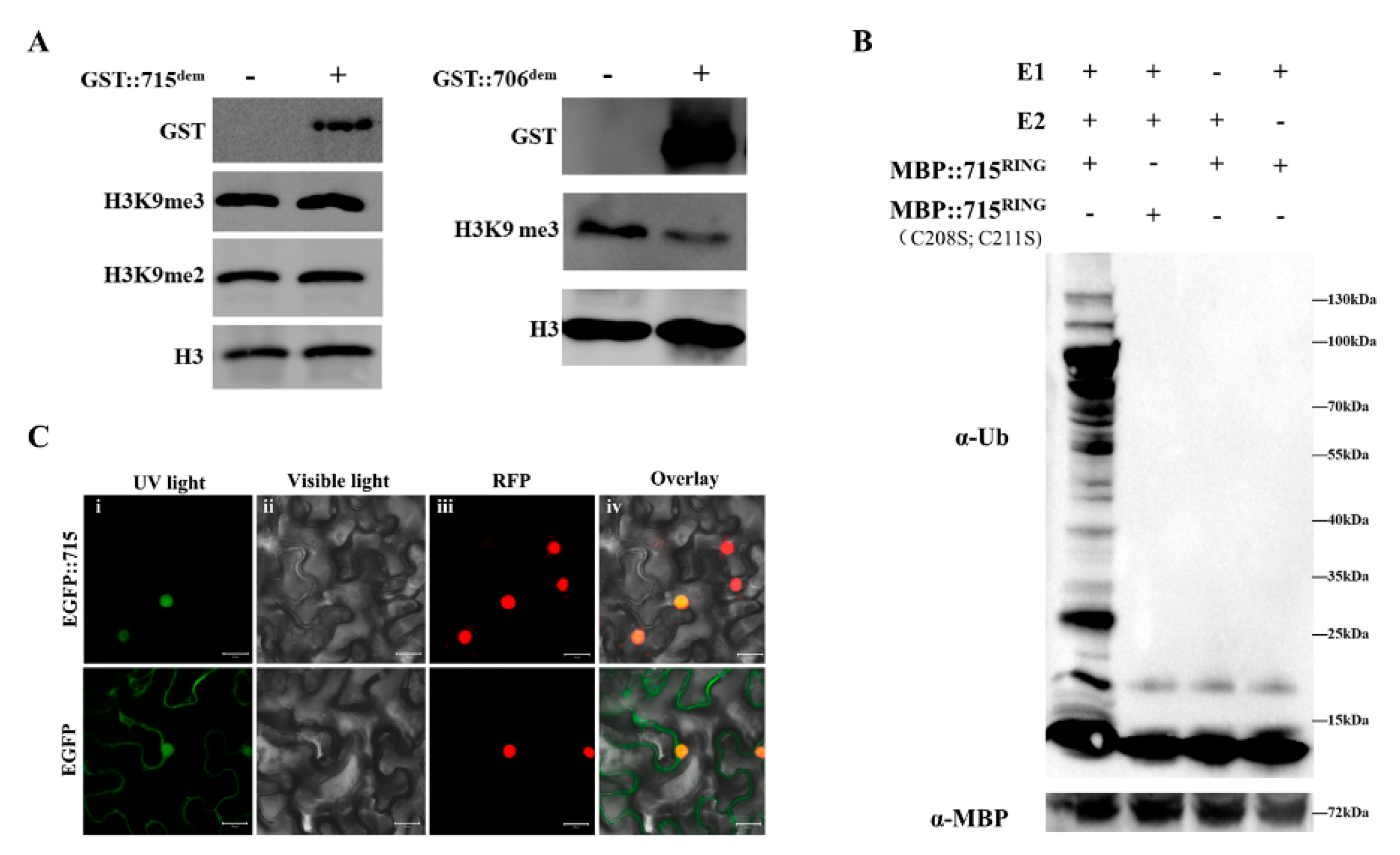
2.1. Isolating and Characterizing OsJMJ715
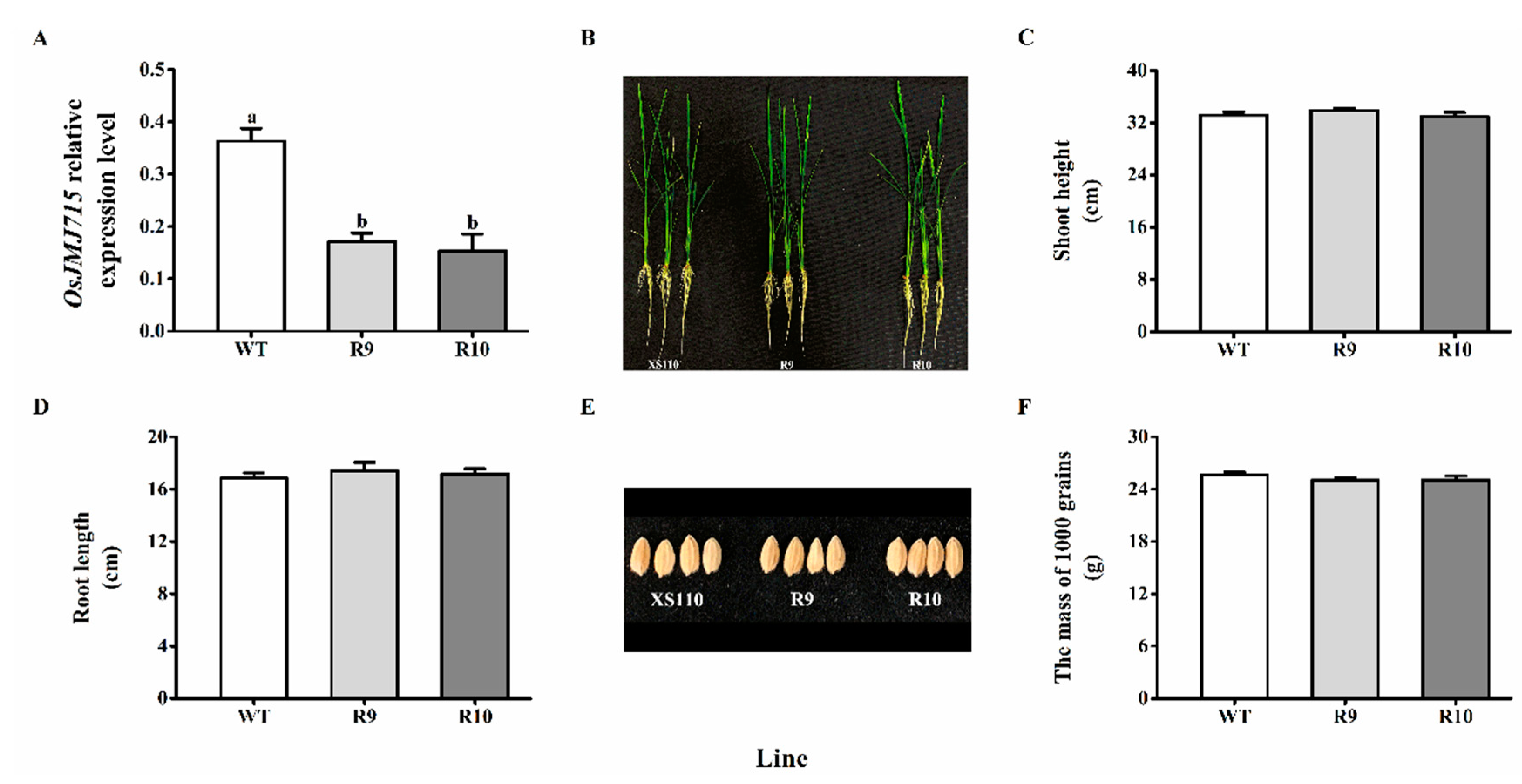
2.2. Silencing OsJMJ715
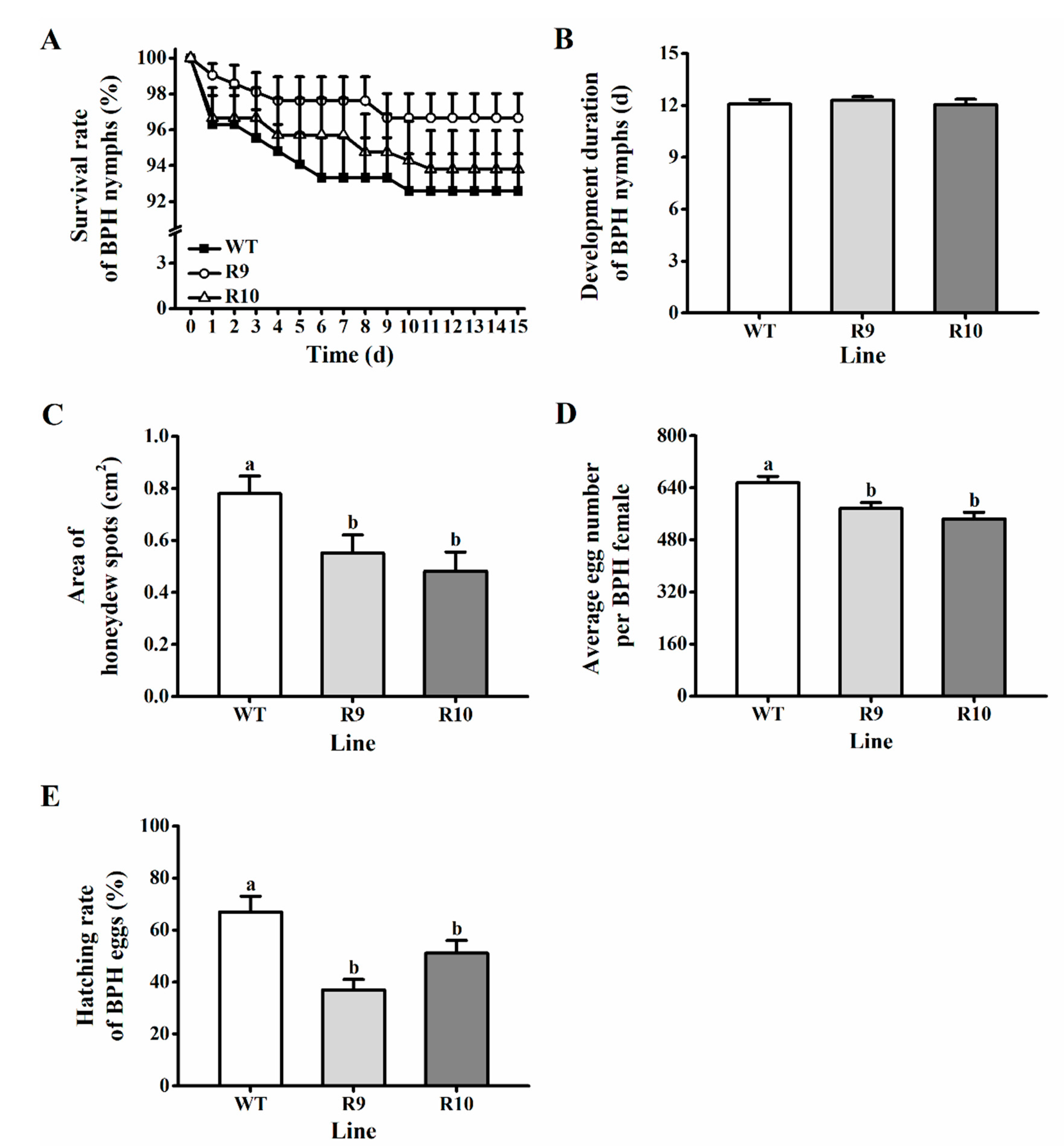
2.3. Silencing OsJMJ715 Enhanced the Resistance of Rice to BPH
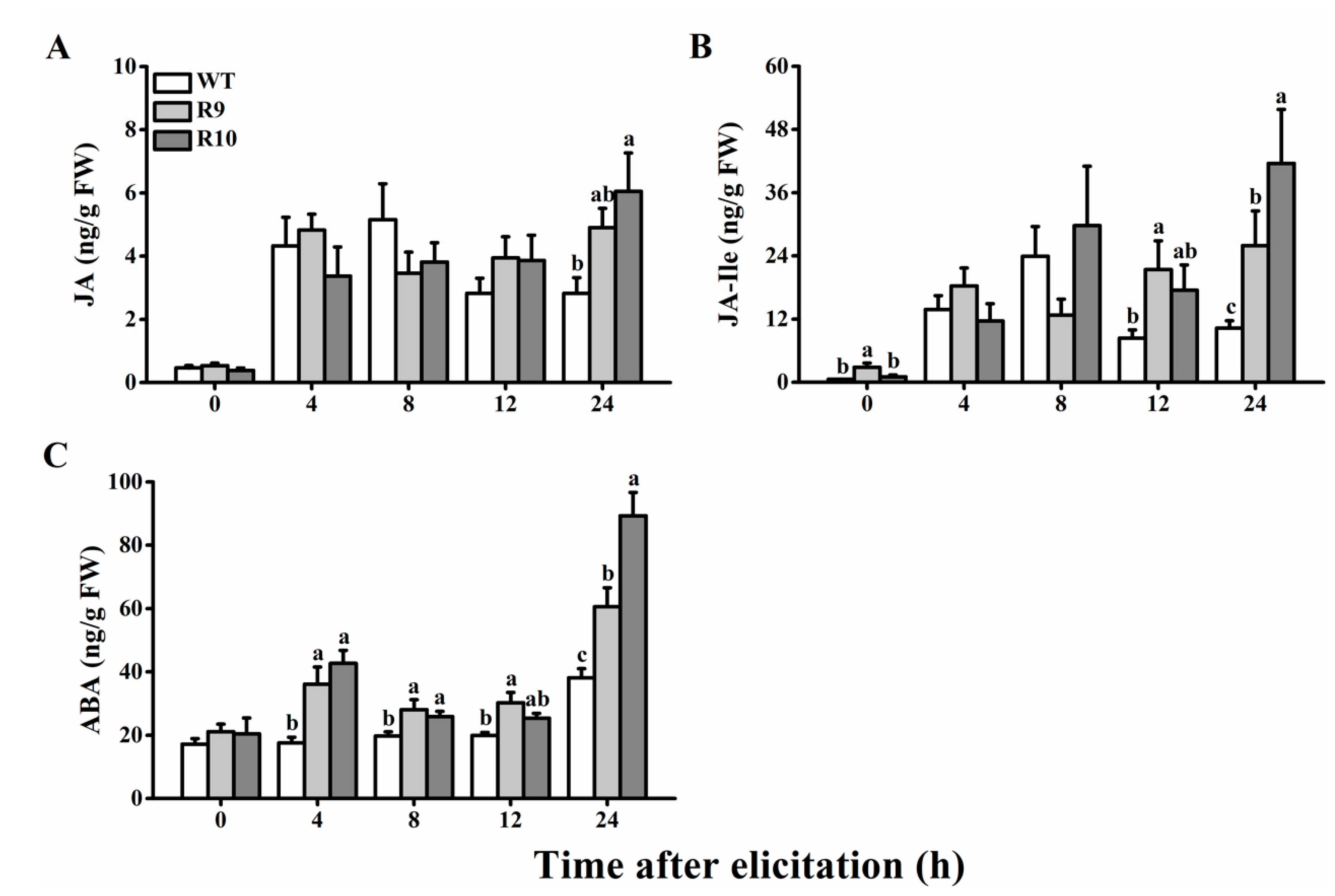
2.4. Silecing OsJMJ715 Enhanced BPH-Induced Levels of JA, JA-Isoleucine, and ABA
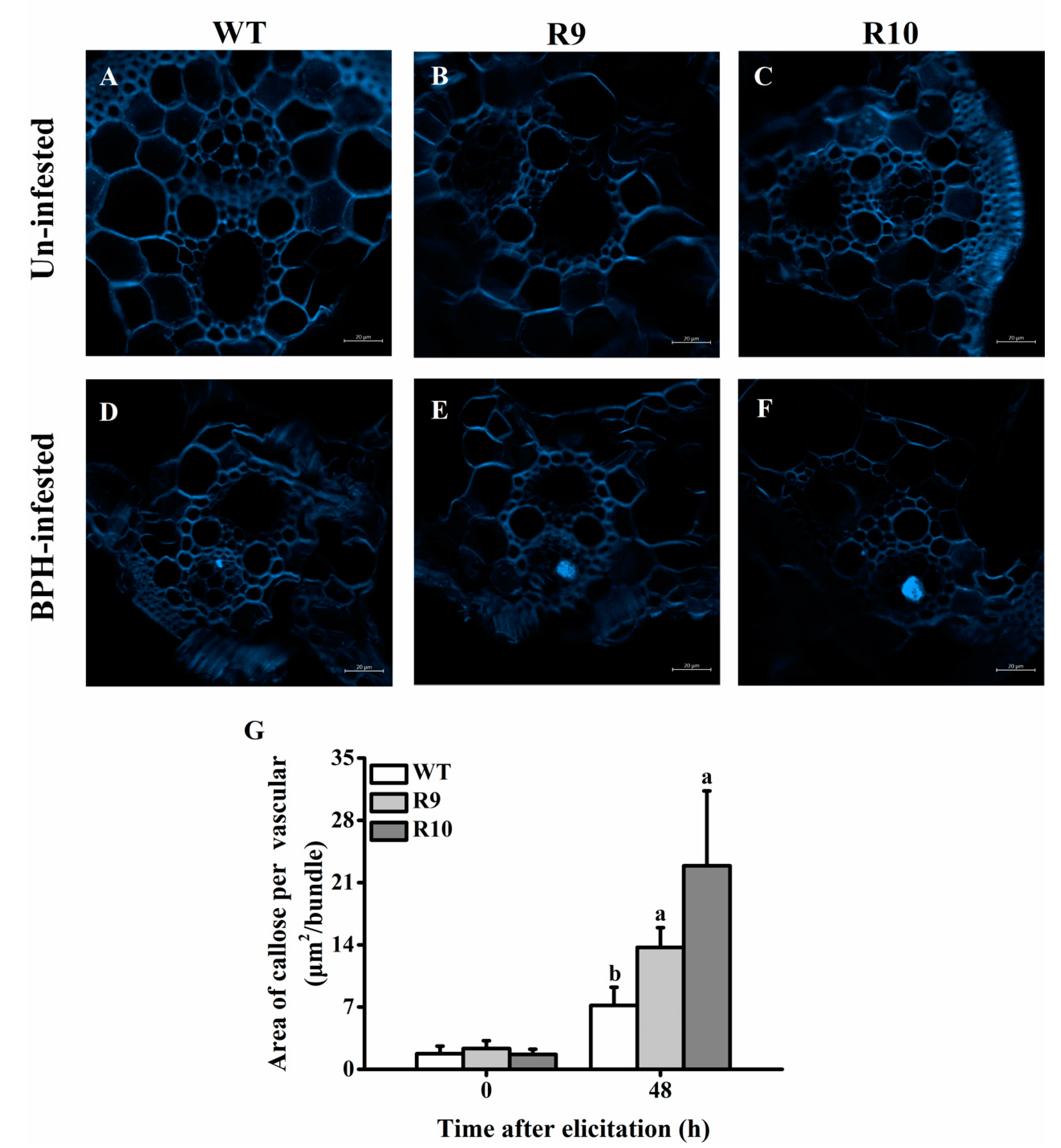
2.5. OsJMJ715 Negatively Mediated BPH-Elicited Callose Deposition
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Insects
4.3. Plant Treatments
4.4. Isolation and Characterization of OsJMJ715
4.5. Quantitative Real-Time PCR
4.6. Subcellular Localization
4.7. Generation of Transgenic Plants
4.8. Histone Demethylation Assay
4.9. Ubiquitination Assay
4.10. JA, JA-Ile, and ABA Measurement
4.11. Callose Measurement
4.12. Herbivore Bioassays
4.13. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Schuman, M.C.; Baldwin, I.T. The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, I.A.; Verhage, A.; Schuurink, R.C.; Watt, L.G.; Pieterse, C.M.; Van Wees, S.C. Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front. Plant Sci. 2013, 4, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahar, K.; Kyndt, T.; Nzogela, Y.B.; Gheysen, G. Abscisic acid interacts antagonistically with classical defense pathways in rice-migratory nematode interaction. New Phytol. 2012, 196, 901–913. [Google Scholar] [CrossRef]
- Dreher, K.; Callis, J. Ubiquitin, hormones and biotic stress in plants. Ann. Bot. 2007, 99, 787–822. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Estelle, M. The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 2010, 61, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.H.G.; Spoel, S.H. The ubiquitin-proteasome system as a transcriptional regulator of plant immunity. J. Exp. Bot. 2018, 69, 4529–4537. [Google Scholar] [CrossRef] [Green Version]
- Vierstra, R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef]
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.R.; Estelle, M. Ubiquitin-mediated control of plant hormone signaling. Plant Physiol. 2012, 160, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miricescu, A.; Goslin, K.; Graciet, E. Ubiquitylation in plants: Signaling hub for the integration of environmental signals. J. Expr. Bot. 2018, 69, 4511–4527. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.X.; Feys, B.F.; James, S.; Nieto-Rostro, M.; Turner, J.G. COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 1998, 280, 1091–1094. [Google Scholar] [CrossRef]
- Trujillo, M.; Ichimura, K.; Casais, C.; Shirasu, K. Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr. Biol. 2008, 18, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Wu, Y.R.; Huang, X.H.; Sun, J.; Xie, Q. AtPUB19, a U-box E3 ubiquitin ligase, negatively regulates abscisic acid and drought responses in Arabidopsis thaliana. Mol. Plant 2011, 4, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Froidure, S.; Canonne, J.; Ben Khaled, S.; Khafif, M.; Pouzet, C.; Jauneau, A.; Roby, D.; Rivas, S. Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nat. Commun. 2013, 10, 1475. [Google Scholar] [CrossRef]
- Li, W.; Zhong, S.H.; Li, G.J.; Li, Q.; Mao, B.Z.; Deng, Y.W.; Zhang, H.J.; Zeng, L.J.; Song, F.M.; He, Z.H. Rice RING protein OsBBI1 with E3 ligase activity confers broad-spectrum resistance against Magnaporthe oryzae by modifying the cell wall defence. Cell Res. 2011, 21, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.E.; Hou, P.; Xiao, F.M.; Liu, Y.S. Overexpressing a novel RING-H2 finger protein gene, OsRHP1, enhances drought and salt tolerance in rice (Oryza sativa L.). J. Plant Biol. 2014, 57, 357–365. [Google Scholar] [CrossRef]
- Park, Y.C.; Chapagain, S.; Jang, C.S. A Negative regulator in response to salinity in rice: Oryza sativa salt-, ABA- and drought-induced RING finger protein 1 (OsSADR1). Plant Cell Physiol. 2018, 59, 575–589. [Google Scholar] [CrossRef] [Green Version]
- Ryu, M.Y.; Cho, S.K.; Kim, W.T. The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiol. 2010, 154, 1983–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.L.; Li, G.L.; Cui, X.; Liu, C.Y.; Wang, X.J.; Cao, X.F. Comparative analysis of JmjC domain-containing proteins reveals the potential histone demethylases in Arabidopsis and rice. J. Integr. Plant Biol. 2008, 50, 886–896. [Google Scholar] [CrossRef]
- Chen, X.S.; Hu, Y.F.; Zhou, D.X. Epigenetic gene regulation by plant Jumonji group of histone demethylase. Biochim. Biophys. Acta 2011, 1809, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, T.; Brzezinka, K.; Friedrich, T.; Gorka, M.; Graf, A.; Kappel, C.; Baurle, I. A JUMONJI protein with E3 ligase and histone H3 binding activities affects transposon silencing in Arabidopsis. Plant Physiol. 2016, 171, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Audonnet, L.; Shen, Y.; Zhou, D.X. JMJ24 antagonizes histone H3K9 demethylase IBM1/JMJ25 function and interacts with RNAi pathways for gene silencing. Gene Expr. Patterns 2017, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Jang, I.C.; Su, L.L.; Xu, J.; Chua, N.H. JMJ24 targets CHROMOMETHYLASE3 for proteasomal degradation in Arabidopsis. Genes Dev. 2016, 30, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.X.; Qi, J.; Ren, N.; Cheng, J.A.; Erb, M.; Mao, B.Z.; Lou, Y.G. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Lu, J.; Ju, H.P.; Zhou, G.X.; Zhu, C.; Erb, M.; Wang, X.; Wang, P.; Lou, Y.G. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011, 68, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.C.; Ju, H.P.; Liu, X.L.; Erb, M.; Wang, X.; Lou, Y.G. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol. Plant 2014, 7, 1670–1682. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhang, J.; Li, J.C.; Zhou, G.X.; Wang, Q.; Bian, W.B.; Erb, M.; Lou, Y.G. Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. Elife 2015, 4, e04805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.F.; Ye, M.; Li, R.; Lou, Y.G. OsWRKY53, a versatile switch in regulating herbivore-induced defense responses in rice. Plant Signal. Behav. 2016, 11, e1169357. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, X.J.; Zu, H.Y.; Zeng, X.; Baldwin, I.T.; Lou, Y.G.; Li, R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol. 2021, 230, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Kuai, P.; Hu, L.F.; Ye, M.F.; Sun, H.; Erb, M.; Lou, Y.G. Suppression of a leucine-rich repeat receptor-like kinase enhances host plant resistance to a specialist herbivore. Plant Cell Environ. 2020, 43, 2571–2585. [Google Scholar] [CrossRef]
- Hao, P.Y.; Liu, C.X.; Wang, Y.Y.; Chen, R.Z.; Tang, M.; Du, B.; Zhu, L.L.; He, G.C. Herbivore-induced callose deposition on the sieve plates of rice: An important mechanism for host resistance. Plant Physiol. 2008, 146, 1810–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.L.; Du, H.T.; Ding, X.; Zhou, Y.D.; Xie, P.F.; Wu, J.C. Mechanisms of callose deposition in rice regulated by exogenous abscisic acid and its involvement in rice resistance to Nilaparvata lugens Stal (Hemiptera: Delphacidae). Pest Manag. Sci. 2017, 73, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.D.; Sun, L.T.; Wang, S.; Xie, P.F.; Liu, J.L. A key ABA hydrolase gene, OsABA8ox3 is involved in rice resistance to Nilaparvata lugens by affecting callose deposition. J. Asia-Pac. Entomol. 2019, 22, 625–631. [Google Scholar] [CrossRef]
- Gao, W.; Liu, W.W.; Zhao, M.; Li, W.X. NERF encodes a RING E3 ligase important for drought resistance and enhances the expression of its antisense gene NFYA5 in Arabidopsis. Nucleic Acids Res. 2015, 43, 607–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, F.L.; Yang, X.F.; Shi, Z.Y.; Miao, X.X. Novel crosstalk between ethylene- and jasmonic acid-pathway responses to a piercing-sucking insect in rice. New Phytol. 2020, 225, 474–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Afsheen, S.; Xin, Z.J.; Han, X.; Lou, Y.G. OsNPR1 negatively regulates herbivore-induced JA and ethylene signaling and plant resistance to a chewing herbivore in rice. Physiol. Plant. 2013, 147, 340–351. [Google Scholar] [CrossRef]
- Zhou, G.X.; Ren, N.; Qi, J.F.; Lu, J.; Xiang, C.Y.; Ju, H.P.; Cheng, J.A.; Lou, Y.G. The 9-lipoxygenase Osr9-LOX1 interacts with the 13-lipoxygenase-mediated pathway to regulate resistance to chewing and piercing-sucking herbivores in rice. Physiol. Plant. 2014, 152, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Ye, M.; Li, R.; Zhang, T.F.; Zhou, G.X.; Wang, Q.; Lu, J.; Lou, Y.G. The Rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of mitogen-activated protein kinase activity. Plant Physiol. 2015, 169, 2907–2921. [Google Scholar] [CrossRef] [Green Version]
- Li, J.C.; Liu, X.L.; Wang, Q.; Huangfu, J.Y.; Schuman, M.C.; Lou, Y.G. A Group D MAPK protects plants from autotoxicity by suppressing herbivore-induced defense signaling. Plant Physiol. 2019, 179, 1386–1401. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.X.; Adachi, Y.; Munemasa, S.; Nakamura, Y.; Mori, I.C.; Murata, Y. Open Stomata 1 kinase is essential for yeast elicitor-induced stomatal closure in Arabidopsis. Plant Cell Physiol. 2015, 56, 1239–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Li, F.; Ali, S.; Sun, X.Q.; Hou, M.L. Silicon amendment to rice plants contributes to reduced feeding in a phloem-sucking insect through modulation of callose deposition. Ecol. Evol. 2018, 8, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Huang, X.; Sun, L.T.; Wu, J.C.; Liu, J.L. Influence of abscisic acid-biosynthesis inhibitor fluridone on the feeding behavior and fecundity of Nilaparvata lugens. Insects 2019, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Zhang, W.L.; Liu, B.F.; Hu, J.; Wei, Z.; Shi, Z.Y.; He, R.F.; Zhu, L.L.; Chen, R.Z.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef]
- Liu, X.L. Functional Characterization of OsMPK4 in the Interaction of Rice with Insect Pests. Ph.D. Thesis, Zhejiang University, Zhejiang, China, 2018. [Google Scholar]
- Wang, W.W.; Zhou, P.Y.; Mo, X.C.; Hu, L.F.; Jin, N.; Chen, X.; Yu, Z.X.; Meng, J.P.; Erb, M.; Shang, Z.C.; et al. Induction of defense in cereals by 4-fluorophenoxyacetic acid suppresses insect pest populations and increases crop yields in the field. Proc. Natl. Acad. Sci. USA 2020, 117, 12017–12028. [Google Scholar] [CrossRef]
- Yoshito, S.; Yoshito, S.; Kazushige, S. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (HORVATH) (Homoptera: Delphacidae). Appl. Entomol. Zool. 1996, 31, 467–473. [Google Scholar]
- Zhou, P.Y.; Li, C.Z.; Wang, X.J.; Fu, W.J.; Wu, Y.T.; Lou, Y.G. Screening of chemical elicitors inducing the resistance of rice to the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Acta Entomol. Sin. 2019, 62, 970–978. [Google Scholar]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Manila, Philippines, 1976; pp. 1–83. [Google Scholar]
- Huang, C.J.; Hu, G.J.; Li, F.F.; Li, Y.Q.; Wu, J.X.; Zhou, X.P. NbPHAN, a MYB transcriptional factor, regulates leaf development and affects drought tolerance in Nicotiana benthamiana. Physiol. Plant. 2013, 149, 297–309. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, D.X. Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc. Natl. Acad. Sci. USA 2008, 105, 13679–13684. [Google Scholar] [CrossRef] [Green Version]
- Whetstine, J.R.; Nottke, A.; Lan, F.; Huarte, M.; Smolikov, S.; Chen, Z.; Spooner, E.; Li, E.; Zhang, G.Y.; Colaiacovo, M.; et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 2006, 125, 467–481. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Guo, H.S.; Dallamn, G.; Fang, S.Y.; Weissman, A.M.; Chua, N.H. SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 2002, 419, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Yang, C.W.; Li, Y.; Zheng, N.Y.; Chen, H.; Zhao, Q.Z.; Gao, T.; Guo, H.S.; Xie, Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 2007, 19, 1912–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Chen, J.M.; Zhang, H.Q.; Zhang, J.F.; He, Y.P. Effects of brown planthopper Nilaparvata lugens Homoptera: (Stål) Delphacidae, feeding on callose deposition rice with different tolerance. Chin. J. Rice Sci. 2013, 27, 624–632. [Google Scholar]
- Paguia, P.; Pathak, M.D.; Heinrichs, E.A. Honeydew excretion measurement techniques for determining differential feeding activity of biotypes of Nilaparvata lugens on rice varieties. J. Econ. Entomol. 1980, 73, 35–40. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, M.; Zhou, S.; Lou, Y.; Lu, J. Silencing an E3 Ubiquitin Ligase Gene OsJMJ715 Enhances the Resistance of Rice to a Piercing-Sucking Herbivore by Activating ABA and JA Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 13020. https://doi.org/10.3390/ijms222313020
Zhang Y, Chen M, Zhou S, Lou Y, Lu J. Silencing an E3 Ubiquitin Ligase Gene OsJMJ715 Enhances the Resistance of Rice to a Piercing-Sucking Herbivore by Activating ABA and JA Signaling Pathways. International Journal of Molecular Sciences. 2021; 22(23):13020. https://doi.org/10.3390/ijms222313020
Chicago/Turabian StyleZhang, Yuebai, Mengting Chen, Shuxing Zhou, Yonggen Lou, and Jing Lu. 2021. "Silencing an E3 Ubiquitin Ligase Gene OsJMJ715 Enhances the Resistance of Rice to a Piercing-Sucking Herbivore by Activating ABA and JA Signaling Pathways" International Journal of Molecular Sciences 22, no. 23: 13020. https://doi.org/10.3390/ijms222313020






