BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-ray Photodynamic Therapy
Abstract
:1. Introduction
2. Results
2.1. BaGdF5:Eu3+ Nanophosphors
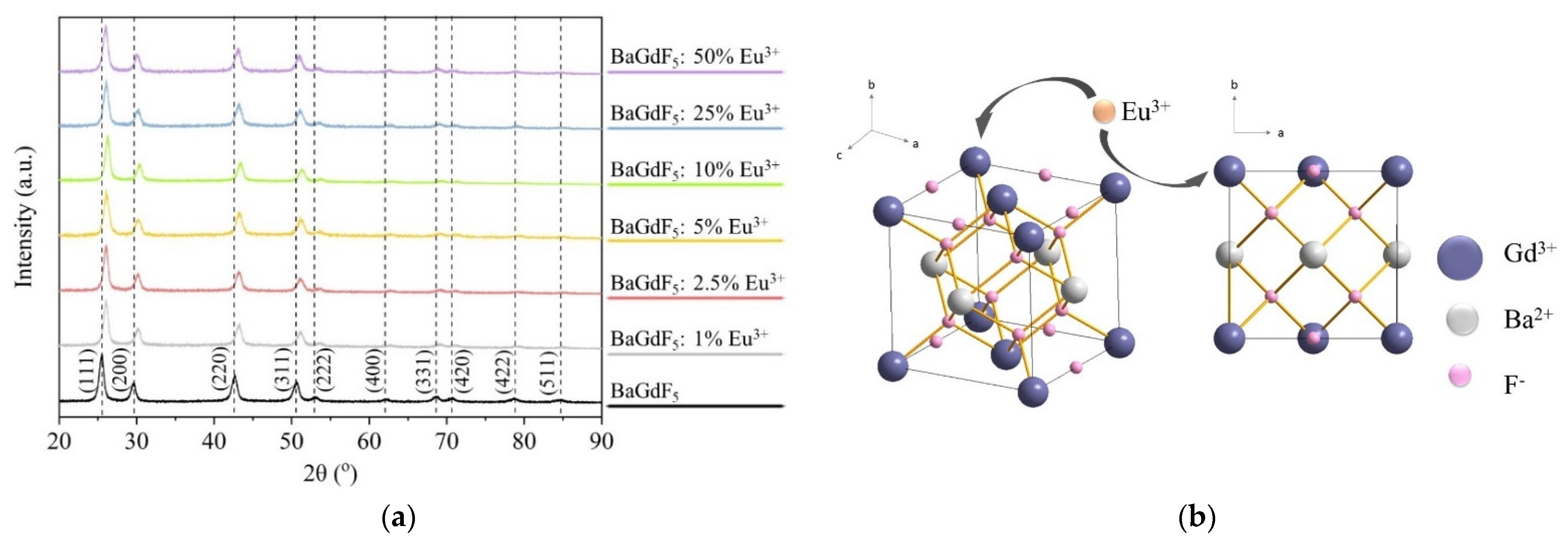
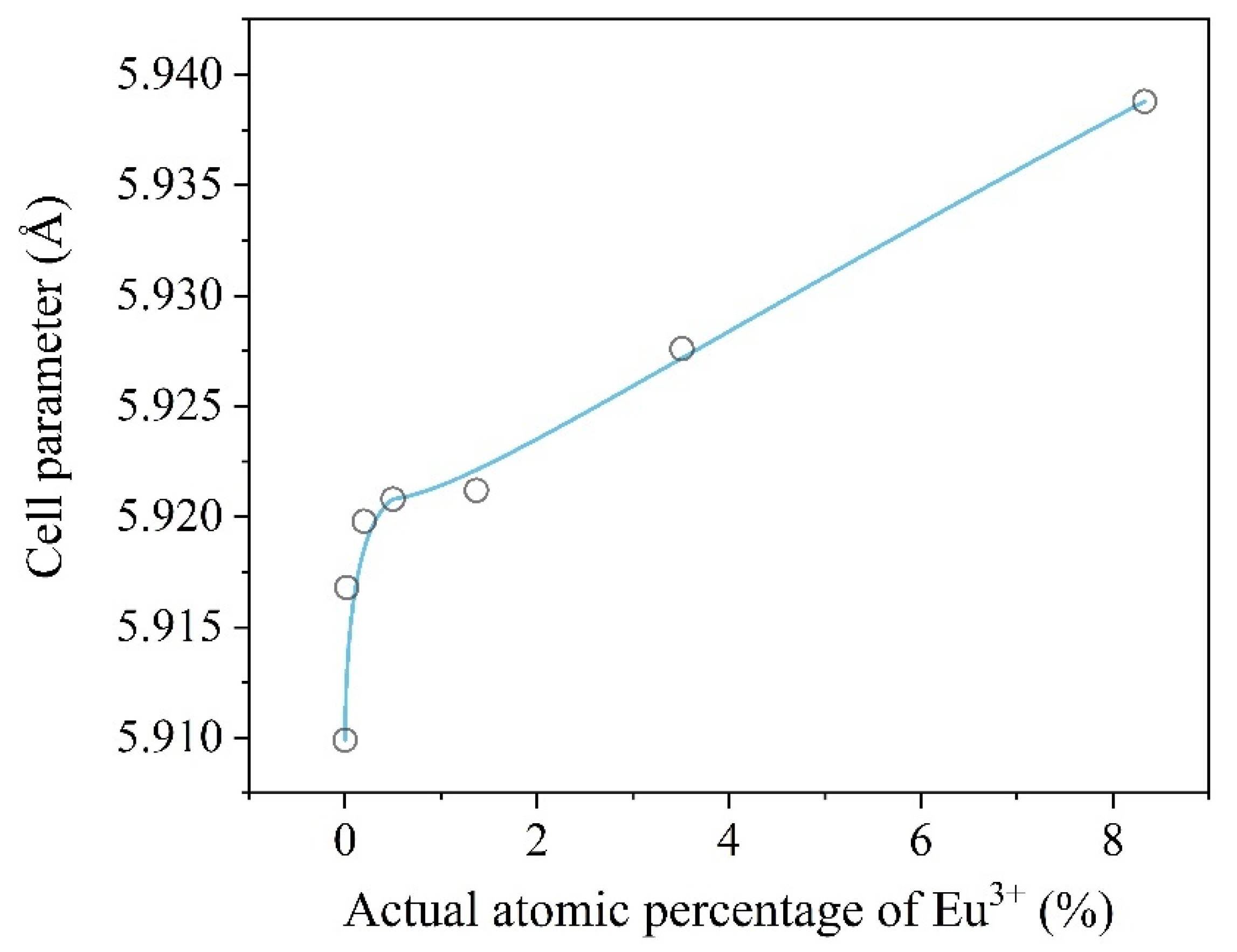
2.1.1. Crystal Structure and Composition
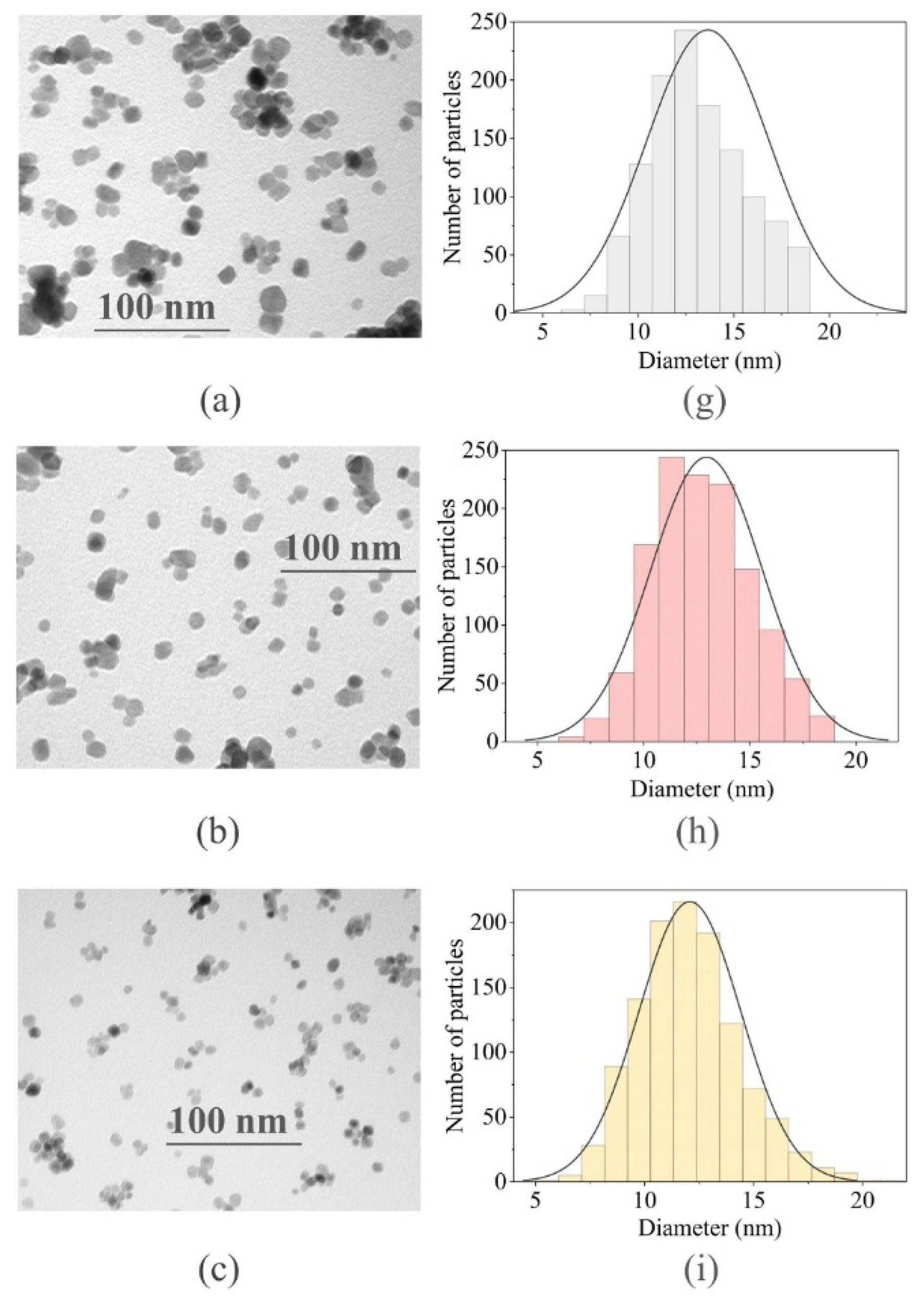
2.1.2. Transmission Electron Microscopy Analysis and Particle Size Distribution
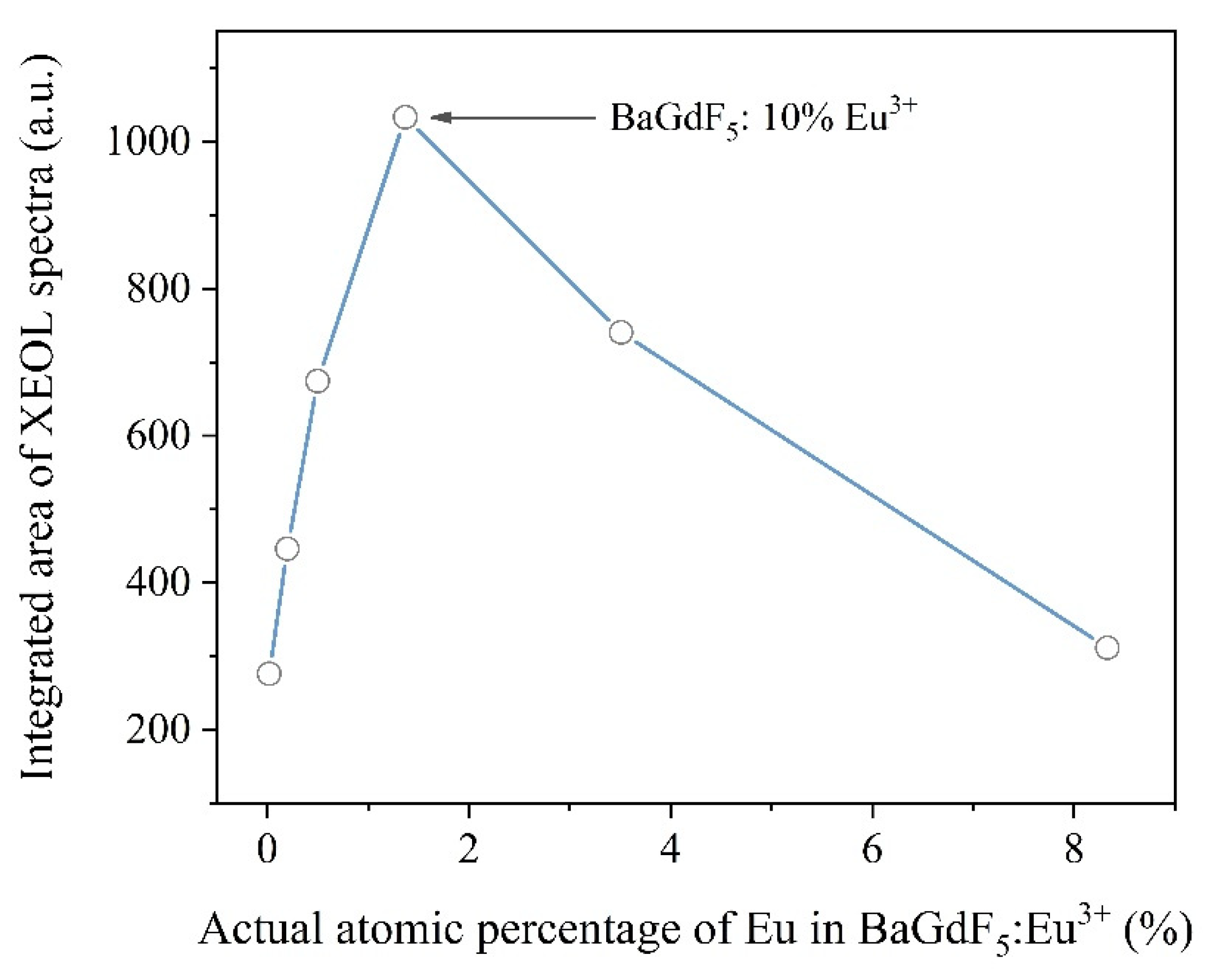
2.1.3. X-ray-Excited Optical Luminescence
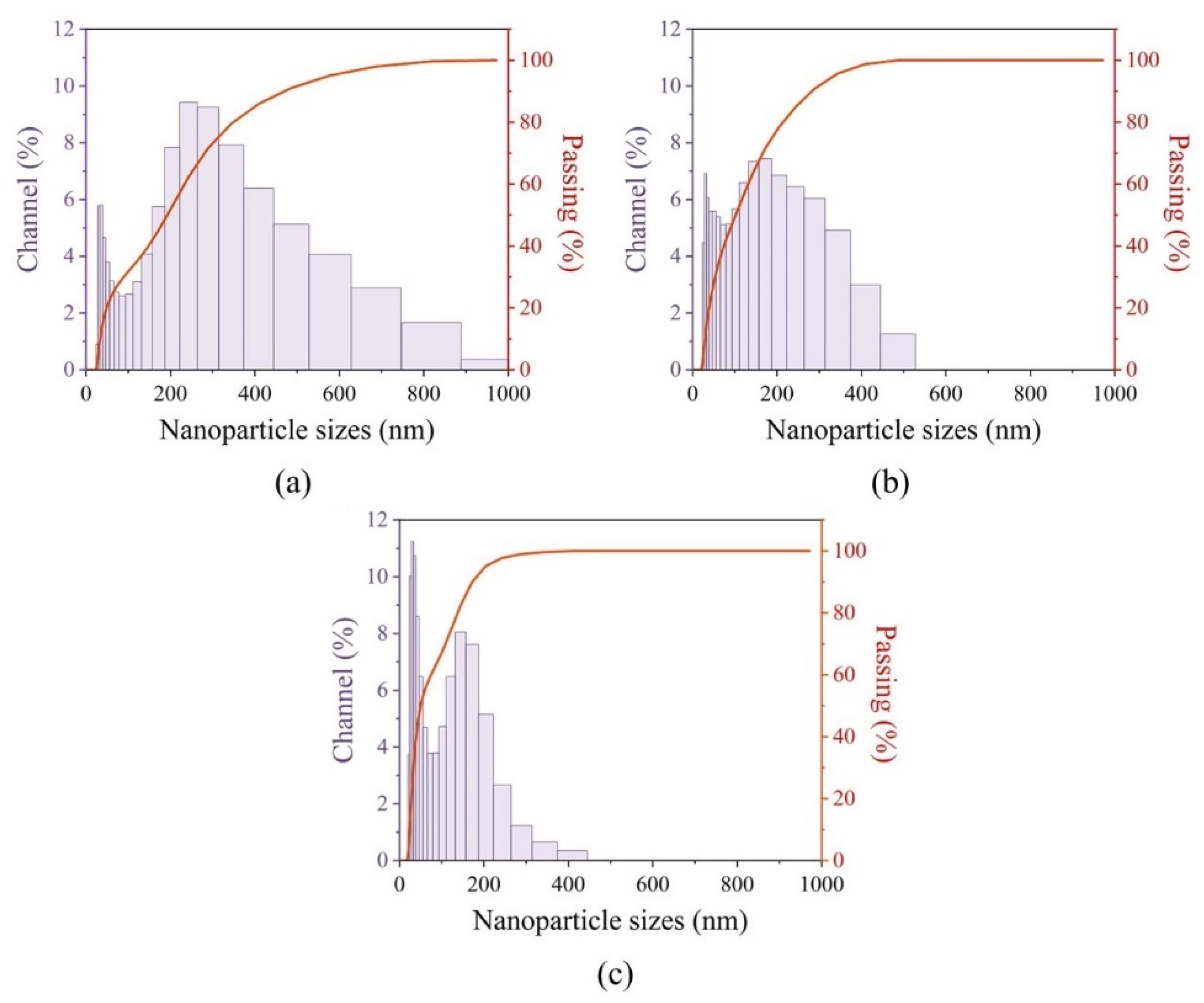
2.1.4. Nanoparticles’ Colloidal Solution Stability
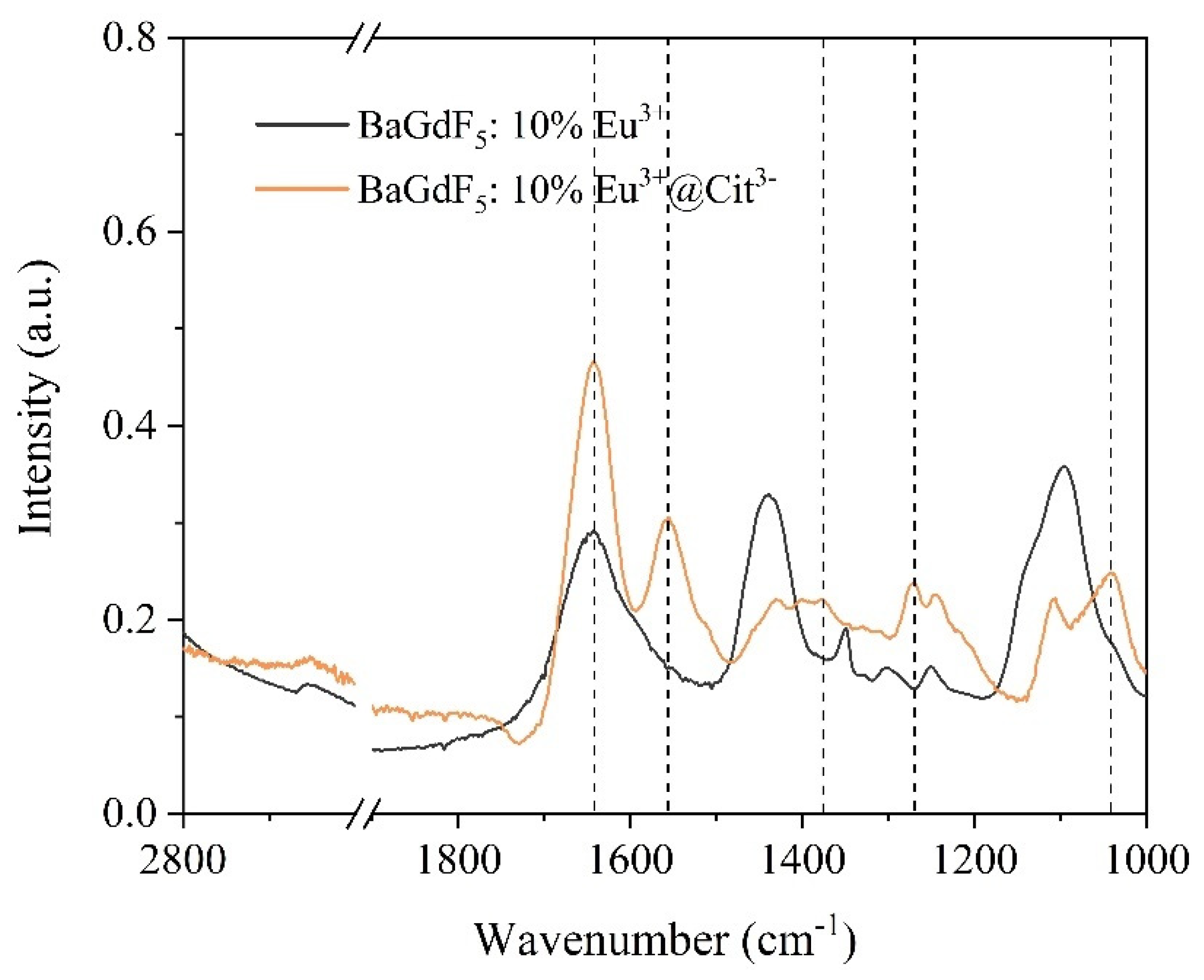
2.2. Citrate Surface Modification
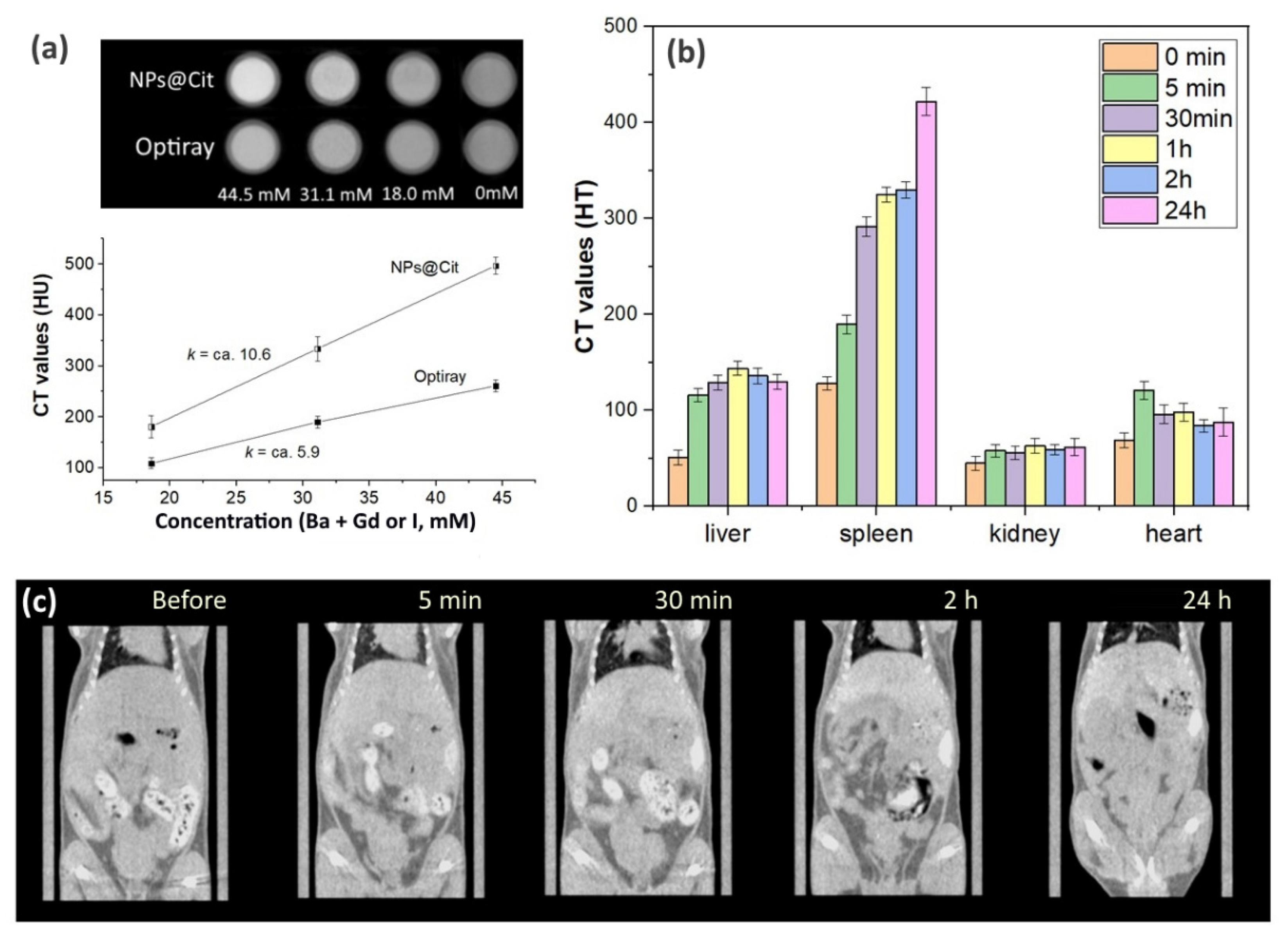
CT Imaging and Biodistribution
2.3. SiO2 Surface Modification
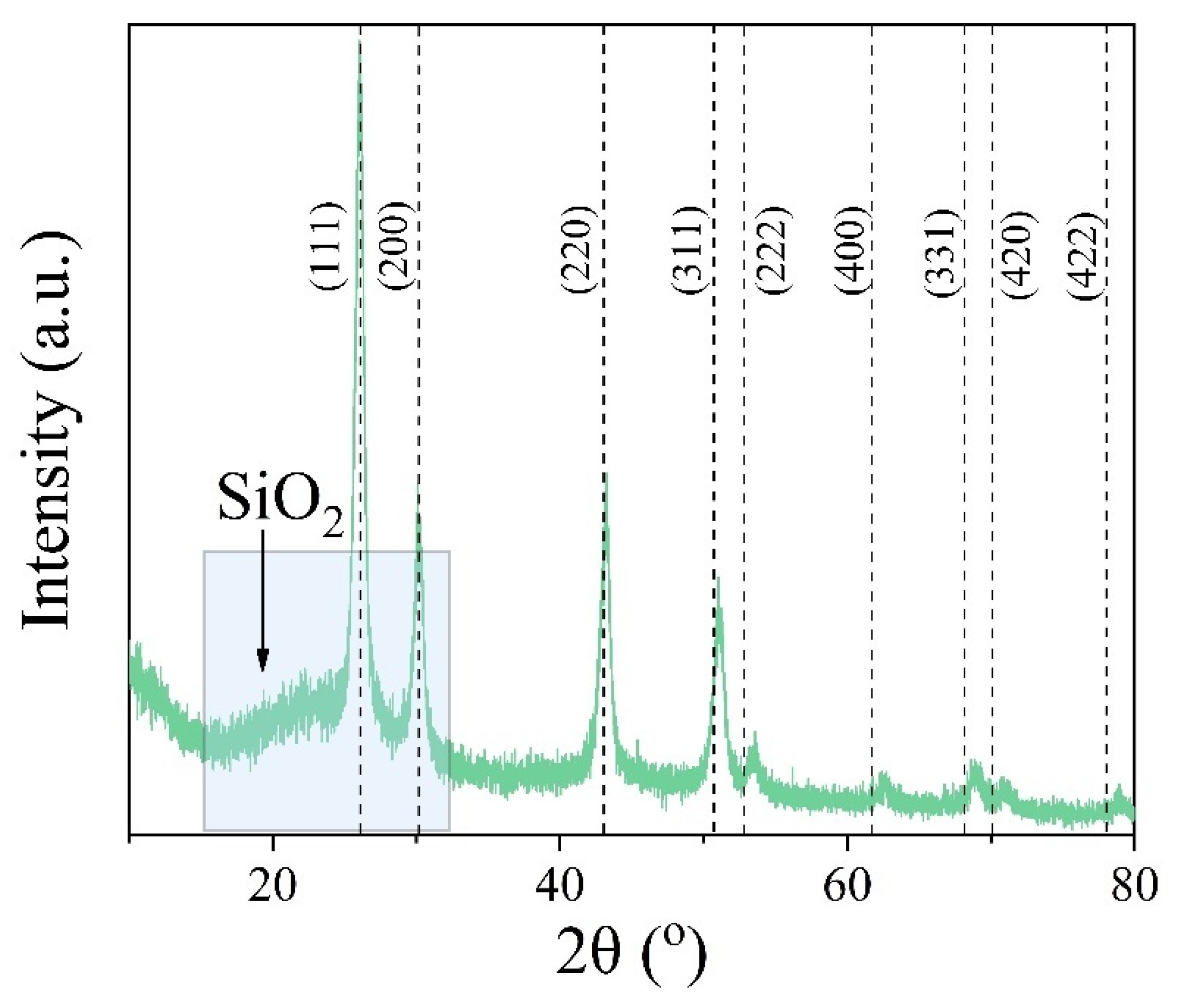
2.3.1. Structure and Morphology
2.3.2. Tunable SiO2 Coating
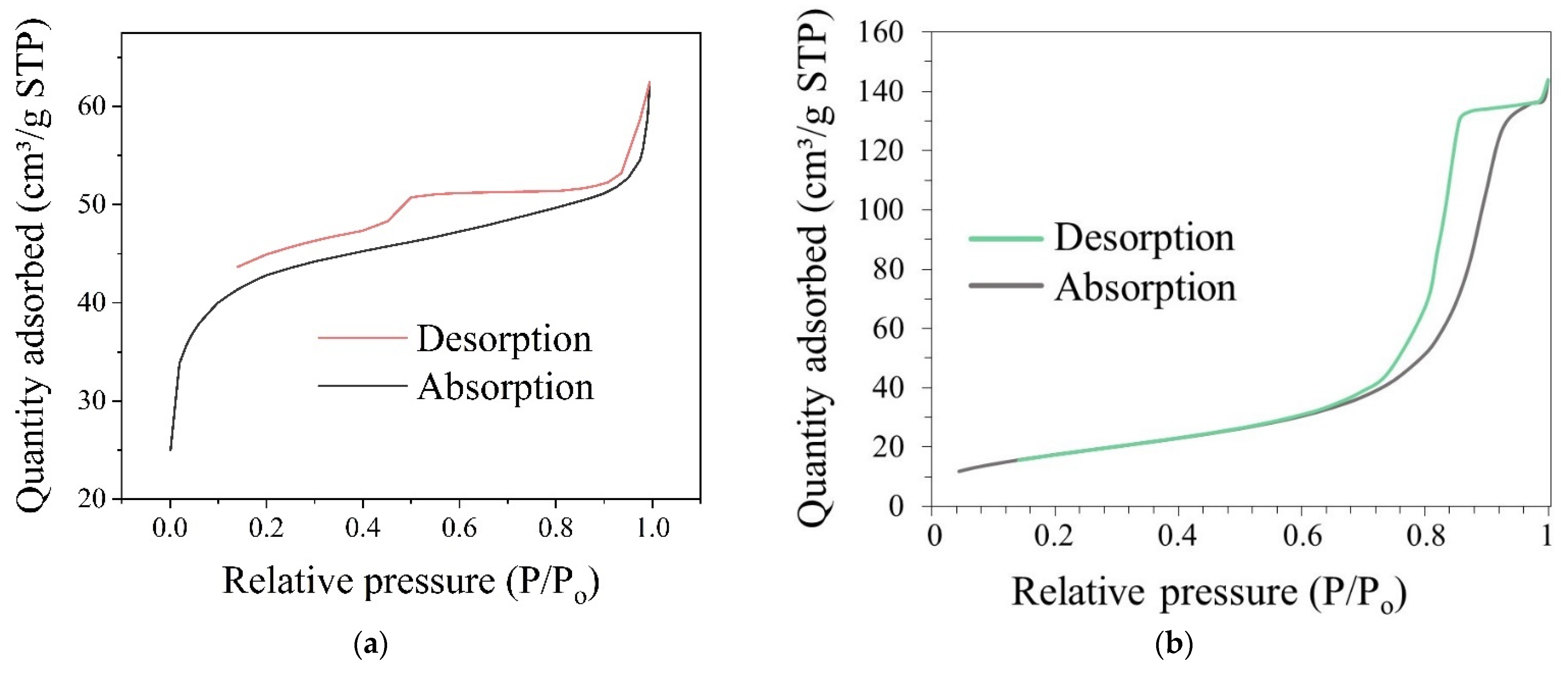
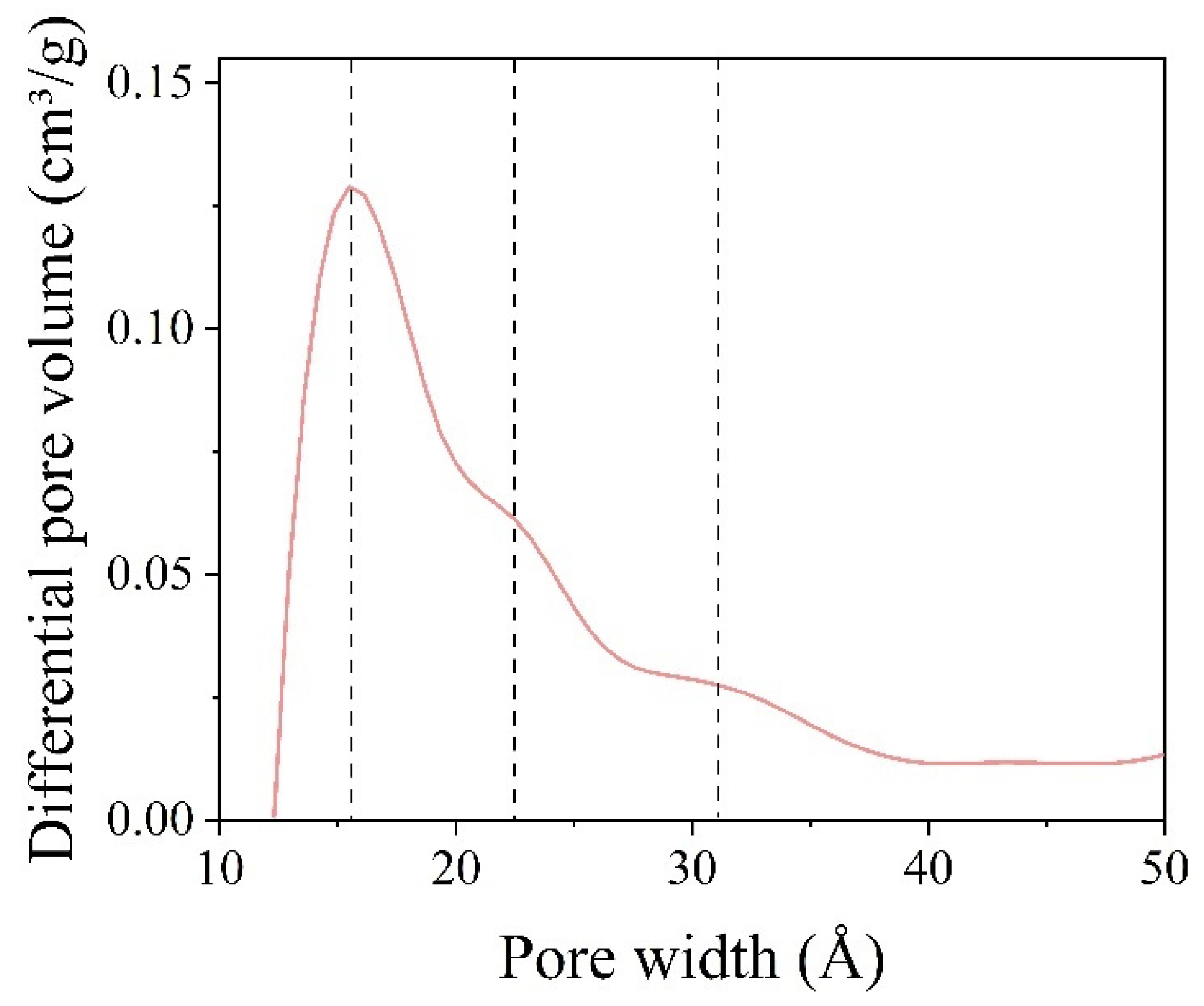
2.3.3. Measurements of the Specific Surface Area and Pore Size Distribution of the Silica Shell Structure
2.3.4. Cytotoxicity Test
2.3.5. Nanocomposite Impregnation by the Methylene Blue Photosensitizer
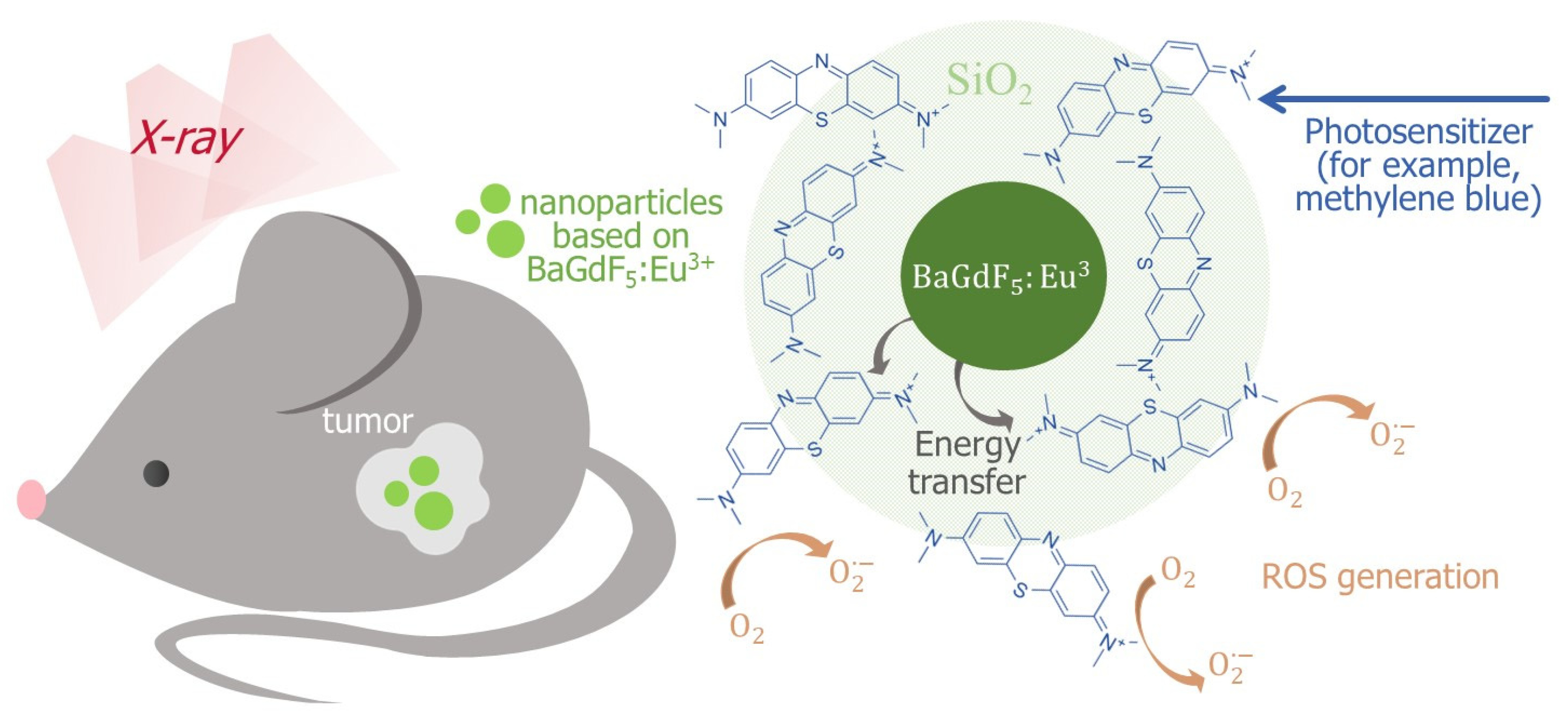
3. Discussion
4. Materials and Methods
4.1. Characterization Techniques
4.2. Synthesis Technique of the BaGdF5: x% Eu3+ Nanophosphors
4.3. Citrate Coating
4.4. SiO2 Coating
4.5. Cytotoxicity Test
4.6. Nanoparticle Impregnation with the Methylene Blue Photosensitizer
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pogue, B.W.; Wilson, B.C. Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine. J. Biomed. Opt. 2018, 23, 121610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Shi, B.; Shangguan, L.; Tong, W.; Yu, G.; Mao, Z.; Huang, F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratx, G.; Kapp, D.S. Is Cherenkov luminescence bright enough for photodynamic therapy? Nat. Nanotechnol. 2018, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-Ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 4004. [Google Scholar] [CrossRef] [PubMed]
- Kirsanova, D.Y.; Gadzhimagomedova, Z.M.; Maksimov, A.Y.; Soldatov, A.V. Nanomaterials for Deep Tumor Treatment. Mini Rev. Med. Chem. 2021, 21, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tang, Y.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef]
- Wilson, B.C. Photodynamic therapy for cancer: Principles. Can. J. Gastroenterol. 2002, 16, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lei, Z.; Huang, S.; Han, X.; Shao, B.; Lu, W.; Jia, Y.; Lv, W.; Jiao, M.; Wang, Z.; et al. Facile fabrication of single-phase multifunctional BaGdF5 nanospheres as drug carriers. ACS Appl. Mater. Interfaces 2014, 6, 12761–12770. [Google Scholar] [CrossRef]
- Yang, D.; Dai, Y.; Liu, J.; Zhou, Y.; Chen, Y.; Li, C.; Ma, P.; Lin, J. Ultra-small BaGdF5-based upconversion nanoparticles as drug carriers and multimodal imaging probes. Biomaterials 2014, 35, 2011–2023. [Google Scholar] [CrossRef]
- He, F.; Li, C.; Zhang, X.; Chen, Y.; Deng, X.; Liu, B.; Hou, Z.; Huang, S.; Jin, D.; Lin, J. Optimization of upconversion luminescence of Nd3+-sensitized BaGdF5-based nanostructures and their application in dual-modality imaging and drug delivery. Dalton Trans. 2016, 45, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Dual-mode, tunable color, enhanced upconversion luminescence and magnetism of multifunctional BaGdF5:Ln3+ (Ln = Yb/Er/Eu) nanophosphors. Phys. Chem. Chem. Phys. 2016, 18, 21518–21526. [Google Scholar] [CrossRef]
- Yang, D.; Kang, X.; Shang, M.; Li, G.; Peng, C.; Li, C.; Lin, J. Size and shape controllable synthesis and luminescent properties of BaGdF5:Ce3+/Ln3+ (Ln = Sm, Dy, Eu, Tb) nano/submicrocrystals by a facile hydrothermal process. Nanoscale 2011, 3, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ma, M.; Yang, L.; Zeng, S.; Yang, Q. Upconversion luminescence and magnetic properties of ligand-free monodisperse lanthanide doped BaGdF5 nanocrystals. J. Lumin. 2011, 131, 2544–2549. [Google Scholar] [CrossRef]
- Zeng, S.; Tsang, M.K.; Chan, C.F.; Wong, K.L.; Hao, J. PEG modified BaGdF5:Yb/Er nanoprobes for multi-modal upconversion fluorescent, in vivo X-ray computed tomography and biomagnetic imaging. Biomaterials 2012, 33, 9232–9238. [Google Scholar] [CrossRef]
- Becerro, A.I.; Gonzalez-Mancebo, D.; Cantelar, E.; Cusso, F.; Stepien, G.; de la Fuente, J.M.; Ocana, M. Ligand-Free Synthesis of Tunable Size Ln:BaGdF5 (Ln = Eu3+ and Nd3+) Nanoparticles: Luminescence, Magnetic Properties, and Biocompatibility. Langmuir 2016, 32, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Eu3+/Tb3+ doped cubic BaGdF5 multifunctional nanophosphors: Multicolor tunable luminescence, energy transfer and magnetic properties. J. Lumin. 2017, 186, 6–15. [Google Scholar] [CrossRef]
- Lee, G.; Struebing, C.; Wagner, B.; Summers, C.; Ding, Y.; Bryant, A.; Thadhani, N.; Shedlock, D.; Star-Lack, J.; Kang, Z. Synthesis and characterization of a BaGdF5:Tb glass ceramic as a nanocomposite scintillator for x-ray imaging. Nanotechnology 2016, 27, 205203. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Zhang, J.; Wang, Y.; Dong, P. Near-infrared quantum cutting in Ho3+, Yb3+-codoped BaGdF5 nanoparticles via firstand second-order energy transfers. Nanoscale Res. Lett. 2012, 7, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Song, Y.; Zheng, K.; Sheng, Y.; Zou, H. BaGdF5:Dy3+,Tb3+,Eu3+ multifunctional nanospheres: Paramagnetic, luminescence, energy transfer, and tunable color. Phys. Chem. Chem. Phys. 2016, 18, 13861–13873. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Wang, Y.; Zhang, J.; Dong, P. Crystal structure and up- and down-conversion properties of Yb3+, Ho3+ codoped BaGdF5 solid-solution with different morphologies. CrystEngComm 2012, 14, 3131–3141. [Google Scholar] [CrossRef]
- Bakhmetyev, V.V.; Dorokhina, A.M.; Keskinova, M.V.; Mjakin, S.V.; Vlasenko, A.B.; Lebedev, L.A.; Malygin, V.V.; Sychov, M.M. Synthesis and characterization of finely dispersed phosphors doped with rare-earth metal ions for enhanced photodynamic therapy of cancer. Chem. Pap. 2019, 74, 787–797. [Google Scholar] [CrossRef]
- Yang, D.; Li, C.; Li, G.; Shang, M.; Kang, X.; Lin, J. Colloidal synthesis and remarkable enhancement of the upconversion luminescence of BaGdF5:Yb3+/Er3+ nanoparticles by active-shell modification. J. Mater. Chem. 2011, 21, 5923–5927. [Google Scholar] [CrossRef]
- Sarkar, S.; Hazra, C.; Mahalingam, V. Scaling down the size of BaLnF5 nanocrystals (Ln = La, Gd, and Lu) with the Ln3+ size. Dalton Trans. 2013, 42, 63–66. [Google Scholar] [CrossRef]
- Yi, Z.; Li, X.; Lu, W.; Liu, H.; Zeng, S.; Hao, J. Hybrid lanthanide nanoparticles as a new class of binary contrast agents for in vivo T1/T2 dual-weighted MRI and synergistic tumor diagnosis. J. Mater. Chem. B 2016, 4, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An Up-to-Date Overview. Pharmaceutics 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murayama, S.; Nishiyama, T.; Takagi, K.; Ishizuka, F.; Santa, T.; Kato, M. Delivery, stabilization, and spatiotemporal activation of cargo molecules in cells with positively charged nanoparticles. Chem. Commun. 2012, 48, 11461–11463. [Google Scholar] [CrossRef]
- Durymanov, M.O.; Rosenkranz, A.A.; Sobolev, A.S. Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines. Theranostics 2015, 5, 1007–1020. [Google Scholar] [CrossRef] [Green Version]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.; Kissel, T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Safronikhin, A.; Ehrlich, H.; Shcherba, T.; Kuzmina, N.; Lisichkin, G. Formation of complexes on the surface of nanosized europium fluoride. Colloids Surfaces A 2011, 377, 367–373. [Google Scholar] [CrossRef]
- Safronikhin, A.; Shcherba, T.; Ehrlich, H.; Lisichkin, G. Preparation and colloidal behaviour of surface-modified EuF3. Appl. Surf. Sci. 2009, 255, 7990–7994. [Google Scholar] [CrossRef]
- Safronikhin, A.V.; Ehrlich, G.V.; Lisichkin, G.V. Synthesis of lanthanum fluoride nanocrystals and modification of their surface. Russ. J. Gen. Chem. 2011, 81, 277–281. [Google Scholar] [CrossRef]
- Safronikhin, A.V.; Ehrlich, H.V.; Shcherba, T.N.; Lisichkin, G.V. Surface complexation onto nanosized lanthanum fluoride. Russ. Chem. Bull. 2011, 60, 1576–1580. [Google Scholar] [CrossRef]
- Safronikhin, A. Chemical Modification of the Surface of Lanthanum and Europium Fluorides. Ph.D. Thesis, Moscow State University, Moscow, Russia, 2013. [Google Scholar]
- Jiang, Q.; Liang, L.H.; Zhao, D.S. Lattice Contraction and Surface Stress of fcc Nanocrystals. J. Phys. Chem. B 2001, 105, 6275–6277. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Yefimova, S.L.; Tkacheva, T.N.; Maksimchuk, P.O.; Bespalova, I.I.; Hubenko, K.O.; Klochkov, V.K.; Sorokin, A.V.; Malyukin, Y.V. GdVO4:Eu3+ nanoparticles—Methylene Blue complexes for PDT: Electronic excitation energy transfer study. J. Lumin. 2017, 192, 975–981. [Google Scholar] [CrossRef]
- King, A.; Singh, R.; Nayak, B.B. Phase and photoluminescence analysis of dual-color emissive Eu3+-doped ZrO2 nanoparticles for advanced security features in anti-counterfeiting. Colloids Surfaces A 2021, 631, 127715. [Google Scholar] [CrossRef]
- Rezende, M.V.D.S.; Montes, P.J.; Valerio, M.E.G.; Jackson, R.A. The optical properties of Eu3+ doped BaAl2O4: A computational and spectroscopic study. Opt. Mater. 2012, 34, 1434–1439. [Google Scholar] [CrossRef]
- Huignard, A.; Buissette, V.; Franville, A.-C.; Gacoin, T.; Boilot, J.-P. Emission Processes in YVO4:Eu Nanoparticles. J. Phys. Chem. B 2003, 107, 6754–6759. [Google Scholar] [CrossRef]
- Jorma, H.; Eija, K. Crystal Fields in REOF:Eu3+ (RE = La, Gd and Y). J. Chem. Soc. Faraday Trans. 1995, 91, 1503–1509. [Google Scholar]
- Kadu, P.J.; Kushare, S.S.; Thacker, D.D.; Gattani, S.G. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS). Pharm. Dev. Technol. 2011, 16, 65–74. [Google Scholar] [CrossRef]
- Biniak, S.; Trykowski, G.; Walczyk, M.; Richert, M. Thermo-Chemical Modification of Low-Dimensional Carbons: An Infrared Study. J. Appl. Spectrosc. 2016, 83, 580–585. [Google Scholar] [CrossRef]
- Thottoli, A.K.; Unni, A.K.A. Effect of trisodium citrate concentration on the particle growth of ZnS nanoparticles. J. Nanostructure Chem. 2013, 3, 56. [Google Scholar] [CrossRef]
- Silva-Silva, M.J.; Mijangos-Ricardez, O.F.; Vázquez-Hipólito, V.; Martinez-Vargas, S.; López-Luna, J. Single and mixed adsorption of Cd(II) and Cr(VI) onto citrate-coated magnetite nanoparticles. Desalination Water Treat. 2014, 57, 4008–4017. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Moglianetti, M.; Pedone, D.; Udayan, G.; Retta, S.F.; Debellis, D.; Marotta, R.; Turco, A.; Rella, S.; Malitesta, C.; Bonacucina, G.; et al. Intracellular Antioxidant Activity of Biocompatible Citrate-Capped Palladium Nanozymes. Nanomaterials 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, W.O.; Fascineli, M.L.; Farias, G.R.; Horst, F.H.; de Andrade, L.R.; Correa, L.H.; Magalhaes, K.G.; Sousa, M.H.; de Almeida, M.C.; Azevedo, R.B.; et al. The influence of female mice age on biodistribution and biocompatibility of citrate-coated magnetic nanoparticles. Int. J. Nanomed. 2019, 14, 3375–3388. [Google Scholar] [CrossRef] [Green Version]
- Fagundes, D.A.; Leonel, L.V.; Fernandez-Outon, L.E.; Ardisson, J.D.; dos Santos, R.G. Radiosensitizing effects of citrate-coated cobalt and nickel ferrite nanoparticles on breast cancer cells. Nanomedicine 2020, 15, 2823–2836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, H.; Wang, J.; Yang, Y.; Wu, D.; Zhang, Y.; Zhang, Y.; Zhou, Z.; Yang, S. Graphene oxide-BaGdF5 nanocomposites for multi-modal imaging and photothermal therapy. Biomaterials 2015, 42, 66–77. [Google Scholar] [CrossRef]
- Wang, T.; Jia, G.; Cheng, C.; Wang, Q.; Li, X.; Liu, Y.; He, C.; Chen, L.; Sun, G.; Zuo, C. Active targeted dual-modal CT/MR imaging of VX2 tumors using PEGylated BaGdF5 nanoparticles conjugated with RGD. New J. Chem. 2018, 42, 11565–11572. [Google Scholar] [CrossRef]
- Zhang, P.; He, Y.; Liu, J.; Feng, J.; Sun, Z.; Lei, P.; Yuan, Q.; Zhang, H. Core-shell BaYbF5:Tm@BaGdF5:Yb, Tm nanocrystals for in vivo trimodal UCL/CT/MR imaging. RSC Adv. 2016, 6, 14283–14289. [Google Scholar] [CrossRef]
- Hubbel, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest. NIST Stand. Ref. Database 126 1995, 1, 1. [Google Scholar]
- Liang, Y.; Ouyang, J.; Wang, H.; Wang, W.; Chui, P.; Sun, K. Synthesis and characterization of core-shell structured SiO2@YVO4:Yb3+, Er3+ microspheres. Appl. Surf. Sci. 2012, 258, 3689–3694. [Google Scholar] [CrossRef]
- Rios, X.; Moriones, P.; Echeverría, J.C.; Luquín, A.; Laguna, M.; Garrido, J.J. Characterisation of hybrid xerogels synthesised in acid media using methyltriethoxysilane (MTEOS) and tetraethoxysilane (TEOS) as precursors. Adsorption 2011, 17, 583–593. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Correlation between physical properties and structure of silica xerogels. J. Non-Cryst. Solids 2004, 347, 128–137. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Schneckenburger, H. Förster resonance energy transfer—What can we learn and how can we use it? Methods Appl. Fluoresc. 2019, 8, 013001. [Google Scholar] [CrossRef]
- Wu, P.G.; Brand, L. Resonance Energy Transfer: Methods and Applications. Anal. Biochem. 1994, 218, 1–13. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Elmenoufy, A.H.; Tang, Y.A.; Hu, J.; Xu, H.; Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 2015, 51, 12247–12250. [Google Scholar] [CrossRef] [Green Version]
- Belanova, A.; Chmykhalo, V.; Beseda, D.; Belousova, M.; Butova, V.; Soldatov, A.; Makarenko, Y.; Zolotukhin, P. A mini-review of X-ray photodynamic therapy (XPDT) nonoagent constituents’ safety and relevant design considerations. Photochem. Photobiol. Sci. 2020, 19, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, J.P.; Gregas, M.K.; Lauly, B.; Zhang, Y.; Vo-Dinh, T. Activity of Psoralen-Functionalized Nanoscintillators against Cancer Cells upon X-ray Excitation. ACS Nano 2011, 5, 4679–4687. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.D.; Chuang, Y.-J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for In Vivo Cancer Treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef] [Green Version]
- Koyu, H.; Haznedaroglu, M.Z. Investigation of impact of storage conditions on Hypericum perforatum L. dried total extract. J. Food Drug Anal. 2015, 23, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaščáková, S.; Giuliani, A.; Lacerda, S.; Pallier, A.; Mercère, P.; Tóth, É.; Réfrégiers, M. X-ray-induced radiophotodynamic therapy (RPDT) using lanthanide micelles: Beyond depth limitations. Nano Res. 2015, 8, 2373–2379. [Google Scholar] [CrossRef] [Green Version]
- Chagovets, V.V.; Kosevich, M.V.; Stepanian, S.G.; Boryak, O.A.; Shelkovsky, V.S.; Orlov, V.V.; Leontiev, V.S.; Pokrovskiy, V.A.; Adamowicz, L.; Karachevtsev, V.A. Noncovalent Interaction of Methylene Blue with Carbon Nanotubes: Theoretical and Mass Spectrometry Characterization. J. Phys. Chem. C 2012, 116, 20579–20590. [Google Scholar] [CrossRef]
- Ronzani, F.; Trivella, A.; Arzoumanian, E.; Blanc, S.; Sarakha, M.; Richard, C.; Oliveros, E.; Lacombe, S. Comparison of the photophysical properties of three phenothiazine derivatives: Transient detection and singlet oxygen production. Photochem. Photobiol. Sci. 2013, 12, 2160–2169. [Google Scholar] [CrossRef]
- Lewis, G.N.; Goldschmid, O.; Magel, T.T.; Bigeleisen, J. Dimeric and Other Forms of Methylene Blue: Absorption and Fluorescence of the Pure Monomer. J. Am. Chem. Soc. 1943, 65, 1150–1154. [Google Scholar] [CrossRef]
- Pepe, G.; Cole, J.M.; Waddell, P.G.; Perry, J.I. Rationalizing the suitability of rhodamines as chromophores in dye-sensitized solar cells: A systematic molecular design study. Mol. Syst. Des. Eng. 2016, 1, 416–435. [Google Scholar] [CrossRef]
- Fernandez-Perez, A.; Marban, G. Visible Light Spectroscopic Analysis of Methylene Blue in Water; What Comes after Dimer? ACS Omega 2020, 5, 29801–29815. [Google Scholar] [CrossRef] [PubMed]
- Martini, I.; Hartland, G.V.; Kamat, P.V. Ultrafast Photophysical Investigation of Cresyl Violet Aggregates Adsorbed onto Nanometer-Sized Particles of SnO2 and SiO2. J. Phys. Chem. B 1997, 101, 4826–4830. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals: Eighth Edition; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Kirsanova, D.Y.; Butova, V.V.; Polyakov, V.A.; Zolotukhin, P.V.; Belanova, A.A.; Legostaev, V.M.; Kuchma, E.A.; Gadzhimagomedova, Z.M.; Soldatov, A.V. X-Ray Nanophosphors based on Bagdf5 for X-ray photodynamic therapy in oncology. Nanotechnol. Russ. 2020, 15, 105–111. [Google Scholar] [CrossRef]
- Qureashi, A.; Pandith, A.H.; Bashir, A.; Manzoor, T.; Malik, L.A.; Sheikh, F.A. Citrate coated magnetite: A complete magneto dielectric, electrochemical and DFT study for detection and removal of heavy metal ions. Surf. Interfaces 2021, 23, 101004. [Google Scholar] [CrossRef]
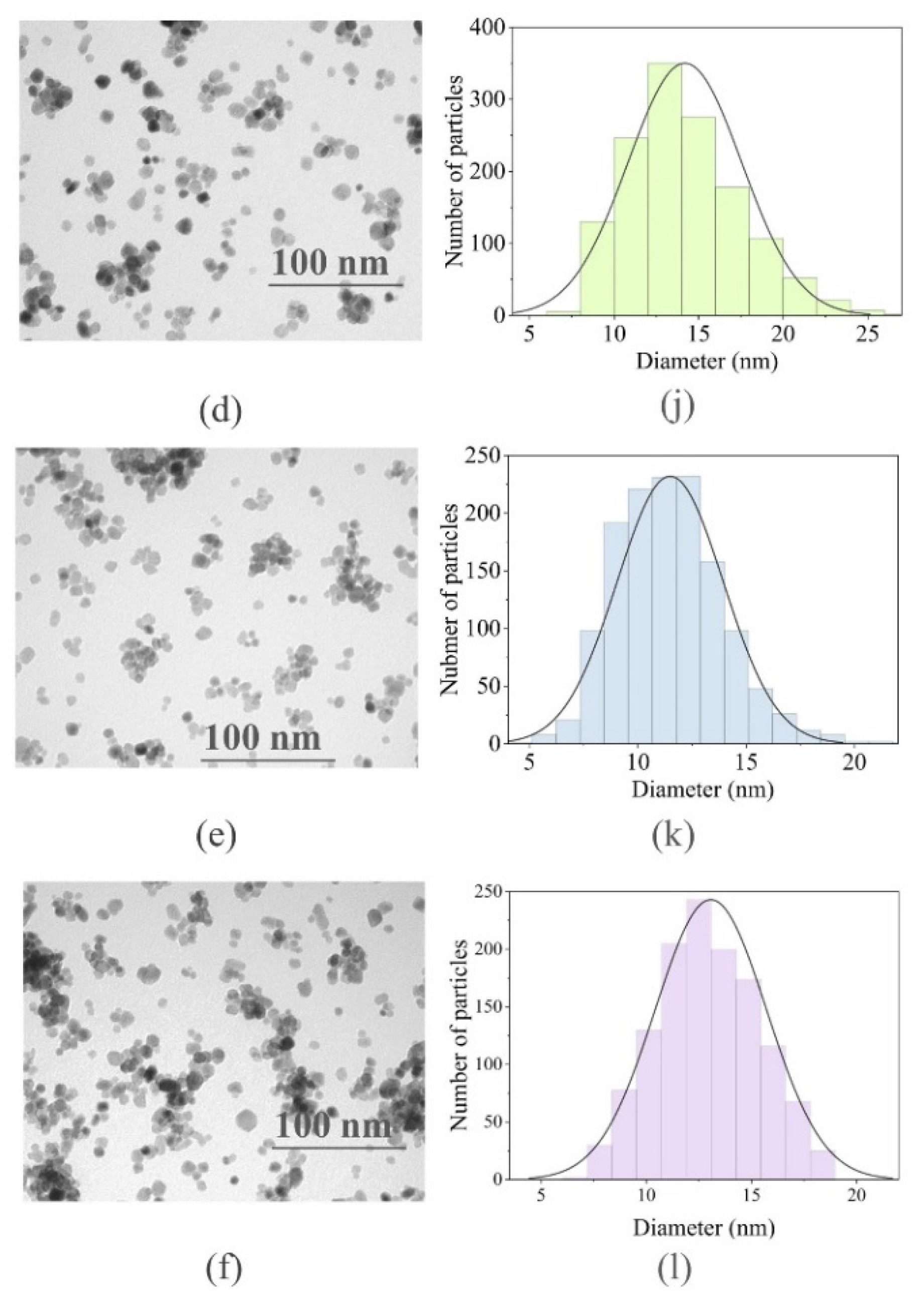






| Sample Names | Initial Elemental Composition at., % | Actual Elemental Composition at., % | Cell Parameters, Å | Cell Volume, Å3 | Crystal Size According to the Scherrer Equation | Crystal Size According to the W–H Analysis |
|---|---|---|---|---|---|---|
| BaGdF5 | 0 | 0 | 5.9099 (5) | 206.41 (3) | 10.71 | 14.20 |
| BaGdF5: 1% Eu3+ | 0.14 | 0.02 | 5.9198 (8) | 207.45 (3) | 10.45 | 13.59 |
| BaGdF5: 2.5% Eu3+ | 0.36 | 0.20 | 5.9168 (8) | 207.13 (8) | 10.49 | 14.20 |
| BaGdF5: 5% Eu3+ | 0.71 | 0.50 | 5.9208 (14) | 207.55 (8) | 9.64 | 11.47 |
| BaGdF5: 10% Eu3+ | 1.43 | 1.37 | 5.9212 (7) | 207.60 (1) | 10.98 | 15.36 |
| BaGdF5: 25% Eu3+ | 3.57 | 3.51 | 5.9276 (9) | 208.27 (4) | 9.61 | 12.64 |
| BaGdF5: 50% Eu3+ | 7.14 | 8.33 | 5.9388 (8) | 209.45 (5) | 9.99 | 12.21 |
| Sample | ζ-Potential, mV |
|---|---|
| BaGdF5 | 26.12 |
| BaGdF5: 1% Eu3+ | 29.89 |
| BaGdF5: 2.5% Eu3+ | 32.04 |
| BaGdF5: 5% Eu3+ | 25.16 |
| BaGdF5: 10% Eu3+ | 32.37 |
| BaGdF5: 25% Eu3+ | 25.49 |
| BaGdF5: 50% Eu3+ | 28.57 |
| Sample | BaCl2·2H2O | GdCl3 | EuCl3·6H2O | NH4F | ||||
|---|---|---|---|---|---|---|---|---|
| mg | mmol | mg | mmol | Mg | mmol | mg | mmol | |
| BaGdF5: 1% Eu | 244.3 | 1S | 261 | 0.99 | 3.7 | 0.01 | 203.7 | 5.5 |
| BaGdF5: 2.5% Eu | 257 | 0.975 | 9.2 | 0.025 | ||||
| BaGdF5: 5% Eu | 250.4 | 0.95 | 18.3 | 0.05 | ||||
| BaGdF5: 10% Eu | 210.9 | 0.8 | 36.6 | 0.1 | ||||
| BaGdF5: 25% Eu | 197.7 | 0.75 | 91.6 | 0.25 | ||||
| BaGdF5: 50% Eu | 131.8 | 0.5 | 183.2 | 0.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadzhimagomedova, Z.; Polyakov, V.; Pankin, I.; Butova, V.; Kirsanova, D.; Soldatov, M.; Khodakova, D.; Goncharova, A.; Mukhanova, E.; Belanova, A.; et al. BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-ray Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 13040. https://doi.org/10.3390/ijms222313040
Gadzhimagomedova Z, Polyakov V, Pankin I, Butova V, Kirsanova D, Soldatov M, Khodakova D, Goncharova A, Mukhanova E, Belanova A, et al. BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-ray Photodynamic Therapy. International Journal of Molecular Sciences. 2021; 22(23):13040. https://doi.org/10.3390/ijms222313040
Chicago/Turabian StyleGadzhimagomedova, Zaira, Vladimir Polyakov, Ilia Pankin, Vera Butova, Daria Kirsanova, Mikhail Soldatov, Darya Khodakova, Anna Goncharova, Elizaveta Mukhanova, Anna Belanova, and et al. 2021. "BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-ray Photodynamic Therapy" International Journal of Molecular Sciences 22, no. 23: 13040. https://doi.org/10.3390/ijms222313040






