Influence of Culture Period on Osteoblast Differentiation of Tissue-Engineered Bone Constructed by Apatite-Fiber Scaffolds Using Radial-Flow Bioreactor
Abstract
:1. Introduction
2. Results
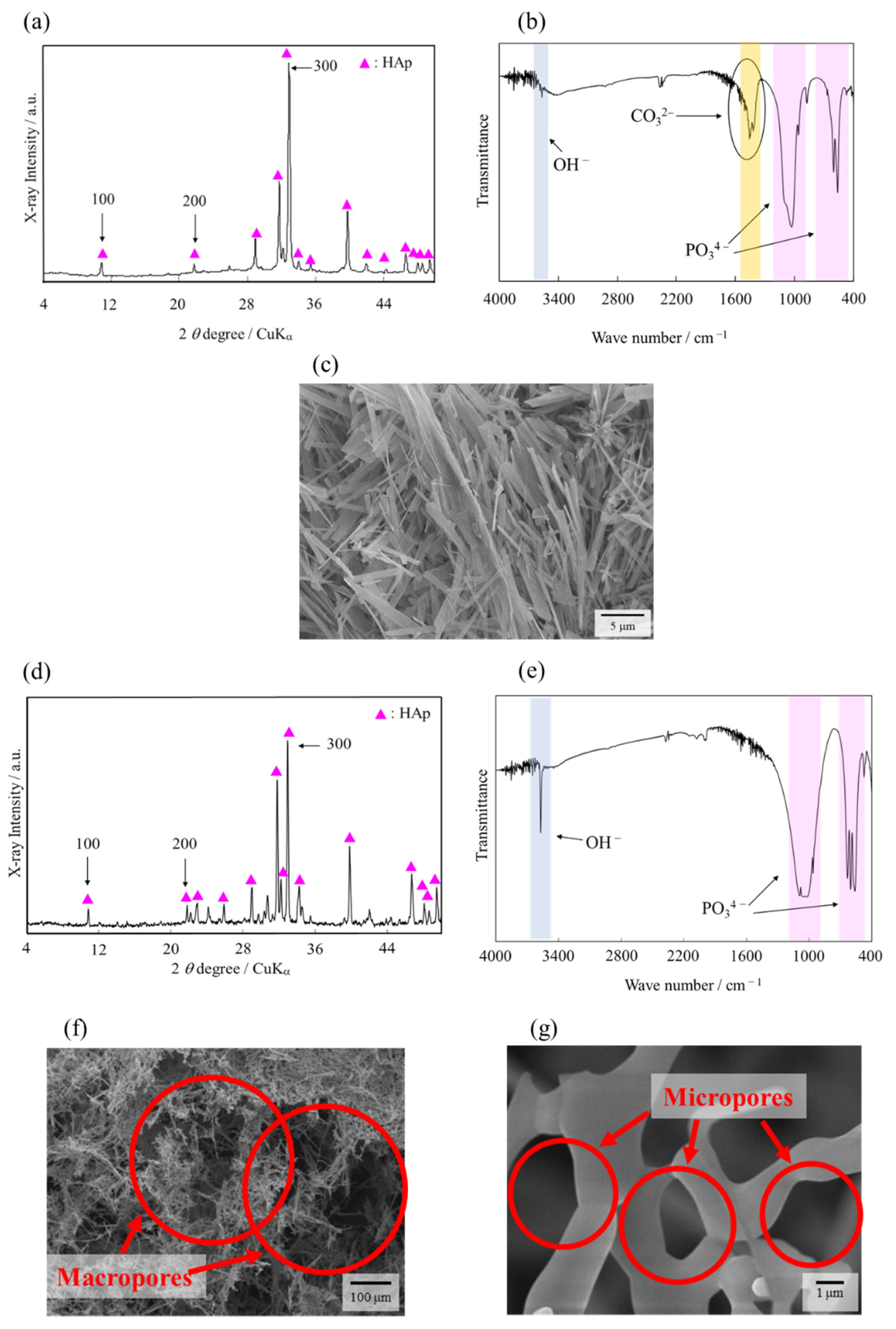
2.1. AFS Properties
2.2. Cell Viability
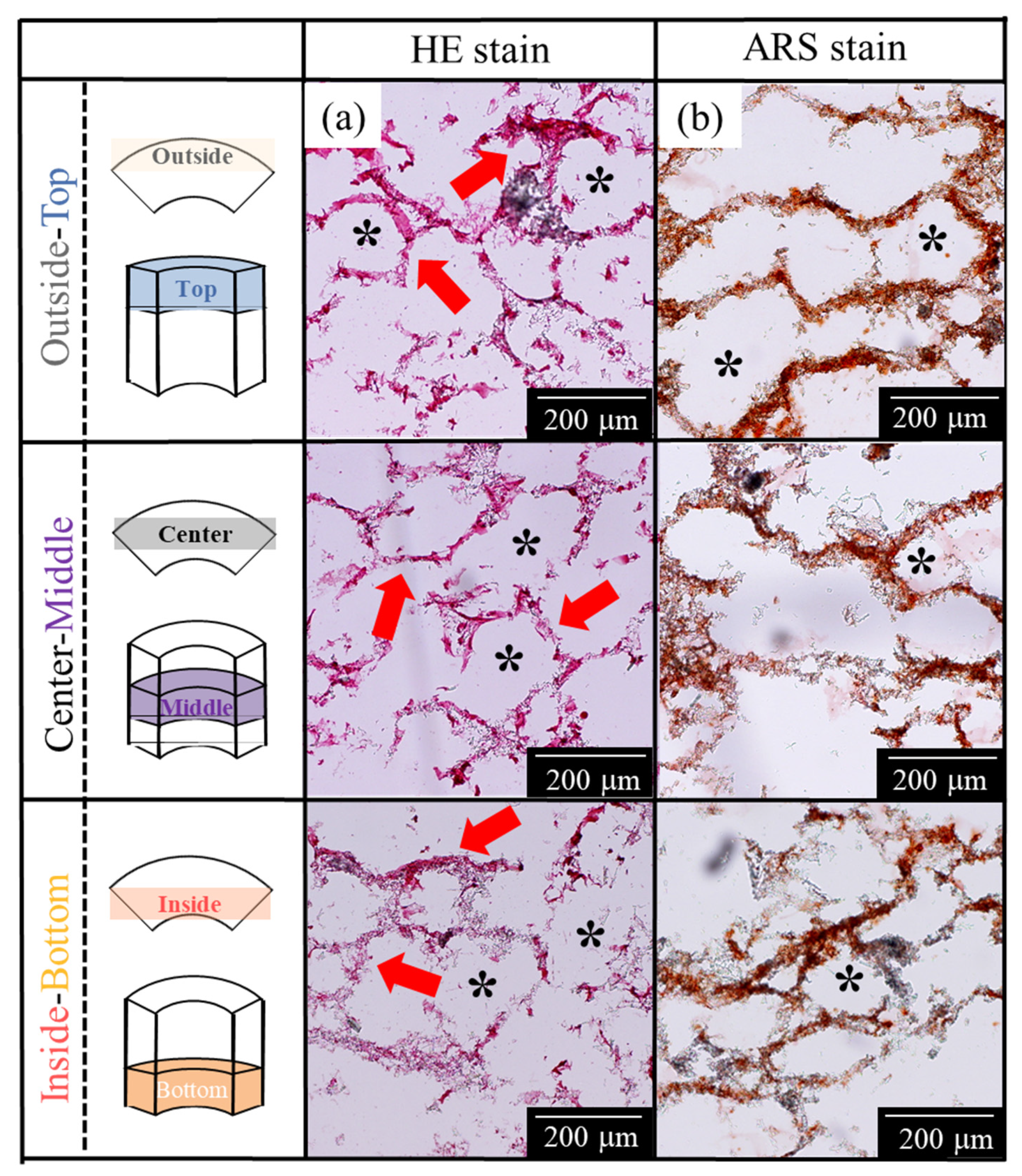
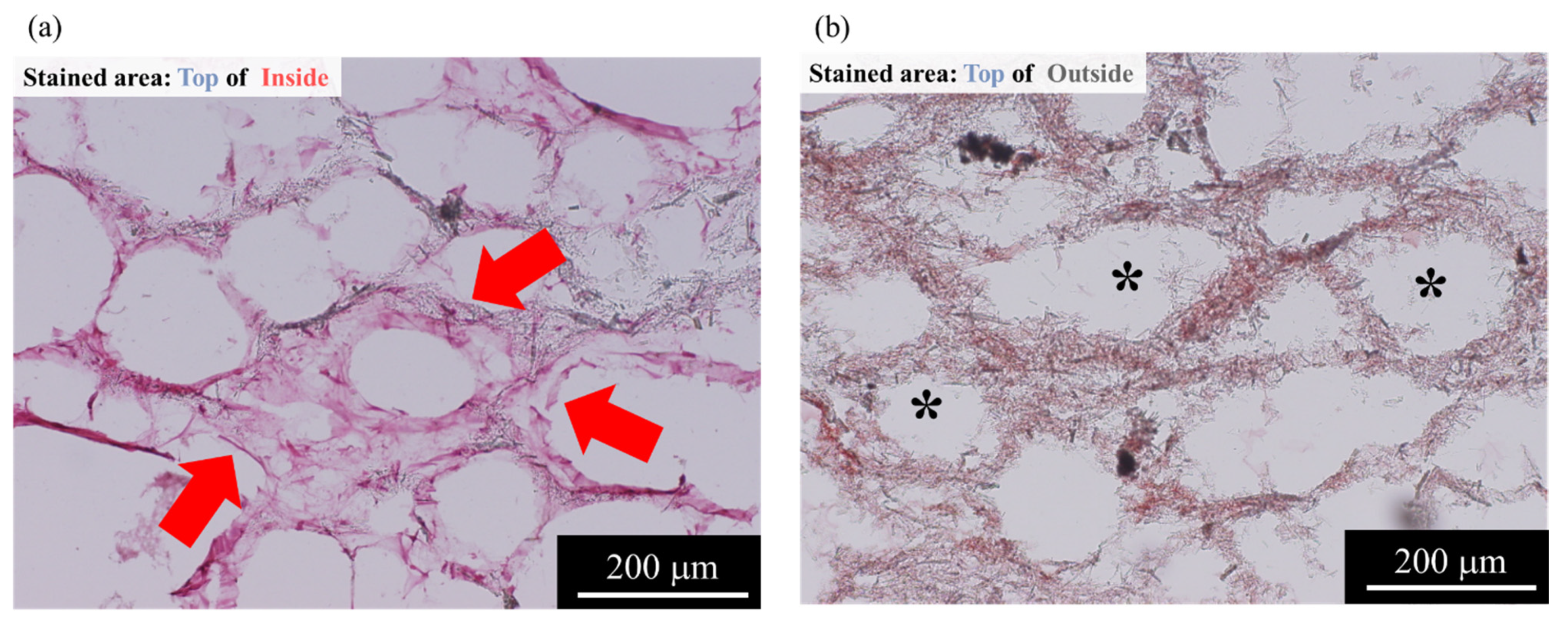
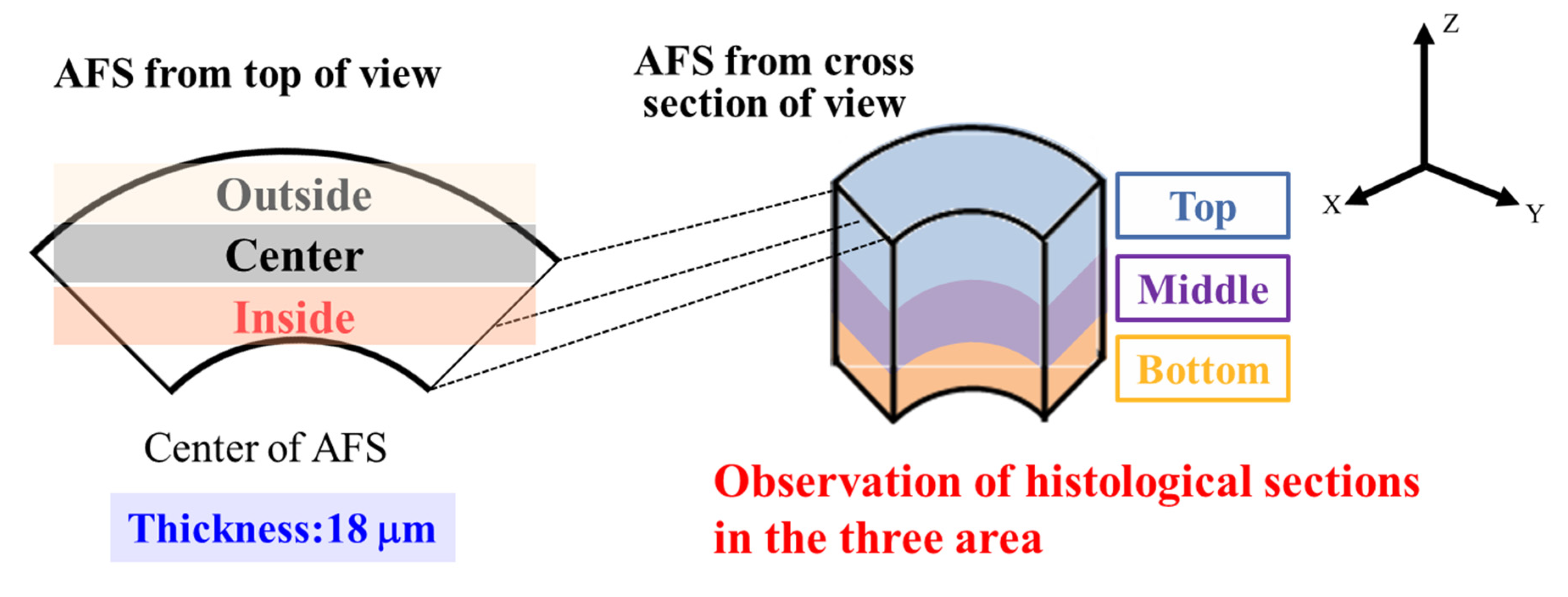
2.3. Histological Evaluations from AFSs after 3D Cell Culture of RBMCs Using a RFB
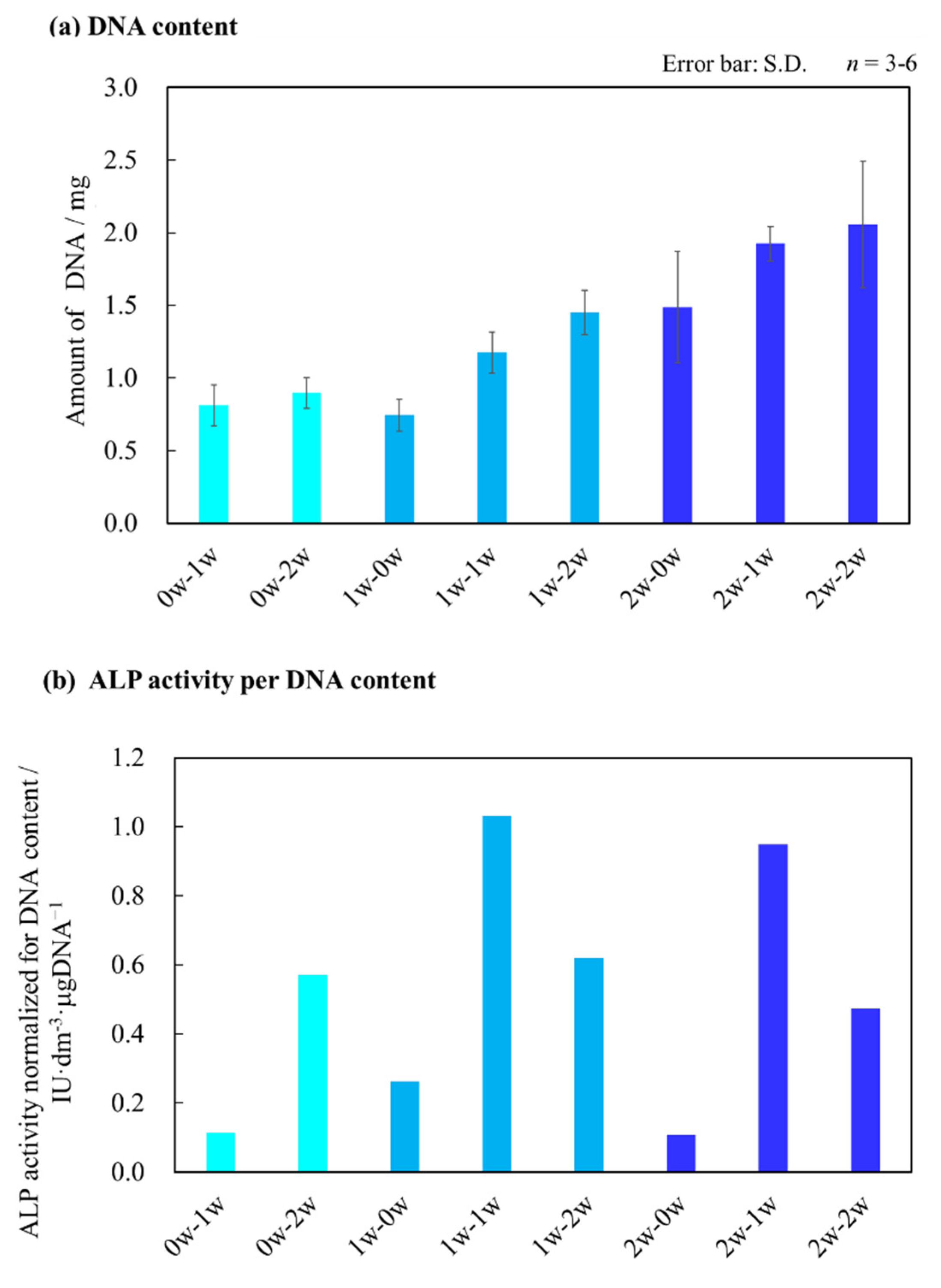
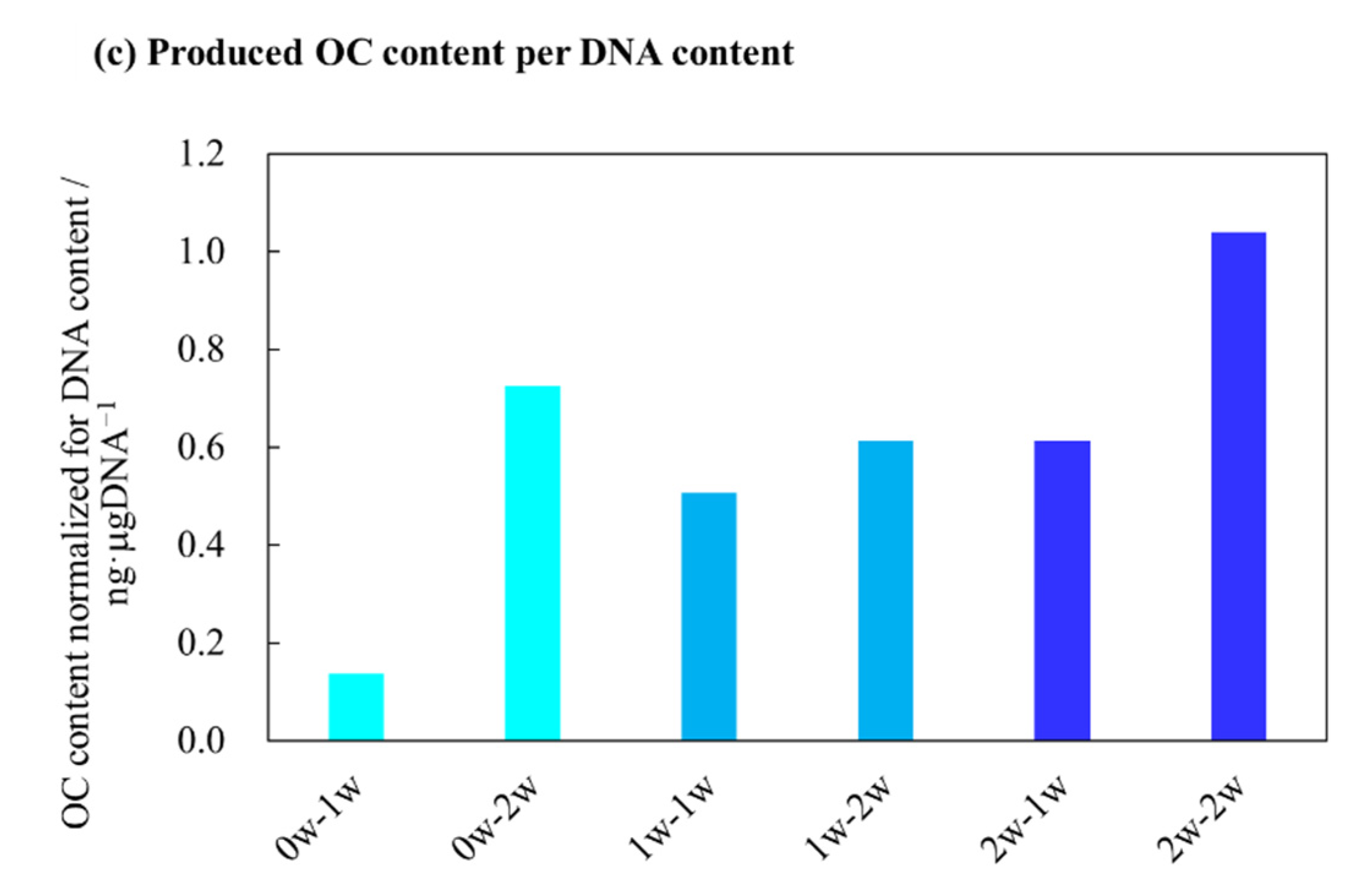
2.4. Quantitative Evaluations of Bone Differentiation Markers for AFS-Cultured RBMCs Using a RFB
3. Discussion
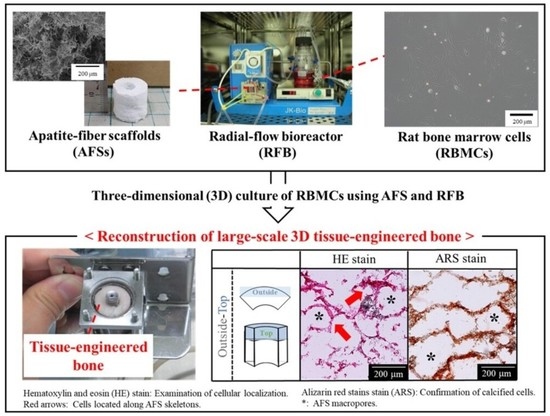
4. Materials and Methods
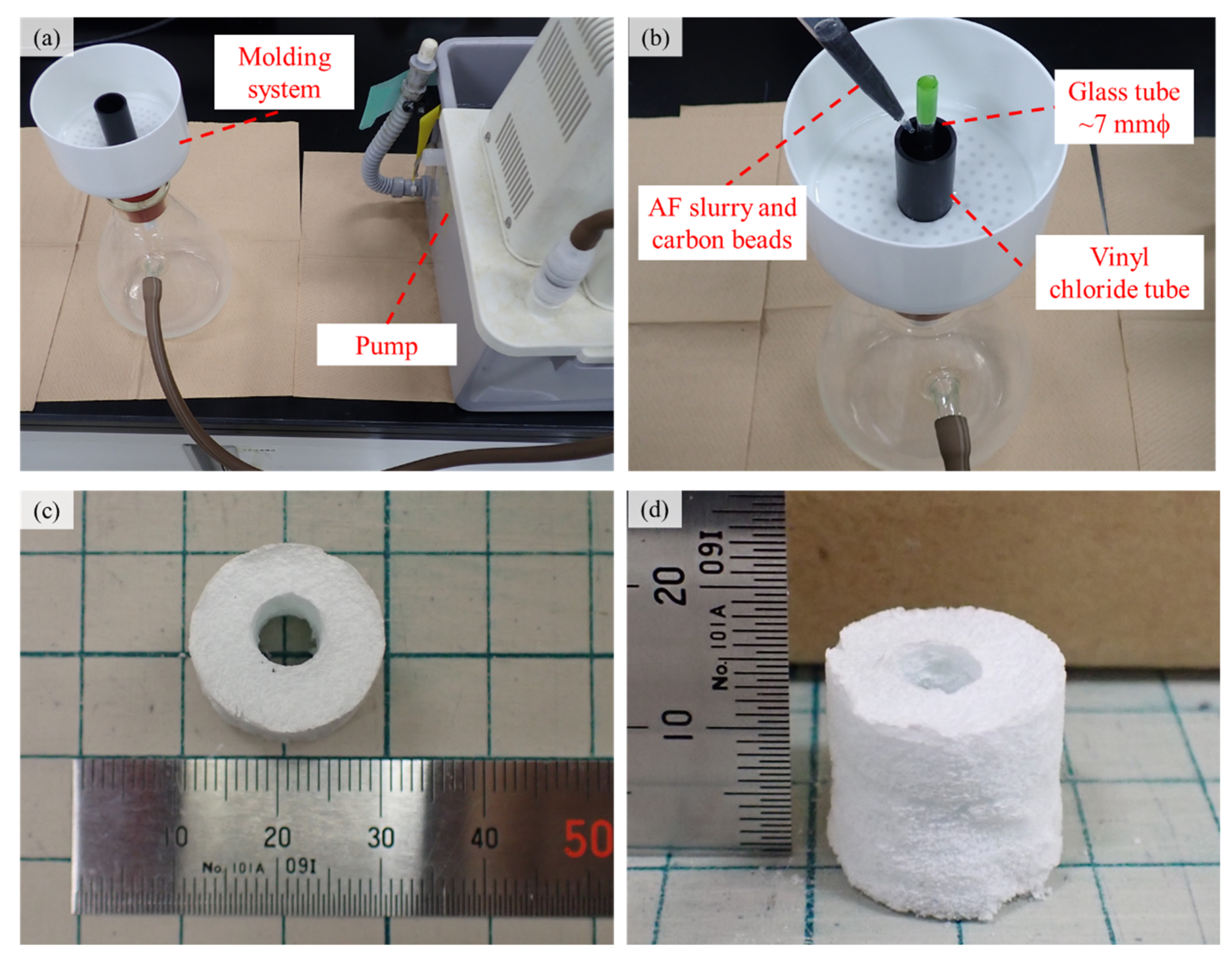
4.1. Fabrication and Characterization of AFSs
4.2. Primary Culture of RBMCs
4.3. The 3D Cell Culture Using a RFB
4.4. Cell Viability Assay
4.5. Harvesting the Tissue-Engineered Bone
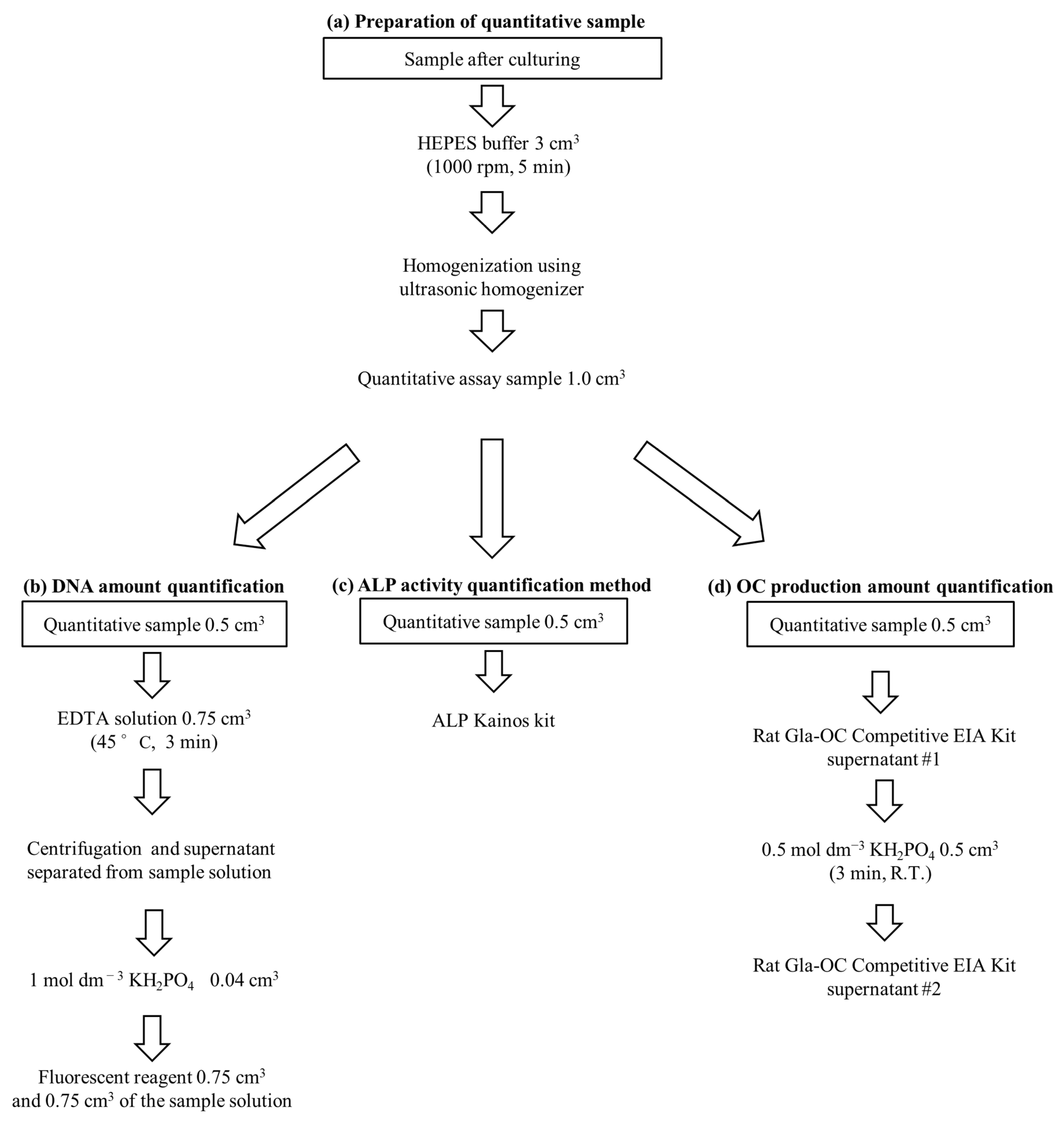
4.5.1. Determination of DNA Amount
4.5.2. Determination of the ALP Activity and OC Amount
4.6. Histological Evaluations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, A.R. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics 2002, 25, s571–s578. [Google Scholar] [CrossRef] [PubMed]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice: The evidence for their use. Bone Jt. J. 2013, 95, 583–597. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, H. Vertebroplasty by using HA Block for osteoporptic spine fracture. J. Lumbar Spine Disord. 2006, 12, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.S.; Cho, J.; Tay, F.; Heng, J.Y.; Ho, R.; Kazarian, S.G.; Williams, D.R.; Boccaccini, A.R.; Polak, J.M.; Mantalaris, A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials 2009, 30, 499–507. [Google Scholar] [CrossRef]
- Farhat, W.; Chatelain, F.; Marret, A.; Faivre, L.; Arakelian, L.; Cattan, P.; Fuchs, A. Trends in 3D bioprinting for esophageal tissue repair and reconstruction. Biomaterials 2021, 267, 120465. [Google Scholar] [CrossRef]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36, 17–28. [Google Scholar] [CrossRef]
- Ishaug-Riley, S.L.; Crane-Kruger, G.M.; Yaszemski, M.J.; Mikos, A.G. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 1998, 19, 1405–1412. [Google Scholar] [CrossRef]
- Mueller, S.M.; Mizuno, S.; Gerstenfeld, L.C.; Glowacki, J. Medium perfusion enhances osteogenesis by murine osteosarcoma cells in three-dimensional collagen sponges. J. Bone Miner. Res. 1999, 14, 2118–2126. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagata, K.; Yokota, T.; Honda, M.; Aizawa, M. Histological evaluations of apatite-fiber scaffold cultured with mesenchymal stem cells by implantation at rat subcutaneous tissue. Biomed. Mater. Eng. 2017, 28, 57–64. [Google Scholar] [CrossRef]
- Berrie, D.M.; Waters, R.C.; Montoya, C.; Chatel, A.; Vela, E.M. Development of a high-yield live-virus vaccine production platform using a novel fixed-bed bioreactor. Vaccine 2020, 38, 3639–3645. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Nishimura, I.; Sato, T.; Katayama, A.; Arano, T.; Ikada, Y.; Yoshinari, M. Dynamic cultivation with radial flow bioreactor enhances proliferation or differentiation of rat bone marrow cells by fibroblast growth factor or osteogenic differentiation factor. Regen. Ther. 2016, 5, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Hongo, T.; Kajikawa, M.; Ishida, S.; Ozawa, S.; Ohno, Y.; Sawada, J.; Umezawa, A.; Ishikawa, Y.; Kobayashi, T.; Honda, H. Three-dimensional high-density culture of HepG2 cells in a 5-ml radial-flow bioreactor for construction of artificial liver. J. Biosci. Bioeng. 2005, 99, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsu, S.H.; Huang, C.E.; Cheng, W.T.; Su, J.M. A scaffold-bioreactor system for a tissue-engineered trachea. Biomaterials 2009, 30, 4117–4126. [Google Scholar] [CrossRef]
- Saito, M.; Matsuura, T.; Masaki, T.; Maehashi, H.; Shimizu, K.; Hataba, Y.; Iwahori, T.; Suzuki, T.; Braet, F. Reconstruction of liver organoid using a bioreactor. World J. Gastroenterol. 2006, 12, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Shinoda, H.; Uchida, H.; Okada, I.; Fujimi, T.J.; Kanzawa, N.; Morisue, H.; Matsumoto, M.; Toyama, Y. In vitro biological evaluations of three-dimensional scaffold developed from single-crystal apatite fibres for tissue engineering of bone. Phosphorus Res. Bull. 2004, 17, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, M.; Porter, A.E.; Best, S.M.; Bonfield, W. Ultrastructural observation of single-crystal apatite fibers. Biomaterials 2005, 26, 3427–3433. [Google Scholar] [CrossRef]
- Zhuang, Z.; Aizawa, M. Protein adsorption on single-crystal hydroxyapatite particles with preferred orientation to a(b)- and c-axes. J. Mater. Sci. Mater. Med. 2013, 24, 1211–1216. [Google Scholar] [CrossRef]
- Honda, M.; Fujimi, T.J.; Izumi, S.; Izawa, K.; Aizawa, M.; Morisue, H.; Tsuchiya, T.; Kanzawa, N. Topographical analyses of proliferation and differentiation of osteoblasts in micro- and macropores of apatite-fiber scaffold. J. Biomed. Mater. Res. Part A 2010, 94A, 937–944. [Google Scholar] [CrossRef]
- Miura, M.; Fukasawa, J.; Yasutomi, Y.; Maehashi, H.; Matsuura, T.; Aizawa, M. Effect of Flow Rate of Medium in Radial-Flow Bioreactor on the Differentiation of Osteoblasts in Tissue-Engineered Bone Reconstructed Using an Apatite-Fiber Scaffold and Rat Bone Marrow Cells. Key Eng. Mater. 2012, 493, 878–883. [Google Scholar] [CrossRef]
- Miura, M.; Fukasawa, J.; Yasutomi, Y.; Maehashi, H.; Matsuura, T.; Aizawa, M. Reconstruction of Tissue-Engineered Bone Using an Apatite-Fiber Scaffold, Rat Bone Marrow Cells and Radial-Flow Bioreactor: Optimization of Flow Rate in Circulating Medium. Key Eng. Mater. 2013, 529, 397–401. [Google Scholar] [CrossRef]
- Tanaka, S.M. Mechanical Loading Promotes Calcification of Tissue-Engineered Bone In Vitro. J. Biomech. Sci. Eng. 2010, 5, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, M.; Howell, F.S.; Itatani, K.; Yokogawa, Y.; Nishizawa, K.; Toriyama, M.; Kameyama, T. Fabrication of Porous Ceramics with Well-Controlled Open Pores by Sintering of Fibrous Hydroxyapatite Particles. J. Ceram. Soc. Jpn. 2000, 108, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Nomoto, H.; Maehashi, H.; Shirai, M.; Nakamura, M.; Masaki, T.; Mezaki, Y.; Park, J.; Aizawa, M.; Ohkawa, K.; Yoshida, K.; et al. Bio-artificial bone formation model with a radial-flow bioreactor for implant therapy-comparison between two cell culture carriers: Porous hydroxyapatite and beta-tricalcium phosphate beads. Hum. Cell 2019, 32, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Holtorf, H.L.; Jansen, J.A.; Mikos, A.G. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J. Biomed. Mater. Res. Part A 2005, 72A, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Janssen, F.W.; Oostra, J.; Oorschot, A.; van Blitterswijk, C.A. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: In vivo bone formation showing proof of concept. Biomaterials 2006, 27, 315–323. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Van Blitterswijk, C.A.; Grote, J.J.; Kuijpers, W.; Daems, W.T.; de Groot, K. Macropore tissue ingrowth: A quantitative and qualitative study on hydroxyapatite ceramic. Biomaterials 1986, 7, 137–143. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Lu, J.X.; Flautre, B.; Anselme, K.; Hardouin, P.; Gallur, A.; Descamps, M.; Thierry, B. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J. Mater. Sci. Mater. Med. 1999, 10, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.; Niemeyer, P.; Salzmann, G.; Sudkamp, N.P.; Hube, R.; Klehm, J.; Menzel, M.; von Eisenhart-Rothe, R.; Bohner, M.; Gorz, L.; et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater. 2013, 9, 7490–7505. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.K.L.; Polak, S.J.; Wheeler, M.B.; Maki, A.J.; Clark, S.G.; Jamison, R.D.; Johnson, A.J.W. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials 2010, 31, 3552–3563. [Google Scholar] [CrossRef] [PubMed]
- Rustom, L.E.; Poellmann, M.J.; Johnson, A.J.W. Mineralization in micropores of calcium phosphate scaffolds. Acta Biomater. 2019, 83, 435–455. [Google Scholar] [CrossRef]
- Maniatopoulos, C.; Sodek, J.; Melcher, A.H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell. Tissue. Res. 1988, 254, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Safadi, F.F.; Barbe, M.F.; Abdelmagid, S.M.; Rico, M.C.; Aswad, R.A.; Litvin, J.; Popoff, S.N. Bone Structure, Development and Bone Biology; Humana Press: Totowa, NJ, USA, 2009; pp. 1–50. [Google Scholar]
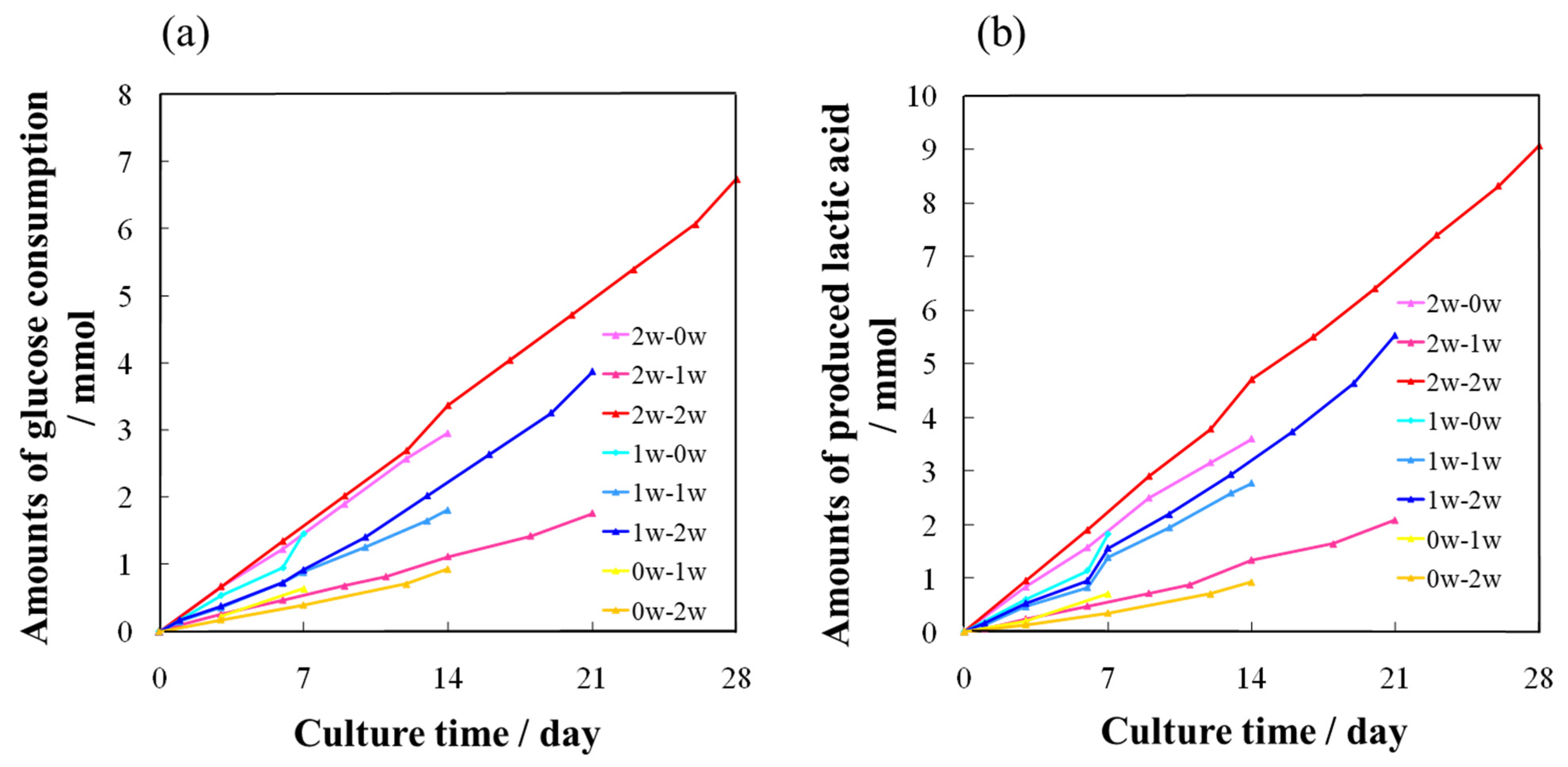

| 0 w Series | 1 w Series | 2 w Series |
|---|---|---|
| - | 1w-0w | 2w-0w |
| 0w-1w | 1w-1w | 2w-1w |
| 0w-2w | 1w-2w | 2w-2w |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Fukasawa, J.; Miura, M.; Lim, P.N.; Honda, M.; Matsuura, T.; Aizawa, M. Influence of Culture Period on Osteoblast Differentiation of Tissue-Engineered Bone Constructed by Apatite-Fiber Scaffolds Using Radial-Flow Bioreactor. Int. J. Mol. Sci. 2021, 22, 13080. https://doi.org/10.3390/ijms222313080
Suzuki K, Fukasawa J, Miura M, Lim PN, Honda M, Matsuura T, Aizawa M. Influence of Culture Period on Osteoblast Differentiation of Tissue-Engineered Bone Constructed by Apatite-Fiber Scaffolds Using Radial-Flow Bioreactor. International Journal of Molecular Sciences. 2021; 22(23):13080. https://doi.org/10.3390/ijms222313080
Chicago/Turabian StyleSuzuki, Kitaru, Jun Fukasawa, Maiko Miura, Poon Nian Lim, Michiyo Honda, Tomokazu Matsuura, and Mamoru Aizawa. 2021. "Influence of Culture Period on Osteoblast Differentiation of Tissue-Engineered Bone Constructed by Apatite-Fiber Scaffolds Using Radial-Flow Bioreactor" International Journal of Molecular Sciences 22, no. 23: 13080. https://doi.org/10.3390/ijms222313080
APA StyleSuzuki, K., Fukasawa, J., Miura, M., Lim, P. N., Honda, M., Matsuura, T., & Aizawa, M. (2021). Influence of Culture Period on Osteoblast Differentiation of Tissue-Engineered Bone Constructed by Apatite-Fiber Scaffolds Using Radial-Flow Bioreactor. International Journal of Molecular Sciences, 22(23), 13080. https://doi.org/10.3390/ijms222313080