Methylmercury plus Ethanol Exposure: How Much Does This Combination Affect Emotionality?
Abstract
1. Introduction
2. Toxicokinetic Interaction
3. Behavioral Alterations
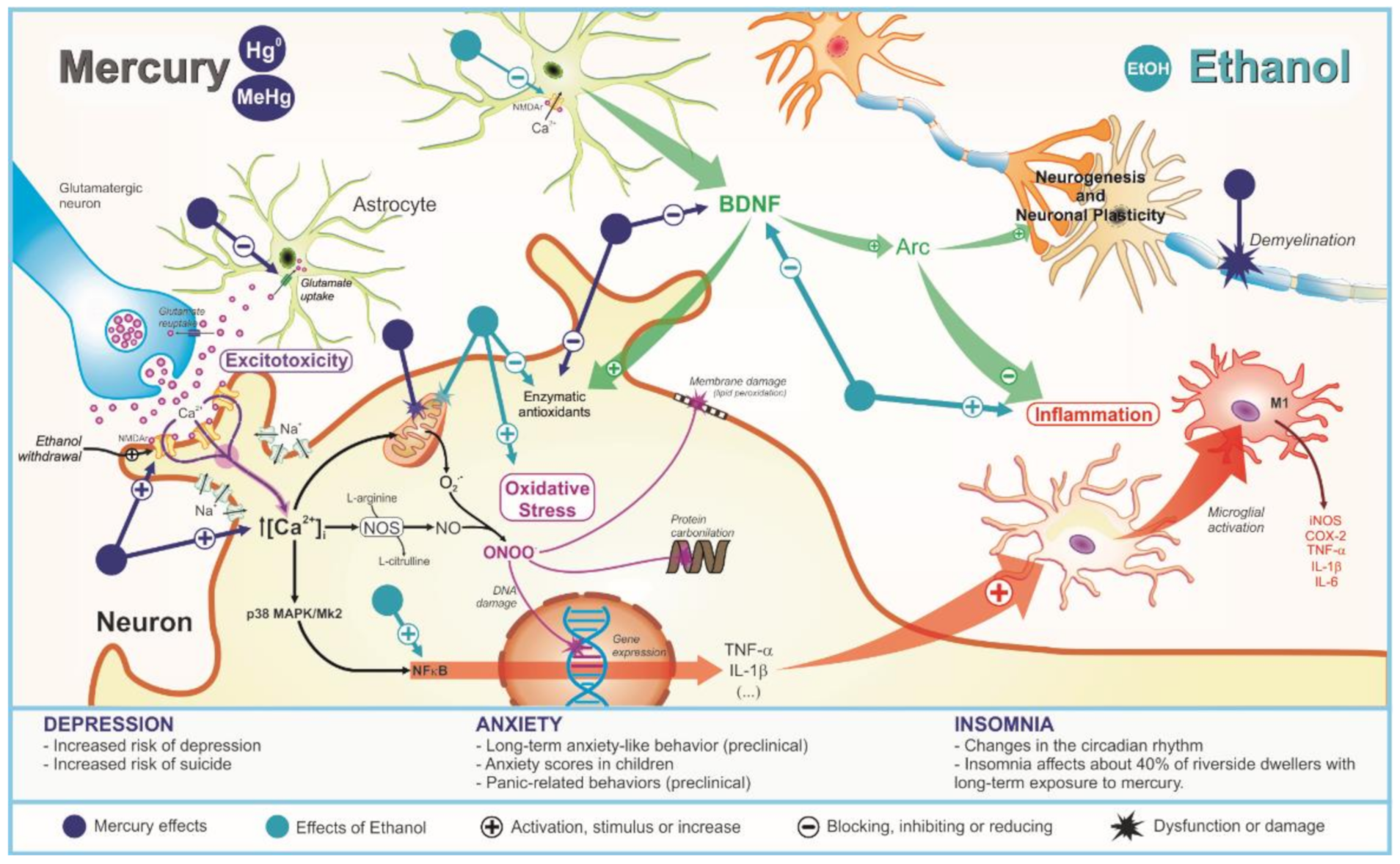
3.1. Depression
3.2. Anxiety
3.3. Insomnia
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belém-Filho, I.J.A.; Ribera, P.C.; Nascimento, A.L.; Gomes, A.R.Q.; Lima, R.R.; Crespo-Lopez, M.E.; Maia, C.S.F. Low doses of methylmercury intoxication solely or associated to ethanol binge drinking induce psychiatric-like disorders in adolescent female rats. Environ. Toxicol. Pharmacol. 2018, 60, 184–194. [Google Scholar] [CrossRef]
- Malm, O. Gold Mining as a Source of Mercury Exposure in the Brazilian Amazon. Environ. Res. 1998, 77, 73–78. [Google Scholar] [CrossRef]
- Nevado, J.J.B.; Martín-Doimeadios, R.C.R.; Bernardo, F.J.G.; Moreno, M.J.; Herculano, A.M.; Nascimento, J.L.M.; Crespo-López, M.E. Mercury in the Tapajós River basin, Brazilian Amazon: A review. Environ. Int. 2010, 36, 593–608. [Google Scholar] [CrossRef]
- Vianna, A.S.; Matos, E.P.; Jesus, I.M.; Asmus, C.I.R.F.; Câmara, V.M. Human exposure to mercury and its hematological effects: A systematic review. Cad. Saúde Pública 2019, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; Martin-Doimeadios, R.D.C.R.; Jiménez-Moreno, M.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Paraense, R.; Crespo-Lopez, M.E. Assessing mercury intoxication in isolated/remote populations: Increased S100B mRNA in blood in exposed riverine inhabitants of the Amazon. Neurotoxicology 2018, 68, 151–158. [Google Scholar] [CrossRef]
- Ye, B.J.; Kim, B.G.; Jeon, M.J.; Kim, S.Y.; Kim, H.C.; Jang, T.W.; Chae, H.J.; Choi, W.J.; Ha, M.N.; Hong, Y.S. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef]
- Farina, M.; Rocha, J.B.T.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sanfeliu, C.; Sebastià, J.; Cristòfol, R.; Rodríguez-Farré, E. Neurotoxicity of organomercurial compounds. Neurotox. Res. 2003, 5, 283–305. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Santos, A.A.; Appel Hort, M.; Culbreth, M.; López-Granero, C.; Farina, M.; Rocha, J.B.T.; Aschner, M. Methylmercury and brain development: A review of recent literature. J. Trace Elem. Med. Biol. 2016, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sacramento, L.; Arrifano, G.P.F.; Lopes-Araújo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Crespo-Lopez, M.E. Human neurotoxicity of mercury in the Amazon: A scoping review with insights and critical considerations. Ecotoxicol. Environ. Saf. 2021, 208, 111686. [Google Scholar] [CrossRef]
- Johansson, C.; Castoldi, A.F.; Onishchenko, N.; Manzo, L.; Vahter, M.; Ceccatelli, S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox. Res. 2007, 11, 241–260. [Google Scholar] [CrossRef]
- Hassan, S.A.; Moussa, E.A.; Abbott, L.C. The effect of methylmercury exposure on early central nervous system development in the zebrafish (Danio rerio) embryo. J. Appl. Toxicol. 2011, 32, 707–713. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Nogara, P.A.; Ardisson-Araújo, D.M.P.; Aschner, M.; Rocha, J.B.T.; Dórea, J.G. Neurodevelopmental Effects of Mercury. Adv. Neurotoxicol. 2018, 2, 27–86. [Google Scholar] [CrossRef]
- Novo, J.P.; Martins, B.; Raposo, R.S.; Pereira, F.C.; Oriá, R.B.; Malva, J.O.; Fontes-Ribeiro, C. Cellular and Molecular Mechanisms Mediating Methylmercury Neurotoxicity and Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 3101. [Google Scholar] [CrossRef]
- Aschner, M.; Yao, C.P.; Allen, J.W.; Tan, K.H. Methylmercury alters glutamate transport in astrocytes. Neurochem. Int. 2000, 37, 199–206. [Google Scholar] [CrossRef]
- Aschner, M.; Du, Y.-L.; Gannon, M.; Kimelberg, H.K. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Res. 1993, 602, 181–186. [Google Scholar] [CrossRef]
- Allen, J.W.; Mutkus, L.A.; Aschner, M. Methylmercury-mediated inhibition of 3H-d-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001, 902, 92–100. [Google Scholar] [CrossRef]
- Lafon-Cazal, M.; Pietri, S.; Culcasi, M.; Bockaert, J. NMDA-dependent superoxide production and neurotoxicity. Nature 1993, 364, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Shanker, G.; Allen, J.W.; Mutkus, L.A.; Aschner, M. Methylmercury inhibits cysteine uptake in cultured primary astrocytes, but not in neurons. Brain Res. 2001, 914, 159–165. [Google Scholar] [CrossRef]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury Toxicity and Neurodegenerative Effects. Rev. Environ. Contam. Toxicol. 2013, 229, 1–18. [Google Scholar] [CrossRef]
- Malqui, H.; Anarghou, H.; Ouardi, F.Z.; Ouasmi, N.; Najimi, M.; Chigr, F. Continuous Exposure to Inorganic Mercury Affects Neurobehavioral and Physiological Parameters in Mice. J. Mol. Neurosci. 2018, 66, 291–305. [Google Scholar] [CrossRef]
- Fernandes, L.M.P.; Lopes, K.S.; Santana, L.N.S.; Fontes-Júnior, E.A.; Ribeiro, C.H.M.A.; Silva, M.C.F.; Maia, C.S.F. Repeated Cycles of Binge-Like Ethanol Intake in Adolescent Female Rats Induce Motor Function Impairment and Oxidative Damage in Motor Cortex and Liver, but Not in Blood. Oxidative Med. Cell. Longev. 2018, 2018, 3467531. [Google Scholar] [CrossRef]
- Fernandes, L.M.P.; Cartágenes, S.C.; Barros, M.A.; Carvalheiro, T.C.V.S.; Castro, N.C.F.; Schamne, M.G.; Maia, C.S.F. Repeated cycles of binge-like ethanol exposure induce immediate and delayed neurobehavioral changes and hippocampal dysfunction in adolescent female rats. Behav. Brain Res. 2018, 350, 99–108. [Google Scholar] [CrossRef]
- Ahmed, S.P.; Bittencourt-Hewitt, A.; Sebastian, C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 2015, 15, 11–25. [Google Scholar] [CrossRef]
- McMakin, D.L.; Alfano, C.A. Sleep and anxiety in late childhood and early adolescence. Curr. Opin. Psychiatry 2015, 28, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Costa Junior, J.M.F.; Lima, A.A.S.; Rodrigues Junior, D.; Khoury, E.D.T.; Souza, G.S.; Silveira, L.C.L.; Pinheiro, M.C.N. Emotional and motor symptoms in riverside dwellers exposed to mercury in the Amazon. Rev. Bras. Epidemiol. 2017, 20, 212–224. [Google Scholar] [CrossRef][Green Version]
- Maia, C.S.F.; Lucena, G.M.; Corrêa, P.B.; Serra, R.B.; Matos, R.W.; Menezes, F.C.; Santos, S.N.; Sousa, J.B.; Costa, E.T.; Ferreira, V.M. Interference of ethanol and methylmercury in the developing central nervous system. Neurotoxicology 2009, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Coldwell, B.B.; Platonow, N. The effect of methylmercuric acetate on the rate of disappearance of ethanol from the blood of swine. Toxicol. Appl. Pharmacol. 1969, 14, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.K.; Wong, M.H.; Chan, H.M.; Lo, S.C.L. Chronic exposure of adult rats to low doses of methylmercury induced a state of metabolic deficit in the somatosensory cortex. J. Proteome Res. 2013, 12, 5233–5245. [Google Scholar] [CrossRef]
- Grandjean, P.; Weihe, P. Neurobehavioral effects of intrauterine mercury exposure: Potential sources of bias. Environ. Res. 1993, 61, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, H.; Arakaki, M.; Akagi, H.; Murao, H.; Hirayama, K.; Smolensky, M.H. Effects of ethanol on methyl mercury toxicity in rats. J. Toxicol. Environ. Health 1986, 18, 595–605. [Google Scholar] [CrossRef]
- Hwang, G.W. Role of intracellular defense factors against methylmercury toxicity. Biol. Pharm. Bull. 2012, 35, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.N.; Pinheiro, A.M.; Belém-Filho, I.J.A.; Fernandes, L.M.P.; Cartágenes, S.C.; Ribera, P.C.; Fontes-Júnior, E.A.; Crespo-Lopez, M.E.; Monteiro, M.C.; Lima, M.O.; et al. Unravelling motor behaviour hallmarks in intoxicated adolescents: Methylmercury subtoxic-dose exposure and binge ethanol intake paradigm in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 21937–21948. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.V.R.; Soares, P.M.G.; Castro Brito, G.A.; de Souza, M.H.L.P. Immunohistochemical approach reveals localization of cystathionine-γ-lyase and cystathionine-β-synthetase in ethanol-induced gastric mucosa damage in mice. Arq. Gastroenterol. 2013, 50, 157–160. [Google Scholar] [CrossRef]
- Turner, C.J.; Bhatnagar, M.K.; Speisky, H. Effect of subchronic administration of ethanol and methylmercury in combination on the tissue distribution of mercury in rats. Can. J. Physiol. Pharm. 1990, 68, 1558–1562. [Google Scholar] [CrossRef]
- Yoshida, E.; Toyama, T.; Shinkai, Y.; Sawa, T.; Akaike, T.; Kumagai, Y. Detoxification of methylmercury by hydrogen sulfide-producing enzyme in mammalian cells. Chem. Res. Toxicol. 2011, 24, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, N.; Tamm, C.; Vahter, M.; Hökfelt, T.; Johnson, J.A.; Johnson, D.A.; Ceccatelli, S. Developmental Exposure to Methylmercury Alters Learning and Induces Depression-like Behavior in Male Mice. Toxicol. Sci. 2007, 97, 428–437. [Google Scholar] [CrossRef]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castrén, E.; Ceccatelli, S. Long-lasting depression-like behavior, and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J. Neurochem. 2008, 106, 1378–1387. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Vos, T. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Alonso, J.; Angermeyer, M.C.; Bernert, S.; Bruffaerts, R.; Brugha, T.S.; Bryson, H.; de Girolamo, G.; Graaf, R.; Demyttenaere, K.; Gasquet, I.; et al. Prevalence of mental disorders in Europe: Prevalence of depression in Brazil results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr. Scand. Suppl. 2004, 420, 21–27. [Google Scholar] [CrossRef]
- Silva, M.J.; Meckinze, K.; Harpham, H.; Hutly, S.R.A. Social capital and mental illness: A systematic review. J. Epidemiol. Community Health 2005, 59, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Breslau, J.; Javaras, K.N.; Blacker, D.; Murphy, J.M.; Normand, S.-L.T. Differential Item Functioning Between Ethnic Groups in the Epidemiological Assessment of Depression. J. Nerv. Ment. Dis. 2008, 196, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, S.; Rehm, J.; Wittchen, H. The economic costs of mental disorders. EMBO Rep. 2016, 17, 1245–1249. [Google Scholar] [CrossRef]
- Maia, C.S.F.; Ferreira, V.M.M.; Diniz, J.S.V.; Carneiro, F.P.; Sousa, J.B.; Costa, E.T.; Tomaz, C. Inhibitory avoidance acquisition in adult rats exposed to a combination of ethanol and methylmercury during central nervous system development. Behav. Brain Res. 2010, 211, 191–197. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Christinal, J.; Sumathi, T. Effect of Bacopa monniera Extract on Methylmercury-Induced Behavioral and Histopathological Changes in Rats. Biol. Trace Elem. Res. 2013, 155, 56–64. [Google Scholar] [CrossRef]
- Nabi, S.; Ara, A.; Rizvi, S.J. Effect of methylmercury on depression like behavior in rats: A study mitigated by exogenous vitamins. Iran J. Pharmacol. Ther. 2012, 11, 1–5. [Google Scholar]
- Castoldi, A.F.; Coccini, T.; Ceccatelli, S.; Manzo, L. Neurotoxicity and molecular effects of methylmercury. Brain Res. Bull. 2001, 55, 197–203. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Baraibar, A.M.; Albiñana, E.; Velasco, P.; Solís, J.M.; Hernández-Guijo, J.M. Methylmercury reduces synaptic transmission and neuronal excitability in rat hippocampal slices. Pflüg. Arch.—Eur. J. Physiol. 2018, 470, 1221–1230. [Google Scholar] [CrossRef]
- Reynolds, J.N.; Racz, W.J. Effects of methylmercury on the spontaneous and potassium-evoked release of endogenous amino acids from mouse cerebellar slices. Can. J. Physiol. Pharmacol. 1987, 65, 791–798. [Google Scholar] [CrossRef]
- Antunes Dos Santos, A.; Ferrer, B.; Marques Gonçalves, F.; Tsatsakis, A.M.; Renieri, E.A.; Skalny, A.V.; Farina, M.; Rocha, J.B.T.; Aschner, M. Oxidative Stress in Methylmercury-Induced Cell Toxicity. Toxics 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System. Antioxidants 2020, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.; Gonçalves, F.M.; Gonçalves, C.L.; Dos Santos, A.A.; Rocha, J.B.T.; Farina, M.; Skalny, A.; Tsatsakis, A.; Bowman, A.B.; Aschner, M. Post-translational modifications in MeHg-induced neurotoxicity. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2068–2081. [Google Scholar] [CrossRef]
- Yuan, Y.; Atchison, W.D. Multiple Sources of Ca2+ Contribute to Methylmercury-Induced Increased Frequency of Spontaneous Inhibitory Synaptic Responses in Cerebellar Slices of Rat. Toxicol. Sci. 2016, 150, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Reardon, A.M.; Bhat, H.K. Methylmercury neurotoxicity: Role of oxidative stress. Toxicol. Environ. Chem. 2007, 89, 535–554. [Google Scholar] [CrossRef]
- Ma, L.; Bi, K.D.; Fan, Y.M.; Jiang, Z.Y.; Zhang, X.Y.; Zhang, J.W.; Zhao, J.; Jiang, F.L.; Dong, J.X. In vitro modulation of mercury-induced rat liver mitochondria dysfunction. Toxicol. Res. 2018, 7, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.; Ferrer, B.; Gonçalves, F.M.; Tsatsakis, A.; Renieri, E.; Skalny, A.; Aschner, M. Oxidative Stress in Methylmercury-Induced Cell Toxicity. Toxics 2018, 6, 47. [Google Scholar] [CrossRef]
- Glaser, V.; Nazari, E.M.; Müller, Y.M.; Feksa, L.; Wannmacher, C.M.; Rocha, J.B.; de Bem, A.F.; Farina, M.; Latini, A. Effects of inorganic selenium administration in methylmercury-induced neurotoxicity in mouse cerebral cortex. Int. J. Dev. Neurosci. 2010, 28, 631–637. [Google Scholar] [CrossRef]
- Giménez-Palomo, A.; Dodd, S.; Anmella, G.; Carvalho, A.F.; Scaini, G.; Quevedo, J.; Pacchiarotti, I.; Vieta, E.; Berk, M. The Role of Mitochondria in Mood Disorders: From Physiology to Pathophysiology and to Treatment. Front. Psychiatry 2021, 12, 546801. [Google Scholar] [CrossRef] [PubMed]
- Zavvarian, M.M. Impacts of Mercury Poisoning on Brain-Derived Neurotrophic Factor: A Review of Recent Literature. J. Undergrad. Life Sci. 2017, 11, 47–50. [Google Scholar]
- Sakaue, M.; Maki, T.; Kaneko, T.; Hemmi, N.; Sekiguchi, H.; Horio, T.; Yamamoto, M. Potentiation of Methylmercury-Induced Death in Rat Cerebellar Granular Neurons Occurs by Further Decrease of Total Intracellular GSH with BDNF via TrkB in Vitro. Biol. Pharm. Bull. 2016, 39, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Kuczewski, N.; Porcher, C.; Gaiarsa, J.L. Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. Eur. J. Neurosci. 2010, 32, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010, 70, 271–288. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived Neurotrophic Factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Sumathi, T. Extenuation of in utero toxic effects of MeHg in the developing neurons by Fisetin via modulating the expression of synaptic transmission and plasticity regulators in hippocampus of the rat offspring. Chem. Biol. Interact. 2019, 305, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, S.; Bose, R.; Edoff, K.; Onishchenko, N.; Spulber, S. Long-lasting neurotoxic effects of exposure to methylmercury during development. J. Intern. Med. 2013, 273, 490–497. [Google Scholar] [CrossRef]
- Andersson, H.; Lindqvist, E.; Olson, L. Downregulation of brain-derived neurotrophic factor mRNA in adult rat brain after acute administration of methylmercury. Mol. Chem. Neuropathol. 1997, 31, 225–233. [Google Scholar] [CrossRef]
- Karpova, N.N.; Lindholm, J.S.O.; Kulesskaya, N.; Onishchenko, N.; Vahter, M.; Popova, D.; Castrén, E. TrkB overexpression in mice buffers against memory deficits and depression-like behavior but not all anxiety- and stress-related symptoms induced by developmental exposure to methylmercury. Front. Behav. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsuda, T.; Inoue, S.; Takao, S.; Harada, M. Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata, Japan. Environ. Int. 2011, 37, 907–913. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsuda, T.; Takao, S.; Harada, M. Long-term exposure to methylmercury and neurologic signs in Minamata and neighboring communities. Epidemiology 2008, 19, 3–9. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). Alcohol Facts and Statistics. 2021. Available online: https://www.niaaa.nih.gov/sites/default/files/publications/NIAAA_Alcohol_Facts_and_Stats_5.pdf (accessed on 10 September 2021).
- Boden, J.M.; Fergusson, D.M. Alcohol and depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Poznański, P.; Łazarczyk, M.; Gorzałczyński, M.; Skiba, D.; Wolińska, R.; Bujalska-Zadrożny, M.; Lutfy, K.; Sadowski, B.; Sacharczuk, M. The Influence of Cross-Fostering on Alcohol Consumption and Depressive-Like Behaviors in HA and LA Mice: The Role of the Endogenous Opioid System. Brain Sci. 2021, 11, 622. [Google Scholar] [CrossRef]
- Fernandes, L.M.P.; de Andrade, E.F.; Monteiro, M.C.; Cartágenes, S.C.; Lima, R.R.; Prediger, R.D.; Maia, C.S.F. Chapter 20—Ethanol: Neurotoxicity and Brain Disorders. In Addictive Substances and Neurological Disease, 1st ed.; Watson, R.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 201–215. [Google Scholar] [CrossRef]
- Jung, M.E.; Metzger, D.B. Alcohol withdrawal and brain injuries: Beyond classical mechanisms. Molecules 2010, 15, 4984–5011. [Google Scholar] [CrossRef]
- Maia, C.S.F.; Ferreira, V.M.M.; Kahwage, R.L.; Amaral, M.N.; Serra, R.B.; Santos, S.N.; Diniz, C.W.P. Adult brain nitrergic activity after concomitant prenatal exposure to ethanol and methyl mercury. Acta Histochem. 2010, 112, 583–591. [Google Scholar] [CrossRef]
- Mah, L.; Szabuniewicz, C.; Fiocco, A.J. Can anxiety damage the brain? Curr. Opin. Psychiatry 2016, 29, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Newland, M.C.; Reed, M.N.; Rasmussen, E. A hypothesis about how early developmental methylmercury exposure disrupts behavior in adulthood. Behav. Process. 2015, 114, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Heimfarth, L.; Delgado, J.; Mignori, M.R.; Gelain, D.P.; Moreira, J.C.F.; Pessoa-Pureur, R. Developmental neurotoxicity of the hippocampus following in utero exposure to methylmercury: Impairment in cell signaling. Arch. Toxicol. 2017, 92, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Carratù, M.R.; Borracci, P.; Coluccia, A.; Giustino, A.; Renna, G.; Tomasini, M.C.; Ferraro, L. Acute exposure to methylmercury at two developmental windows: Focus on neurobehavioral and neurochemical effects in rat offspring. Neuroscience 2016, 141, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Lewis, R.; Curtis, J.T. Chronic exposure to inorganic mercury alters stress responses in male prairie voles (Microtus ochrogaster). Horm Behav. 2019, 109, 53–55. [Google Scholar] [CrossRef]
- Patel, N.B.; Xu, Y.; McCandless, L.C.; Chen, A.; Yolton, K.; Braun, J.; Lanphear, B.P. Very low-level prenatal mercury exposure and behaviors in children: The HOME Study. Environ. Health 2019, 18, 4. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Pereira, M.C.; Santana, L.N.S.; Fernandes, R.M.; Teixeira, F.B.; Oliveira, G.B.; Maia, C.S.F. Chronic ethanol exposure during adolescence through early adulthood in female rats induces emotional and memory deficits associated with morphological and molecular alterations in hippocampus. J. Psychopharmacol. 2015, 29, 712–724. [Google Scholar] [CrossRef]
- Brancato, A.; Plescia, F.; Lavanco, G.; Cavallaro, A.; Cannizzaro, C. Continuous and Intermittent Alcohol Free-Choice from Pre-gestational Time to Lactation: Focus on Drinking Trajectories and Maternal Behavior. Front. Behav. Neurosci. 2016, 10, 31. [Google Scholar] [CrossRef]
- Marco, E.M.; Peñasco, S.; Hernández, M.D.; Gil, A.; Borcel, E.; Moya, M.; Giné, E.; López-Moreno, J.A.; Guerri, C.; López-Gallardo, M.; et al. Long-Term Effects of Intermittent Adolescent Alcohol Exposure in Male and Female Rats. Front. Behav. Neurosci. 2017, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Suarez, A.; D’Addario, C.; Pautassi, R.M.; Fabio, M.C. Transient serotonin depletion at adolescence, but not at early infancy, reduced subsequent anxiety-like behavior and alcohol intake in female mice. Psychopharmacology 2021, 238, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Plescia, F.; Brancato, A.; Marino, R.A.; Vita, C.; Navarra, M.; Cannizzaro, C. Effect of Acetaldehyde Intoxication and Withdrawal on NPY Expression: Focus on Endocannabinoidergic System Involvement. Front. Psychiatry 2014, 5, 138. [Google Scholar] [CrossRef][Green Version]
- Cacace, S.; Plescia, F.; La Barbera, M.; Cannizzaro, C. Evaluation of chronic alcohol self-administration by a 3-bottle choice paradigm in adult male rats. Effects on behavioural reactivity, spatial learning and reference memory. Behav. Brain Res. 2011, 219, 213–220. [Google Scholar] [CrossRef][Green Version]
- Chauhan, V.; Chauhan, A. Effects of methylmercury and alcohol exposure in Drosophila melanogaster: Potential risks in neurodevelopmental disorders. Int. J. Dev. Neurosci. 2016, 51, 36–41. [Google Scholar] [CrossRef]
- Fuller, P.M.; Gooley, J.J.; Saper, C.B. Neurobiology of the sleep-wake cycle: Sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythms 2006, 21, 482–493. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Falup-Pecurariu, C.; Diaconu, S.; ȚÎNȚ, D.; Falup-Pecurariu, O. Neurobiology of sleep (Review). Exp. Ther. Med. 2021, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; Alfano, C.A. Sleep and emotion regulation: An organizing, integrative review. Sleep Med. Rev. 2017, 31, 6–16. [Google Scholar] [CrossRef]
- Allada, R.; White, N.E.; So, W.V.; Hall, J.C.; Rosbash, M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 1998, 93, 791–804. [Google Scholar] [CrossRef]
- Murphy, K.; Delanty, N. Sleep deprivation. A clinical perspective. Sleep Biol. Rhythms 2007, 5, 2–14. [Google Scholar] [CrossRef]
- Gruber, R.; Cassoff, J. The interplay between sleep and emotion regulation: Conceptual framework empirical evidence and future directions. Curr. Psychiatry Rep. 2014, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Rivera-Bermudez, M.A.; Gerdin, M.J.; Masana, M.I. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, d1093–d1108. [Google Scholar] [CrossRef]
- Yamada, H.; Yatsushiro, S.; Ishio, S.; Hayashi, M.; Nishi, T.; Yamamoto, A.; Futai, M.; Yamaguchi, A.; Moriyama, Y. Metabotropic glutamate receptors negatively regulate melatonin synthesis in rat pinealocytes. J. Neurosci. 1998, 18, 2056–2062. [Google Scholar] [CrossRef]
- Basu, N.; Scheuhammer, A.M.; Rouvinen-Watt, K.; Grochowina, N.; Klenavic, K.; Evans, R.D.; Chan, H.M. Methylmercury impairs components of the cholinergic system in captive mink (Mustela vison). Toxicol. Sci. 2006, 91, 202–209. [Google Scholar] [CrossRef]
- Qiu, M.H.; Vetrivelan, R.; Fuller, P.M.; Lu, J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur. J. Neurosci. 2010, 31, 499–507. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Christensen, M.-K.; Poulsen, E.H. Mercury in the Rat Hypothalamic Arcuate Nucleus and Median Eminence after Mercury Vapor Exposure. Exp. Mol. Pathol. 1993, 58, 205–214. [Google Scholar] [CrossRef]
- Møller-Madsen, B.; Danscher, G. Localization of mercury in CNS of the rat. Toxicol. Appl. Pharmacol. 1991, 108, 457–473. [Google Scholar] [CrossRef]
- Lach, H.; Srebro, Z.; Dziubek, K.; Krawczyk, S.; Szaroma, W. The influence of mercury and lead compounds on the circadian rhythm of cytoplasmic RNA in hypothalamic neurons of mice. Folia Histochem. Cytochem. 1983, 21, 231–237. [Google Scholar]
- Falnoga, I.; Tusek-Znidaric, M.; Horvat, M.; Stegnar, P. Mercury, selenium, and cadmium in human autopsy samples from Idrija residents and mercury mine workers. Environ. Res. 2000, 84, 211–218. [Google Scholar] [CrossRef]
- Falnoga, I.; Kobal, A.B.; Stibilj, V.; Horvat, M. Selenoprotein P in subjects exposed to mercury and other stress situations such as physical load or metal chelation treatment. Biol. Trace Elem. Res. 2002, 89, 25–33. [Google Scholar] [CrossRef]
- Rodenbek e Hajak Rodenbeck, A.; Hajak, G. Neuroendocrine dysregulation in primary insomnia. Rev. Neurol. 2001, 157, S57–S61. [Google Scholar]
- Nylander, M. Mercury in pituitary glands of dentists. Lancet 1986, 327, 442. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, O.; Møller-Madsen, B.; Danscher, G. Intracellular accumulation of mercury in the anterior pituitary of rats exposed to mercuric chloride. Exp. Mol. Pathol. 1985, 42, 278–286. [Google Scholar] [CrossRef]
- Kabuto, M. Acute Endocrine Effects of a Single Administration of Methylmercury Chloride (MMC) in Rats. Endocrinol. Japon. 1986, 33, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.L.; Thorlacius-Ussing, O. Ultrastructural localization of mercury in adrenals from rats exposed to methyl mercury. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1987, 52, 529–538. [Google Scholar] [CrossRef]
- Ekman, A.C.; Leppäluoto, J.; Huttunen, P.; Aranko, K.; Vakkuri, O. Ethanol inhibits melatonin secretion in healthy volunteers in a dose-dependent randomized double blind cross-over study. J. Clin. Endocrinol. Metab. 1993, 77, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.; Amaral, F.G.; Madrigrano, T.C.; Scialfa, J.H.; Bordin, S.; Afeche, S.C.; Cipolla-Neto, J. Ethanol consumption and pineal melatonin daily profile in rats. Addict. Biol. 2011, 16, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.; Gastfriend, D.R.; Coyle, J.T. The glutamatergic basis of human alcoholism. Am. J. Psychiatry 1995, 152, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, K.P.; Salinas, A.G.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238. [Google Scholar] [CrossRef]
- Haddad, J.J. Alcoholism and neuro-immune-endocrine interactions: Physiochemical aspects. Biochem. Biophys. Res. Commun. 2004, 323, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Torres, J.M.; Miranda, M.T.; Ruiz, E.; Ortega, E. Effects of acute alcohol intoxication on pituitary-gonadal axis hormones, pituitary-adrenal axis hormones, beta-endorphin and prolactin in human adults of both sexes. Alcohol. Alcohol. 2002, 37, 169–173. [Google Scholar] [CrossRef]
- Cobb, C.F.; Thiel, D.H.V. Mechanism of Ethanol-Induced Adrenal Stimulation. Alcoholi. Clin. Exp. Res. 1982, 6, 202–206. [Google Scholar] [CrossRef]
- Adinoff, B.; Risher-Flowers, D.; De Jong, J.; Ravitz, B.; Bone, G.H.; Nutt, D.J.; Roehrich, L.; Martin, P.R.; Linnoila, M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am. J. Psychiatry 1991, 148, 1023–1025. [Google Scholar] [CrossRef]
- Čupić, Ž.; Stanojević, A.; Marković, V.M.; Kolar-Anić, L.; Terenius, L.; Vukojević, V. The HPA axis and ethanol: A synthesis of mathematical modelling and experimental observations. Addict. Biol. 2016, 22, 1486–1500. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luz, D.A.; Cartágenes, S.d.C.; da Silveira, C.C.S.d.M.; Pinheiro, B.G.; Ferraro, K.M.M.M.; Fernandes, L.d.M.P.; Fontes-Júnior, E.A.; Maia, C.d.S.F. Methylmercury plus Ethanol Exposure: How Much Does This Combination Affect Emotionality? Int. J. Mol. Sci. 2021, 22, 13131. https://doi.org/10.3390/ijms222313131
Luz DA, Cartágenes SdC, da Silveira CCSdM, Pinheiro BG, Ferraro KMMM, Fernandes LdMP, Fontes-Júnior EA, Maia CdSF. Methylmercury plus Ethanol Exposure: How Much Does This Combination Affect Emotionality? International Journal of Molecular Sciences. 2021; 22(23):13131. https://doi.org/10.3390/ijms222313131
Chicago/Turabian StyleLuz, Diandra Araújo, Sabrina de Carvalho Cartágenes, Cinthia Cristina Sousa de Menezes da Silveira, Bruno Gonçalves Pinheiro, Kissila Márvia Matias Machado Ferraro, Luanna de Melo Pereira Fernandes, Enéas Andrade Fontes-Júnior, and Cristiane do Socorro Ferraz Maia. 2021. "Methylmercury plus Ethanol Exposure: How Much Does This Combination Affect Emotionality?" International Journal of Molecular Sciences 22, no. 23: 13131. https://doi.org/10.3390/ijms222313131
APA StyleLuz, D. A., Cartágenes, S. d. C., da Silveira, C. C. S. d. M., Pinheiro, B. G., Ferraro, K. M. M. M., Fernandes, L. d. M. P., Fontes-Júnior, E. A., & Maia, C. d. S. F. (2021). Methylmercury plus Ethanol Exposure: How Much Does This Combination Affect Emotionality? International Journal of Molecular Sciences, 22(23), 13131. https://doi.org/10.3390/ijms222313131







