CsAGA1 and CsAGA2 Mediate RFO Hydrolysis in Partially Distinct Manner in Cucumber Fruits
Abstract
:1. Introduction
2. Results
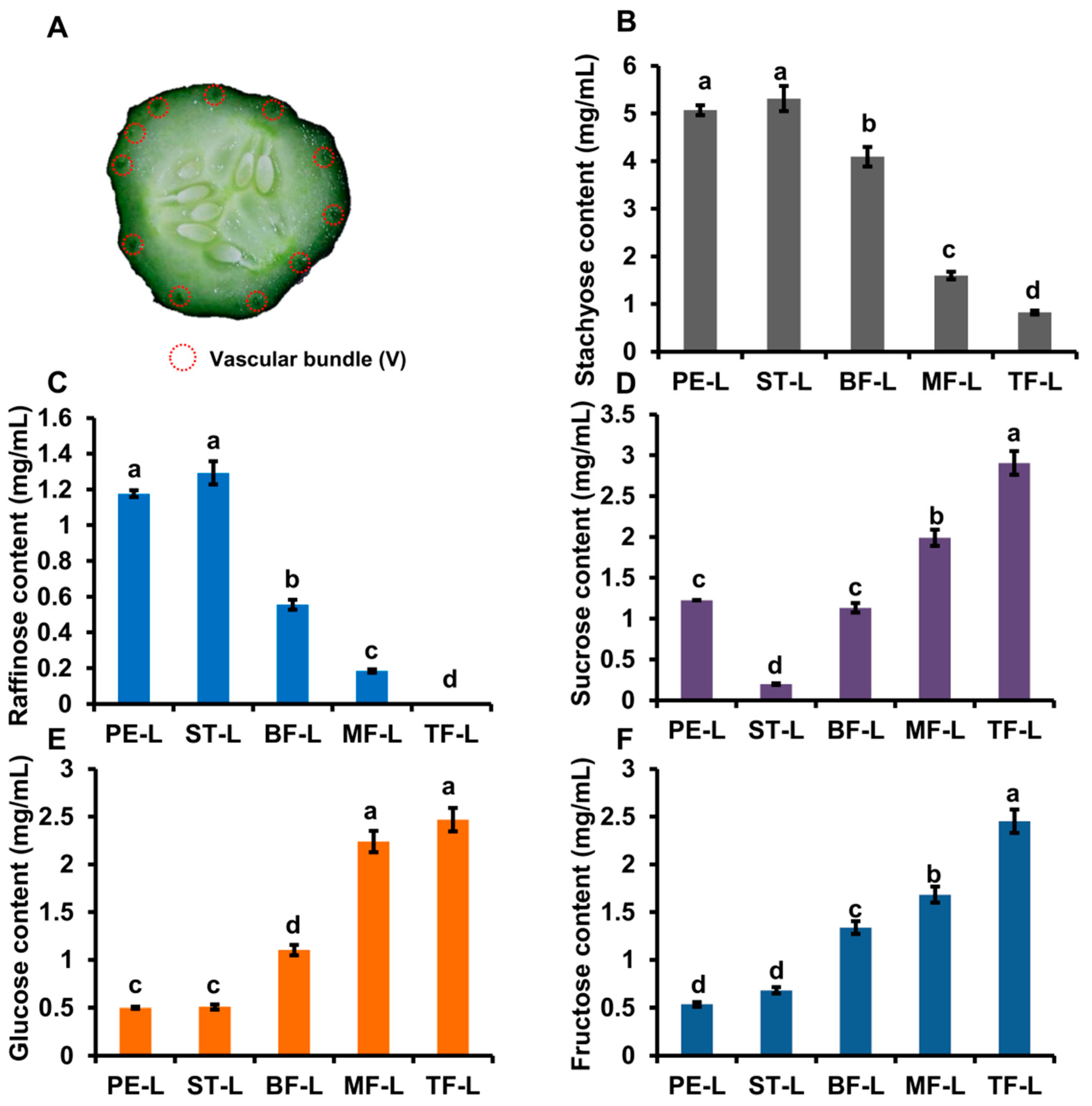
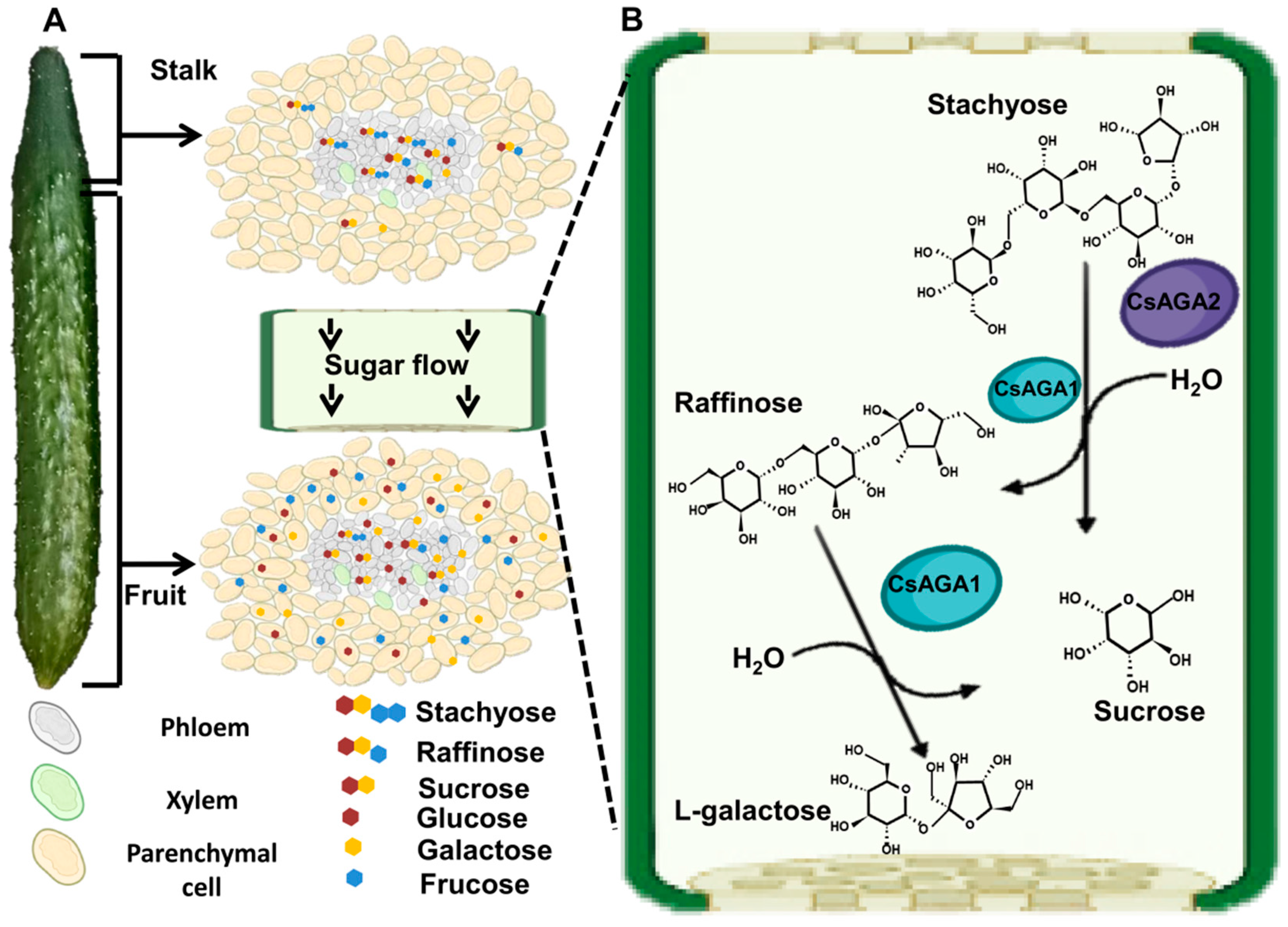
2.1. The Distribution Pattern of Soluble Sugars
2.2. The Distribution of Soluble Sugars in Phloem Sap
2.3. The Expression Pattern of α-Gals in Fruits
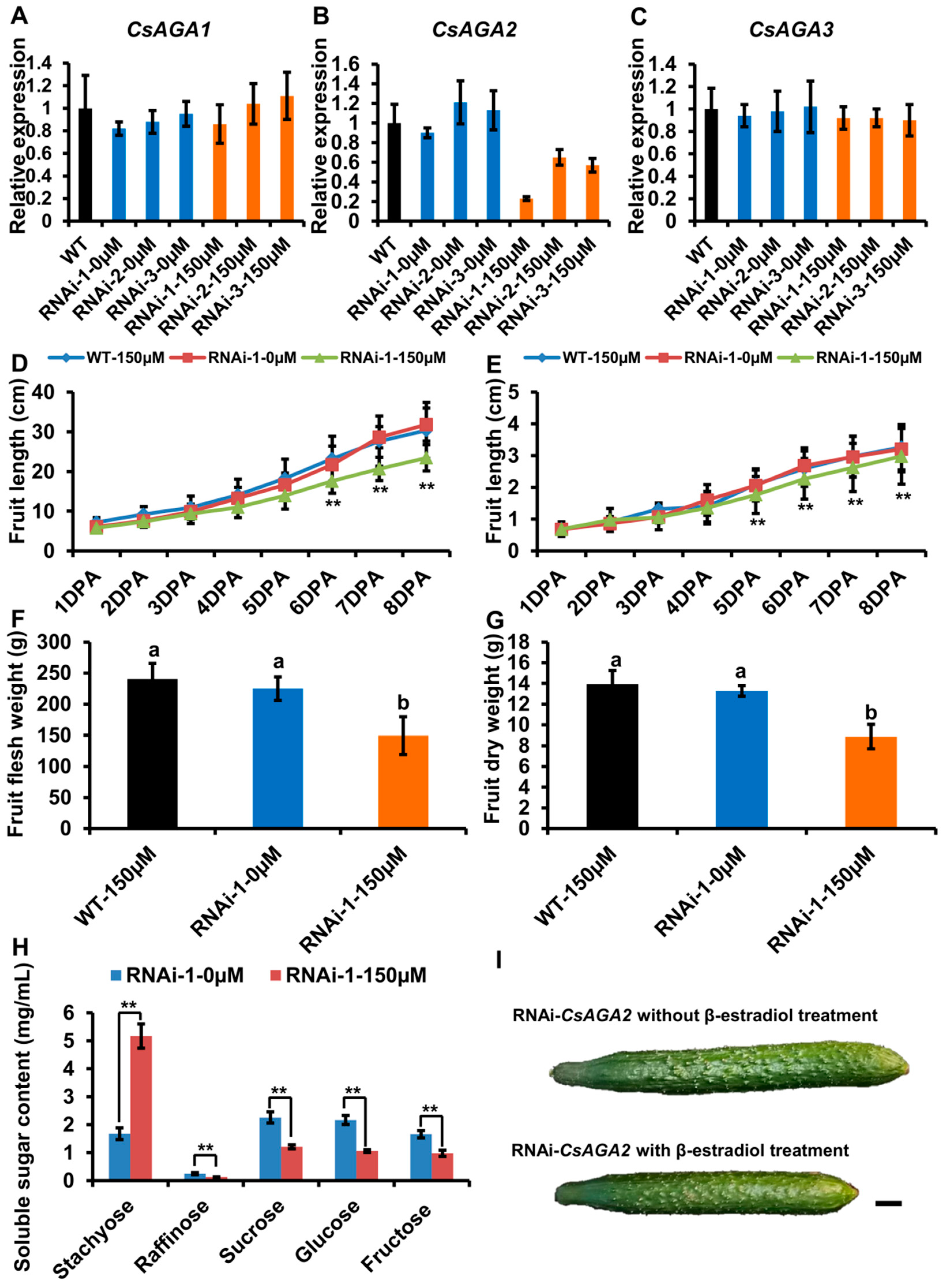
2.4. CsAGA2 Mediates Stachyose Hydrolysis
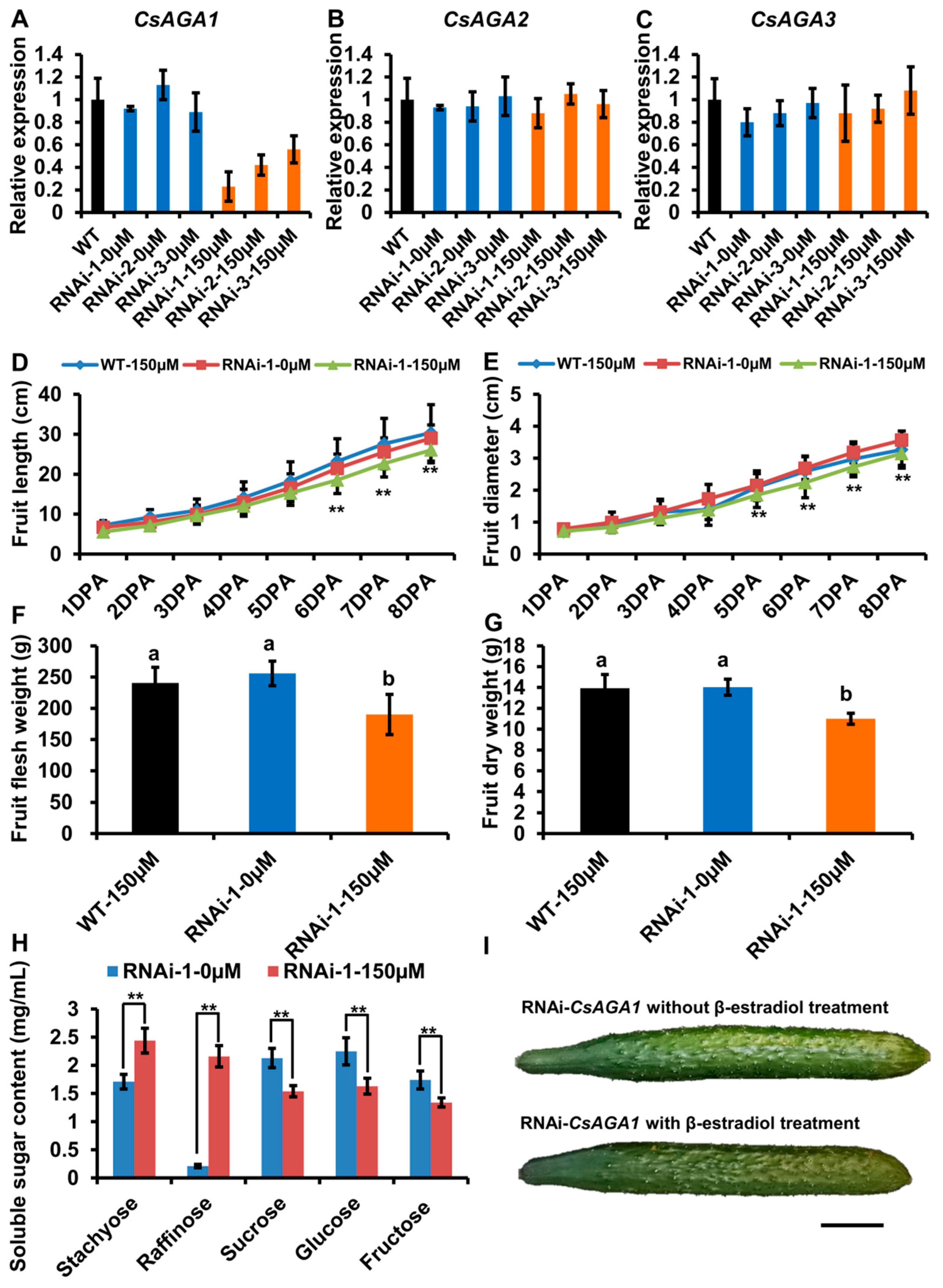
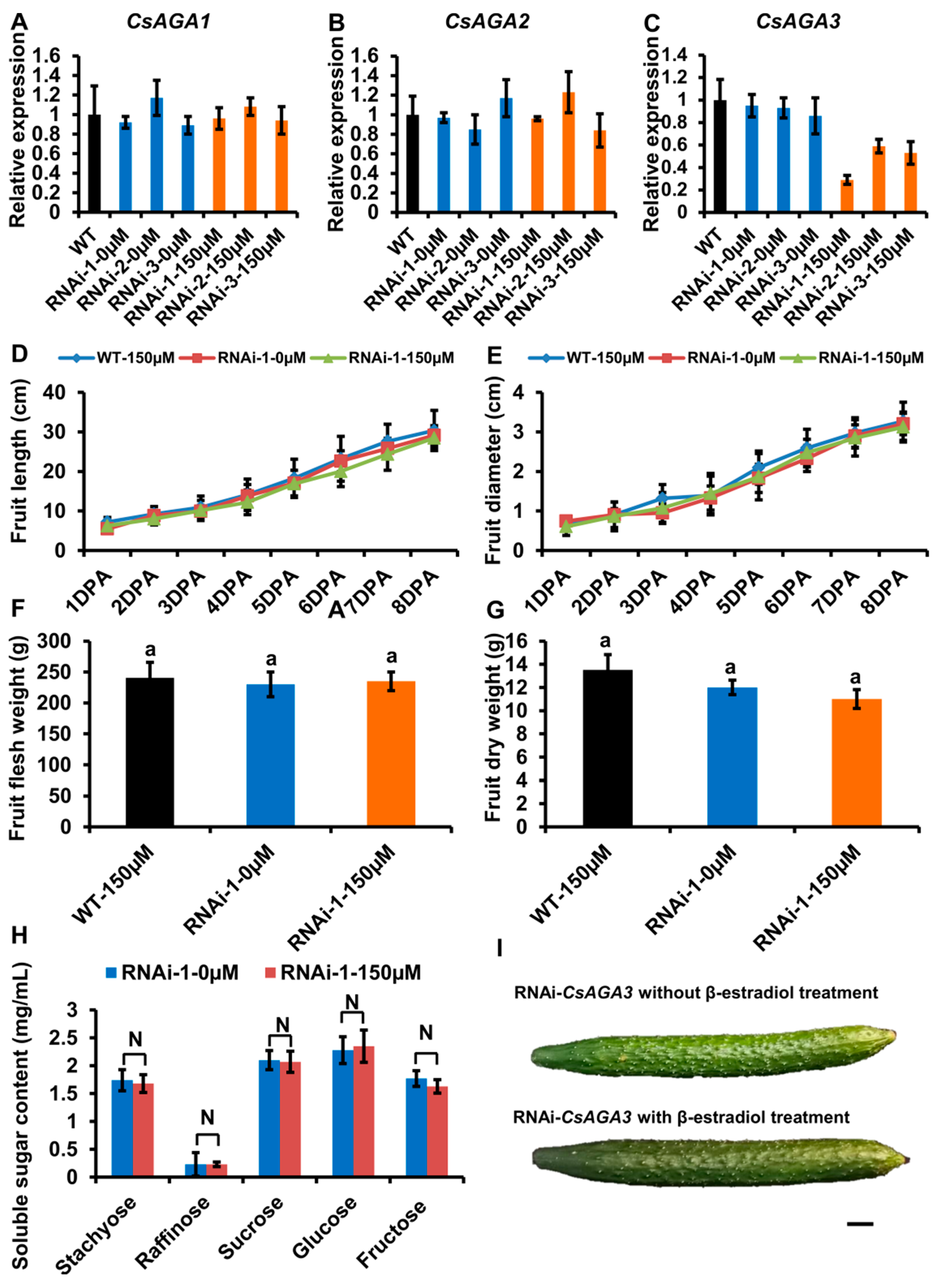
2.5. CsAGA1 Mediates Both Stachyose and Raffinose Hydrolysis
3. Discussion
3.1. A Chemical-Inducible Gene Silencing System in Pedicel of Cucumber Fruit
3.2. Hydrolysis of RFOs in Fruits
3.3. The Distinct Functions of AGAs in RFOs Hydrolysis of Cucurbitaceous Plants
3.4. The Non-Sweet Motif within the Promoter of ClAGA2 Is Widely Distributed in Cucurbitaceous Plants
4. Materials and Methods
4.1. Plant Growth Conditions and Materials
4.2. Vascular Sap Sampling
4.3. Determination of Soluble Sugar
4.4. Enzyme Activity Determination
4.5. Total RNA Isolation and Expression Analysis
4.6. In Situ Hybridization
4.7. Vector Construction
4.8. Generation of Gene Interference Lines
4.9. β-Estradiol Treatment
4.10. Characterization of Fruit Length, Diameter and Weight
4.11. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Che, G.; Zhang, X. Molecular basis of cucumber fruit domestication. Curr. Opin. Plant Biol. 2019, 47, 38–46. [Google Scholar] [CrossRef]
- Gross, K.C.; Pharr, D.M. A Potential Pathway for Galactose Metabolism in Cucumis sativus L.; A Stachyose Transporting Species. Plant Physiol. 1982, 69, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Yu, X.; Ayre, B.G.; Turgeon, R. The origin and composition of cucurbit “phloem” exudate. Plant Physiol. 2012, 158, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Li, M.; Guo, S.; Sun, H.; Zhao, J.; Zhang, J.; Liu, G.; He, H.; Tian, S.; Yu, Y.; et al. Evolutionary gain of oligosaccharide hydrolysis and sugar transport enhanced carbohydrate partitioning in sweet watermelon fruits. Plant Cell. 2021, 33, 1554–1573. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat. Genet. 2019, 51, 1616–1623. [Google Scholar] [CrossRef]
- Sonnewald, U.; Fernie, A.R. Next-generation strategies for understanding and influencing source-sink relations in crop plants. Curr. Opin. Plant Biol. 2018, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Meng, F.; Wang, S.; Sui, X.; Li, W.; Wei, Y.; Sun, J.L.; Zhang, Z.X. Changes in carbohydrate levels and their metabolic enzymes in leaves, phloem sap and mesocarp during cucumber (Cucumis sativus L.) fruit development. Sci. Hortic. 2009, 121, 131–137. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Ding, L.; Yan, S.; Liu, M.; Jiang, L.; Zhao, W.; Wang, Q.; Yan, L.; Liu, R.; et al. Phloem transcriptome signatures underpin the physiological differentiation of the pedicel, stalk and fruit of cucumber (Cucumis sativus L.). Plant Cell Physiol. 2016, 57, 19–34. [Google Scholar] [CrossRef] [Green Version]
- Chrost, B.; Schmitz, K. Changes in soluble sugar and activity of a-galactosidases and acid invertase during muskmelon (Cucumis melo L.) fruit development. J. Plant Physiol. 1997, 151, 41–50. [Google Scholar] [CrossRef]
- Dai, N.; Cohen, S.; Portnoy, V.; Tzuri, G.; Harel-Beja, R.; Pompan-Lotan, M.; Carmi, N.; Zhang, G.; Diber, A.; Pollock, S.; et al. Metabolism of soluble sugars in developing melon fruit: A global transcriptional view of the metabolic transition to sucrose accumulation. Plant Mol. Biol. 2011, 76, 1–18. [Google Scholar] [CrossRef]
- Pharr, D.; Hubbard, N. Melons: Biochemical and physiological control of sugar accumulation. In Encyclopedia of Agricultural Science; Arntzen, C.J., Ritteret, E.M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1994; Volume 3, pp. 25–37. [Google Scholar]
- Handley, L.W.; Pharr, D.M.; McFeeters, R.F. Carbohydrate changes during maturation of cucumber fruit: Implications for sugar metabolism and transport. Plant Physiol. 1983, 72, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Guo, S.; He, H.; Zhang, H.; Gong, G.; Ren, Y.; Xu, Y. Dynamic characteristics of sugar accumulation and related enzyme activities in sweet and non-sweet watermelon fruits. Acta Physiol. Plant. 2013, 35, 3213–3222. [Google Scholar] [CrossRef]
- Irving, D.E.; Hurst, P.L.; Ragg, J.S. changes in carbohydrates and carbohydrate metabolizing enzymes during the development, maturation, and ripening of buttercup squash (Cucurbita maxima D. ‘Delica’). J. Am. Soc. Hortic. Sci. 1997, 122, 310. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H.; Kim, W.D.; Kaneko, S.; Prema, P.A.; Nakajima, M.; Kobayashi, H. Expression of rice (Oryza sativa L. var. Nipponbare) alpha-galactosidase genes in Escherichia coli and characterization. Biosci. Biotechnol. Biochem. 2007, 71, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Zamski, E.; Schaffer, A.A. Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships; Marcel Dekker Inc.: New York, NY, USA, 1996. [Google Scholar]
- Gao, Z.F.; Schaffer, A.A. A novel alkaline alpha-galactosidase from melon fruit with a substrate preference for raffinose. Plant Physiol. 1999, 119, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmi, N.; Zhang, G.; Petreikov, M.; Gao, Z.; Eyal, Y.; Granot, D.; Schaffer, A.A. Cloning and functional expression of alkaline alpha-galactosidase from melon fruit: Similarity to plant SIP proteins uncovers a novel family of plant glycosyl hydrolases. Plant J. 2003, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Katrolia, P.; Rajashekhara, E.; Yan, Q.; Jiang, Z. Biotechnological potential of microbial alpha-galactosiases. Crit. Rev. Biotechnol. 2014, 34, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Keller, F.; Pharr, D.M. Metabolism of carbohydrates in sinks and sources:galactosyl-sucrose oligosaccharides. In Photoassimilate Distribution in Plants and Crops: Source Sink Relationships; Zamski, E., Schaffer, A.A., Eds.; Routledge: London, UK, 1996; pp. 157–183. [Google Scholar]
- Blochl, A.; Peterbauer, T.; Hofmann, J.; Richter, A. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta 2008, 228, 99–110. [Google Scholar] [CrossRef]
- Soh, C.P.; Ali, Z.M.; Lazan, H. Characterisation of an alpha-galactosidase with potential relevance to ripening related texture changes. Phytochemistry 2006, 67, 242–254. [Google Scholar] [CrossRef]
- Gu, H.; Lu, M.; Zhang, Z.P.; Xu, J.J.; Cao, W.H.; Miao, M.M. Metabolic process of raffinose family oligosaccharides during cold stress and recovery in cucumber leaves. J. Plant Physiol. 2018, 224, 112–120. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Liu, Y.C.; Dai, H.B.; Miao, M.M. Characteristics and expression patterns of six α-galactosidases in cucumber (Cucumis sativus L.). PLoS ONE 2021, 16, e0244714. [Google Scholar] [CrossRef]
- Ruiz-Medrano, R.; Xoconostle-Cazares, B.; Lucas, W.J. The phloem as a conduit for inter-organ communication. Curr. Opin. Plant Biol. 2001, 4, 202–209. [Google Scholar] [CrossRef]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.-R.; Helariutta, Y.; He, X.-Q.; Fukuda, H.; Kang, J.; Brady, S.; et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef]
- Crafts, A.S. Phloem anatomy, exudation, and transport of organic nutrients in cucurbits. Plant Physiol. 1932, 7, i4-225. [Google Scholar] [CrossRef]
- Eschrich, W.; Evert, R.F.; Heyser, W. Proteins of the sieve-tube exudate of Cucurbita maxima. Planta 1971, 100, 208–221. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef] [Green Version]
- Ivamoto-Suzuki, S.T.; Reis, O.; Domingues, D.; Santos, T.; De Oliveira, F.F.; Pot, D.; Leroy, T.; Vieira, L.G.E.; Carazzolle, M.F.; Pereira, G.A.G.; et al. Transcriptome Analysis of Leaves, Flowers and Fruits Perisperm of Coffea arabica L. Reveals the Differential Expression of Genes Involved in Raffinose Biosynthesis. PLoS ONE 2017, 12, e0169595. [Google Scholar] [CrossRef] [Green Version]
- Marton, L.; Wullems, G.J.; Molendijk, L.; Schilperoort, R.A. In vitro transformation of Cultured Cells from Nicotiana Tabacum by Agrobacterium Tumefaciens. Nature 1979, 277, 129–131. [Google Scholar] [CrossRef]
- Moore, I.; Samalova, M.; Kurup, S. Transactivated and chemically inducible gene expression in plants. Plant J. 2006, 45, 651–683. [Google Scholar] [CrossRef]
- Zuo, J.; Chua, N.H. Chemical-inducible systems for regulated expression of plant genes. Curr. Opin. Biotechnol. 2000, 11, 146–151. [Google Scholar] [CrossRef]
- Sun, J.; Niu, Q.W.; Tarkowski, P.; Zheng, B.; Tarkowska, D.; Sandberg, G.; Chua, N.H.; Zuo, J. The Arabidopsis AtIPT8/PGA22 gene encodes an isopentenyl transferase that is involved in de novo cytokinin biosynthesis. Plant Physiol. 2003, 131, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Dong, J. Enhancing terpenoid indole alkaloid production by inducible expression of mammalian Bax in Catharanthus roseus cells. Sci. China C Life Sci. 2007, 50, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Okuzaki, A.; Konagaya, K.; Nanasato, Y.; Tsuda, M.; Tabei, Y. Estrogen-inducible GFP expression patterns in rice (Oryza sativa L.). Plant Cell Rep. 2011, 30, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Phoeurk, C.; Somana, J.; Sornwatana, T.; Udompaisarn, S.; Traewachiwiphak, S.; Sirichaiyakul, P.; Phongsak, T.; Arthan, D. Three novel mutations in α-galactosidase gene involving in galactomannan degradation in endosperm of curd coconut. Phytochemistry 2018, 156, 33–42. [Google Scholar] [CrossRef]
- Grandis, A.; Leite, D.C.C.; Tavares, E.Q.P.; Arenque-Musa, B.C.; Gaiarsa, J.; Martins, M.C.M.; de Souza, A.P.; Gomez, L.D.; Fabbri, C.; Mattei, B.; et al. Cell wall hydrolases act in concert during aerenchyma development in sugarcane roots. Ann. Bot. 2019, 124, 1067–1089. [Google Scholar] [CrossRef]
- Chrost, B.; Kolukisaoglu, U.; Schulz, B.; Krupinska, K. An alpha-galactosidase with an essential function during leaf development. Planta 2007, 225, 11–20. [Google Scholar]
- Mitchell, D.E.; Madore, M.A. Patterns of Assimilate Production and Translocation in Muskmelon (Cucumis melo L.): II. Low Temperature Effects. Plant Physiol. 1992, 99, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Miao, M.M.; Xu, X.F.; Chen, X.H.; Xue, L.B.; Cao, B.S. Cucumber carbohydrate metabolism and translocation under chilling night temperature. J. Plant Physiol. 2007, 164, 621–628. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, Q.; Lam, E.; Tian, F.; Chaieb, S.; Hemar, Y. In situ study starch gelatinization under ultra-high hydrostatic pressure using synchrotron SAXS Food Hydrocolloids. Food Hydrocoll. 2016, 56, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Weigel, D.; Glazebrook, J. Immunohistochemistry on sections of plant tissues using enzyme-coupled avidin-biotin complex. Cold Spring Harb. Protoc. 2008, 2008, prot4945. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Niu, Q.W.; Møller, S.G.; Chua, N.H. Chemical-regulated, site-specific DNA excision in transgenic plants. Nat. Biotechnol. 2001, 19, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.S.; Fei, J.F.; Xie, Q.; Chua, N.H. A chemical-regulated inducible RNAi system in plants. Plant J. 2003, 34, 383–392. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, B.; Zhang, M.; Zhang, J.; Dai, H.; Zhang, Z.; Miao, M. CsAGA1 and CsAGA2 Mediate RFO Hydrolysis in Partially Distinct Manner in Cucumber Fruits. Int. J. Mol. Sci. 2021, 22, 13285. https://doi.org/10.3390/ijms222413285
Hua B, Zhang M, Zhang J, Dai H, Zhang Z, Miao M. CsAGA1 and CsAGA2 Mediate RFO Hydrolysis in Partially Distinct Manner in Cucumber Fruits. International Journal of Molecular Sciences. 2021; 22(24):13285. https://doi.org/10.3390/ijms222413285
Chicago/Turabian StyleHua, Bing, Mengying Zhang, Jinji Zhang, Haibo Dai, Zhiping Zhang, and Minmin Miao. 2021. "CsAGA1 and CsAGA2 Mediate RFO Hydrolysis in Partially Distinct Manner in Cucumber Fruits" International Journal of Molecular Sciences 22, no. 24: 13285. https://doi.org/10.3390/ijms222413285






