Lupinus albus Protein Components Inhibit MMP-2 and MMP-9 Gelatinolytic Activity In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of the L. albus MMPI
2.2. Nature and Composition of the L. albus MMPI
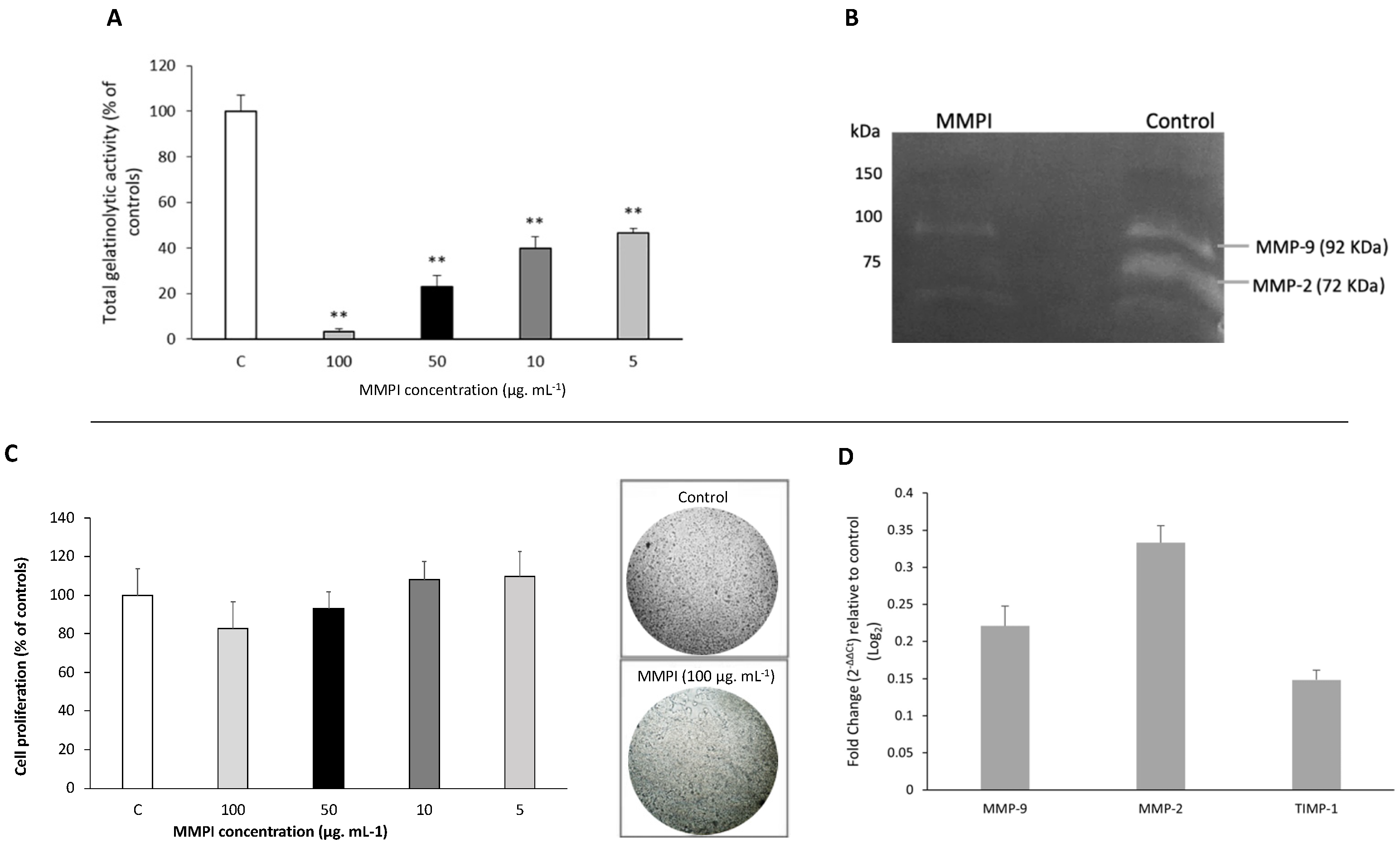
2.3. The L. albus MMPI Inhibits Both MMP-2 and MMP-9 In Vitro, in a Dose-Dependent Manner, without Impairing Cell Viability or Gene Expression
2.4. The L. albus MMPI Inhibits MMP-2 and MMP-9 In Vivo and Reduces Colitis Injuries
2.5. L. albus MMPI May Present the Features of an Ideal MMP-9 Inhibitor for Gut-Related Diseases
3. Materials and Methods
3.1. Protein Isolation and Identification
3.2. Fast Protein Liquid Chromatography
3.3. High-Performance Liquid Chromatography
3.4. 1-D Electrophoresis
3.5. 2-D Electrophoresis
3.6. MALDI-TOF TOF
3.7. MS/MS Analysis
3.8. MMP-9 and MMP-2 Catalytic Activities
3.8.1. DQ-Gelatin Assay
3.8.2. Gelatin Zymography
3.9. In Vitro Colon Cancer Cell Assays
3.9.1. HT29 Cell Cultures
3.9.2. Cell Viability Assay
3.10. Assessment of Gene Expression by Quantitative Real-Time PCR (RT-qPCR)
RN Extraction and cDNA Synthesis
3.11. In Vivo Animal Model of Colitis
3.11.1. Animals
3.11.2. Animal Care and Maintenance
3.11.3. Induction of Colitis
3.11.4. Experimental Groups
3.11.5. Macroscopic Evaluation of Colitis Severity
3.11.6. Gelatin Zymography of Colon Extracts
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Patent
References
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Glade, M.J. Food, nutrition and the prevention of cancer: A global perspective: World Cancer Research Fund/American Institute for Cancer Research. Nutrition 1999, 15, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Vansaun, M.N.; Shim, J.H.; Matrisian, L.M.; Gorden, D.L. Increased metastases are associated with inflammation and matrix metalloproteinase-9 activity at incision sites in a murine model of peritoneal dissemination of colorectal cancer. J. Surg. Res. 2013, 180, 252–259. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Dunay, I.R.; Fuchs, D.; Trautmann, D.; Fischer, A.; Kühl, A.A.; Loddenkemper, C.; Siegmund, B.; Batra, A.; Bereswill, S.; et al. The distinct roles of MMP-2 and MMP-9 in acute DSS colitis. Eur. J. Microbiol. Immunol. 2011, 1, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Baugh, M.D.; Perry, M.J.; Hollander, A.P.; Davies, D.R.; Cross, S.S.; Lobo, A.J.; Taylor, C.J.; Evans, G.S. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 1999, 117, 814–822. [Google Scholar] [CrossRef]
- Garg, P.; Vijay-Kumar, M.; Wang, L.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taleban, S.; Elquza, E.; Gower-Rousseau, C.; Peyrin-Biroulet, L. Cancer and inflammatory bowel disease in the elderly. Dig. Liver Dis. 2016, 48, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Patel, N.R.; Walter, L.; Ingersoll, S.; Sitaraman, S.V.; Garg, P. Constitutive expression of MMP9 in intestinal epithelium worsens murine acute colitis and is associated with increased levels of proinflammatory cytokine Kc. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, 793–803. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, S.; Gilmer, J.F.; Medina, C. Matrix metalloproteinases in inflammatory bowel disease: An update. Mediators Inflamm. 2015, 2015, 964131. [Google Scholar] [CrossRef]
- Medina, C.; Radomski, M.W. Role of matrix metalloproteinases in intestinal inflammation. J. Pharmacol. Exp. Ther. 2006, 318, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Bourguet, E.; Hornebeck, W.; Sapi, J.; Alix, A.J.P.; Moroy, G.; Sharma, R.R. Pharmacomodulation of Broad Spectrum Matrix Metalloproteinase Inhibitors Towards Regulation of Gelatinases. In Enzyme Inhibition and Bioapplications; IntechOpen: London, UK, 2012; Available online: https://www.intechopen.com/books/enzyme-inhibition-and-bioapplications/pharmacomodulation-of-broad-spectrum-matrix-metalloproteinase-inhibitors-towards-regulation-of-gelat (accessed on 5 May 2021).
- Ndinguri, M.W.; Bhowmick, M.; Tokmina-Roszyk, D.; Robichaud, T.; Fields, G. Peptide-Based Selective Inhibitors of Matrix Metalloproteinase-Mediated Activities. Molecules 2012, 17, 14230–14248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, X.; Zhang, S.; Lu, W.; Liao, S.; Liu, X.; Shan, L.; Shen, X.; Jiang, H.; Zhang, W.; et al. Natural products as a gold mine for selective matrix metalloproteinases inhibitors. Bioorg. Med. Chem. 2012, 20, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Gregg, B.F. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Abdul Amin, S.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef]
- De Leo, F.; Volpicella, M.; Licciulli, F.; Liuni, S.; Gallerani, R.; Cecil, R. PLANT-PIs: A database for plant protease inhibitors and their genes. Nucleic Acids Res. 2002, 30, 347–348. [Google Scholar] [CrossRef] [Green Version]
- Mota, J.; Figueira, M.E.; Ferreira, R.B.; Lima, A. An Up-Scalable and Cost-Effective Methodology for Isolating a Polypeptide Matrix Metalloproteinase-9 Inhibitor from Lupinus albus Seeds. Foods 2021, 10, 1663. [Google Scholar] [CrossRef]
- Kennedy, A.R. The evidence for soybean products as cancer preventive agents. J. Nutr. 1995, 125, 733S–743S. [Google Scholar] [CrossRef]
- Jiang, Q.; Pan, Y.; Cheng, Y.; Li, H.; Liu, D.; Li, H. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-κB signaling pathways. Oncol. Rep. 2016, 36, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, S.; Freitas, R.; Rajasekhar, B.T.; Teixeira, A.R.; Ferreira, R.B. The unique biosynthetic route from Lupinus β-conglutin gene to blad. PLoS ONE 2010, 5, e8542. [Google Scholar] [CrossRef]
- Sironi, E.; Sessa, F.; Duranti, M. A simple procedure of lupin seed protein fractionation for selective food applications. Eur. Food Res. Technol. 2005, 221, 145–150. [Google Scholar] [CrossRef]
- Salmanowicz, B.; Weder, J. Primary structure of 2S albumin from seeds of Lupinus albus. Zeitschrift für Lebensmitteluntersuchung und -Forschung A 1997, 204, 129–135. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Duranti, M.; Guerrieri, N.; Cerletti, P.; Vecchio, G. The legumin precursor from white lupin seed. Identity of the subunits, assembly and proteolysis. Eur. J. Biochem. 1992, 206, 941–947. [Google Scholar] [CrossRef]
- Duranti, M.; Cucchetti, E.; Cerletti, P. Changes in composition and subunits in the storage proteins of germinating lupine seeds. J. Agric. Food Chem. 1984, 32, 490–493. [Google Scholar] [CrossRef]
- Monteiro, S.; Carreira, A.; Freitas, R.; Pinheiro, A.M.; Ferreira, R.B. A nontoxic polypeptide oligomer with a fungicide potency under agricultural conditions which is equal or greater than that of their chemical counterparts. PLoS ONE 2015, 10, e0122095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, R.B.; Freitas, R.L.; Teixeira, A.R. Self-aggregation of legume seed storage proteins inside the protein storage vacuoles is electrostatic in nature, rather than lectin-mediated. FEBS Lett. 2003, 534, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S.K. Curcumin: A potential candidate for matrix metalloproteinase inhibitors. Expert Opin. Ther. Targets 2012, 16, 959–972. [Google Scholar] [CrossRef]
- Honari, M.; Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Asemi, Z. Resveratrol is a promising agent for colorectal cancer prevention and treatment: Focus on molecular mechanisms. Cancer Cell Int. 2019, 19, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Han, Z.; Tian, L.; Chen, K.; Fan, Y.; Ye, B.; Huang, W.; Wang, C.; Huang, Z. Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J. Transl. Med. 2014, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3 expression. Mol. Med. Rep. 2017, 16, 4710–4720. [Google Scholar] [CrossRef] [Green Version]
- Gweon, E.J.; Kim, S.J. Resveratrol attenuates matrix metalloproteinase-9 and-2-regulated differentiation of HTB94 chondrosarcoma cells through the p38 kinase and JNK pathways. Oncol. Rep. 2014, 32, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.; Araújo, J.E.; Gómez-Meire, S.; Lodeiro, C.; Perez-Melon, C.; Iglesias-Lamas, E.; Otero-Glez, A.; Capelo, J.L.; Santos, H.M. Proteomics analysis of the peritoneal dialysate effluent reveals the presence of calcium-regulation proteins and acute inflammatory response. Clin. Proteom. 2014, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Toth, M.; Sohail, A.; Fridman, R. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography. In Metastasis Research Protocols. Methods in Molecular Biology (Methods and Protocols); Dwek, M., Brooks, S., Schumacher, U., Eds.; Springer Protocols: Totowa, NJ, USA, 2012; p. 878. [Google Scholar] [CrossRef] [Green Version]
- Coito, J.L.; Rocheta, M.; Carvalho, L.; Amâncio, S. Microarray-based uncovering reference genes for quantitative real time PCR in grapevine under abiotic stress. BMC Res. Notes 2012, 5, 220. [Google Scholar] [CrossRef] [Green Version]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, R.B.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.; Alves, P.; Bronze, R.; et al. Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 2017, 46, 100–108. [Google Scholar] [CrossRef]
- Castaneda, F.E.; Walia, B.; Vijay-Kumar, M.; Patel, N.R.; Roser, S.; Kolachala, V.L.; Rojas, M.; Wang, L.; Oprea, G.; Garg, P.; et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: Central role of epithelial-derived MMP. Gastroenterology 2005, 129, 1991–2008. [Google Scholar] [CrossRef] [PubMed]




| Length of Colons | Size of Lesion | Presence/Consistency of Diarrhea | |
|---|---|---|---|
| Control | 14.5 ± 0.082 | 0 | 0 |
| TNBS + EtOH 50% | 11.8 ± 0.19 # | 3.6 ± 0.14 # | 3 # |
| TNBS + MMPI p.o. | 14.8 ± 0.33 * | 2.44 ± 0.84 | 1.13 ± 0.35 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.; Direito, R.; Rocha, J.; Fernandes, J.; Sepodes, B.; Figueira, M.E.; Raymundo, A.; Lima, A.; Ferreira, R.B. Lupinus albus Protein Components Inhibit MMP-2 and MMP-9 Gelatinolytic Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 13286. https://doi.org/10.3390/ijms222413286
Mota J, Direito R, Rocha J, Fernandes J, Sepodes B, Figueira ME, Raymundo A, Lima A, Ferreira RB. Lupinus albus Protein Components Inhibit MMP-2 and MMP-9 Gelatinolytic Activity In Vitro and In Vivo. International Journal of Molecular Sciences. 2021; 22(24):13286. https://doi.org/10.3390/ijms222413286
Chicago/Turabian StyleMota, Joana, Rosa Direito, João Rocha, João Fernandes, Bruno Sepodes, Maria Eduardo Figueira, Anabela Raymundo, Ana Lima, and Ricardo Boavida Ferreira. 2021. "Lupinus albus Protein Components Inhibit MMP-2 and MMP-9 Gelatinolytic Activity In Vitro and In Vivo" International Journal of Molecular Sciences 22, no. 24: 13286. https://doi.org/10.3390/ijms222413286








