Paracrine Shear-Stress-Dependent Signaling from Endothelial Cells Affects Downstream Endothelial Function and Inflammation
Abstract
:1. Introduction
2. Results
2.1. Shear-Stress-Challenged HUVEC
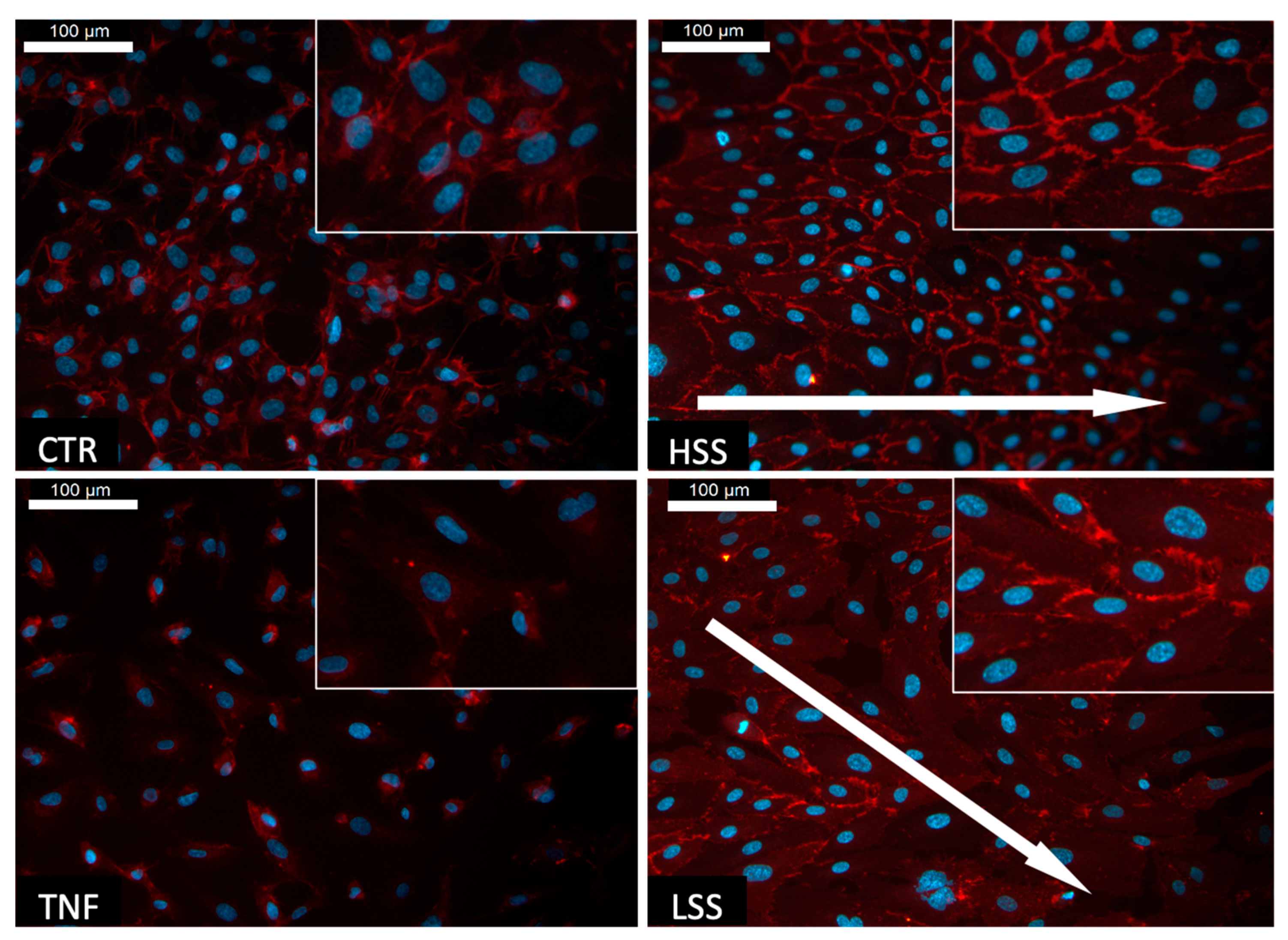
2.1.1. Shear Stress Induces the Alignment of HUVECs
2.1.2. PECAM-1 (CD31) Expression Depends on Shear Stress Magnitudes
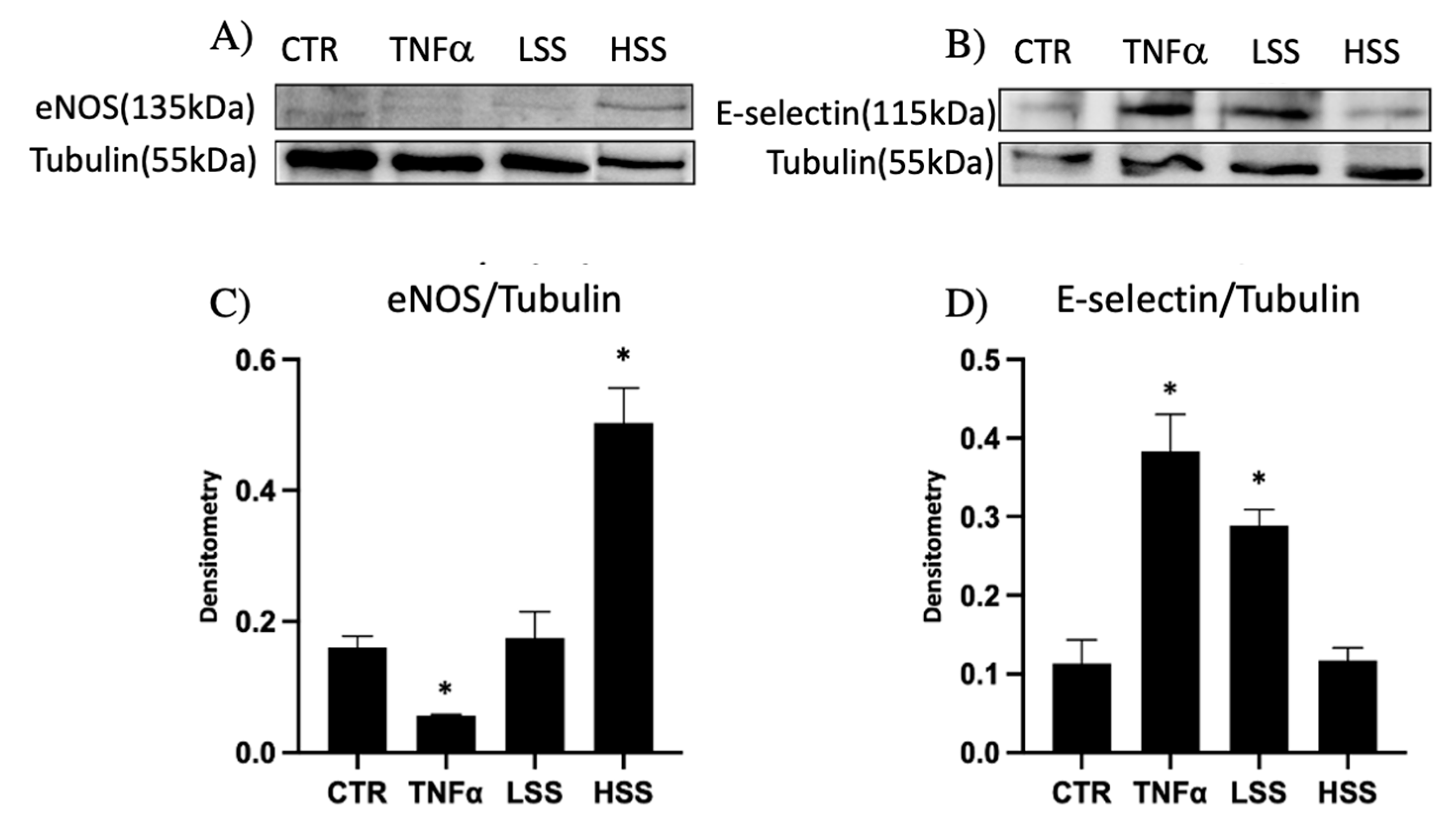
2.1.3. eNOS and E-selectin Protein Expression Depends on Shear Stress
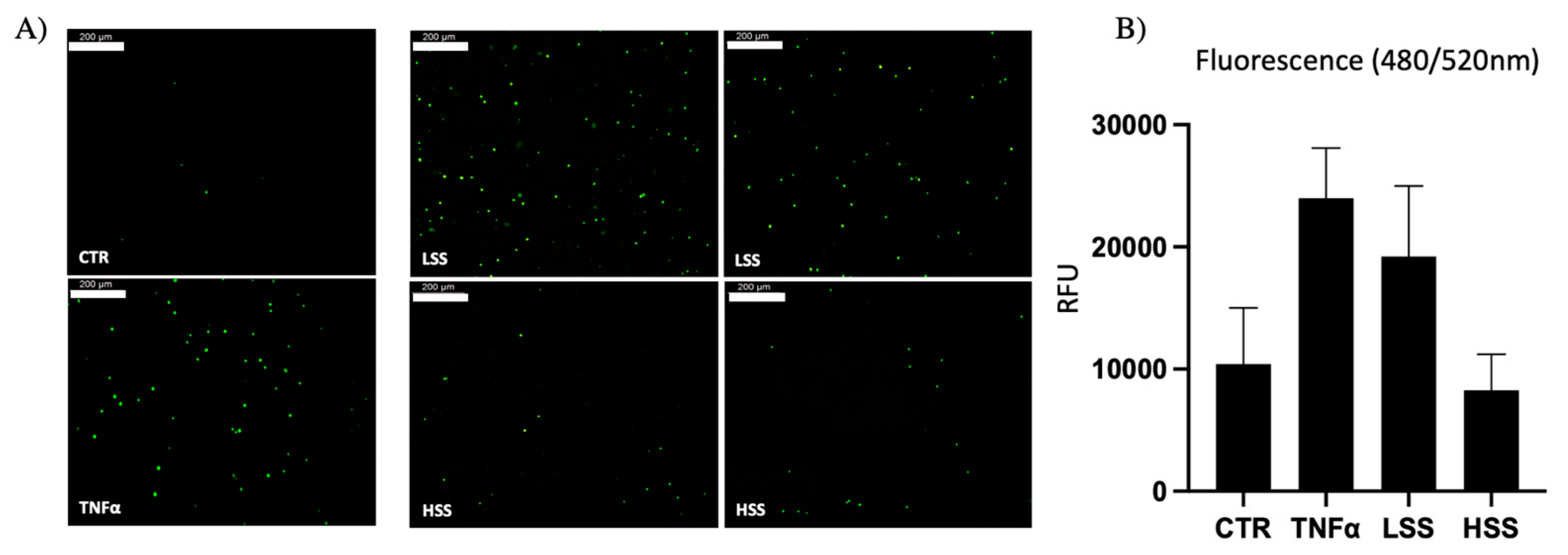
2.1.4. PBMC Adhesion Rate Is Dissimilar in Differently Sheared HUVEC Monolayers
2.2. Conditioned Media Stimulation on HUVEC Cells
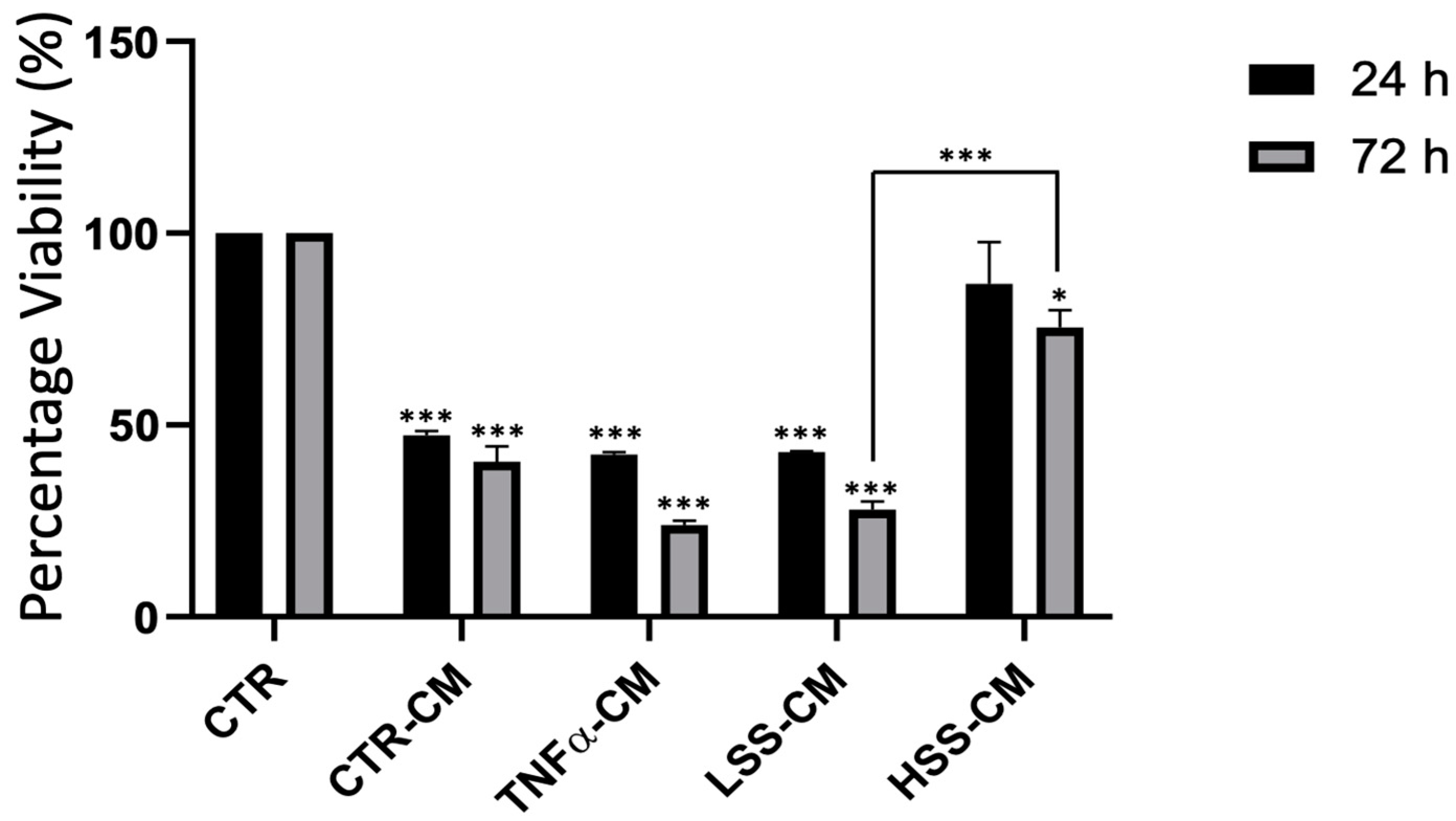
2.2.1. HSS-CM and LSS-CM Have Opposite Effects on Cellular Viability
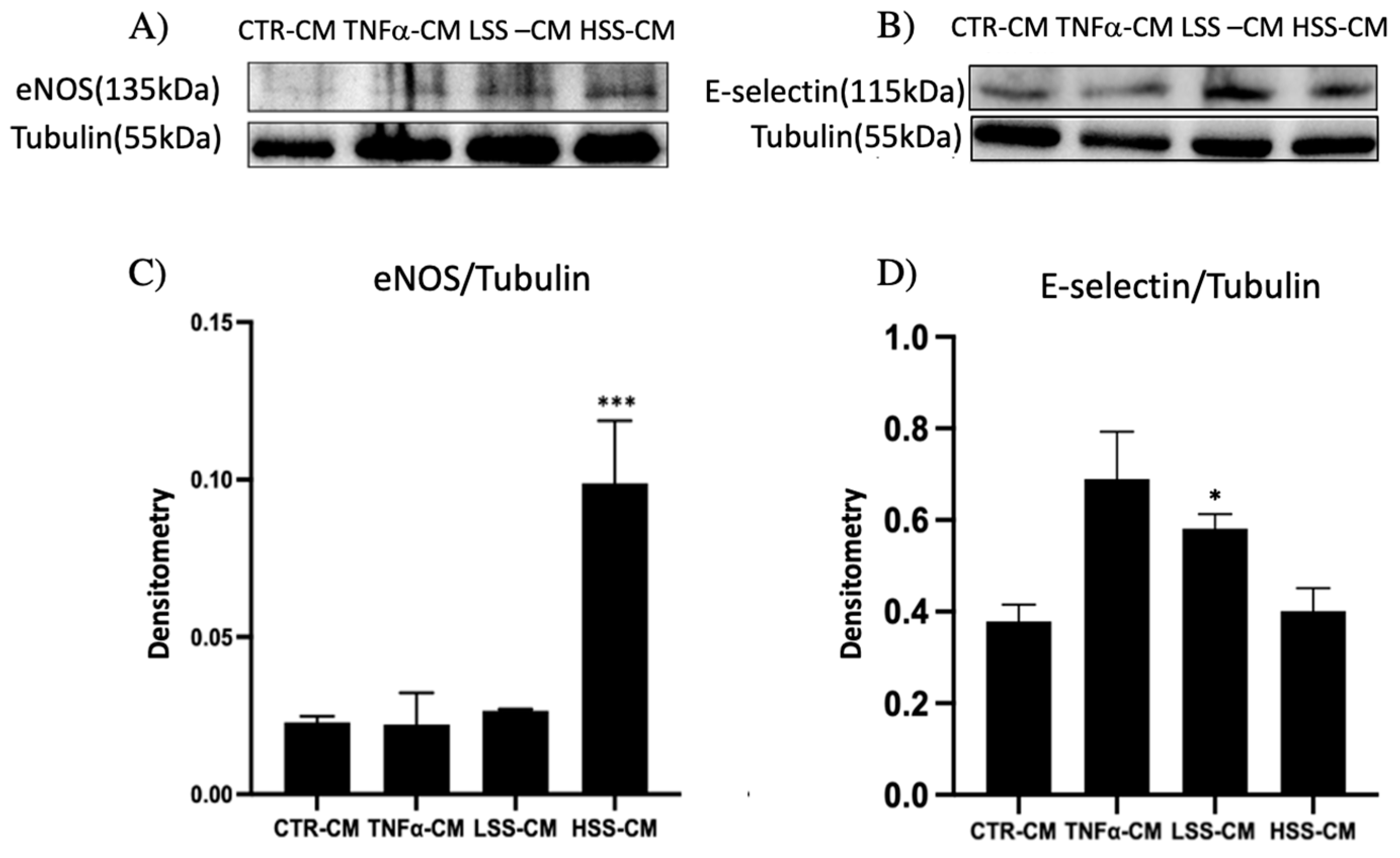
2.2.2. eNOS and E-selectin Protein Expression in Different CMs Culture
2.2.3. ICAM-1 mRNA Expression Is Upregulated by LSS-CM
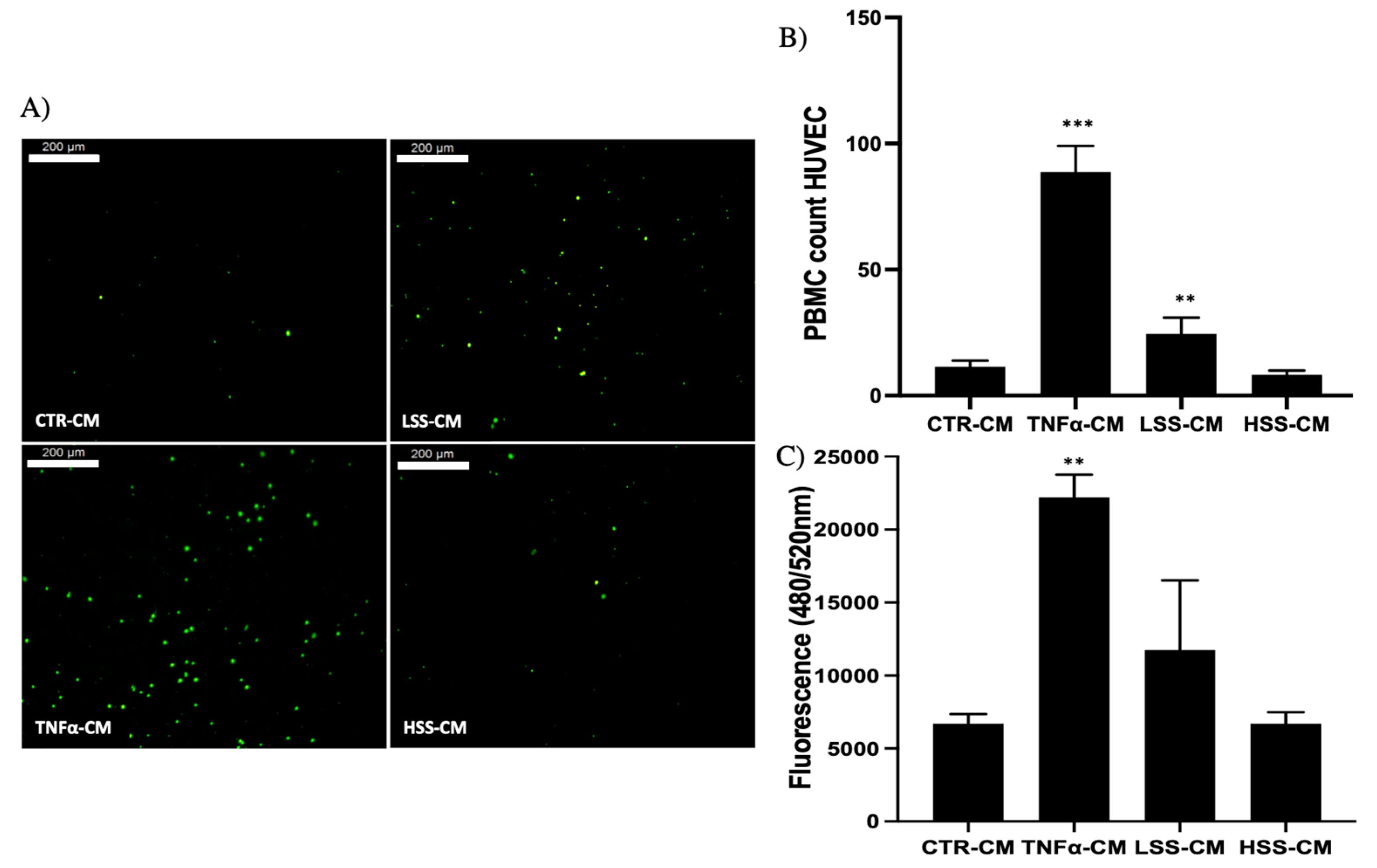
2.2.4. PBMC Adhesion Rate Is Higher in LSS-CM-Cultured HUVECs
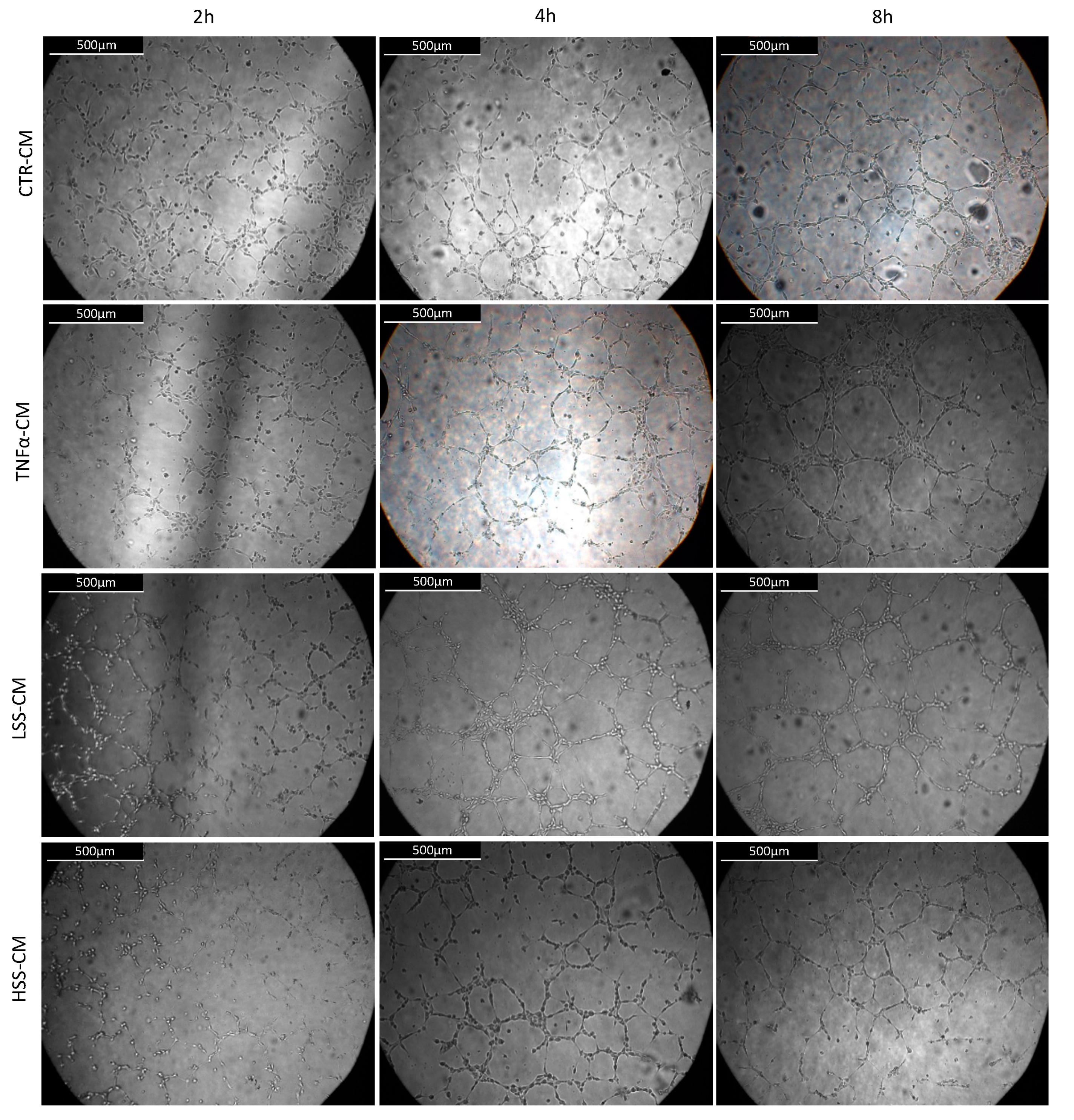
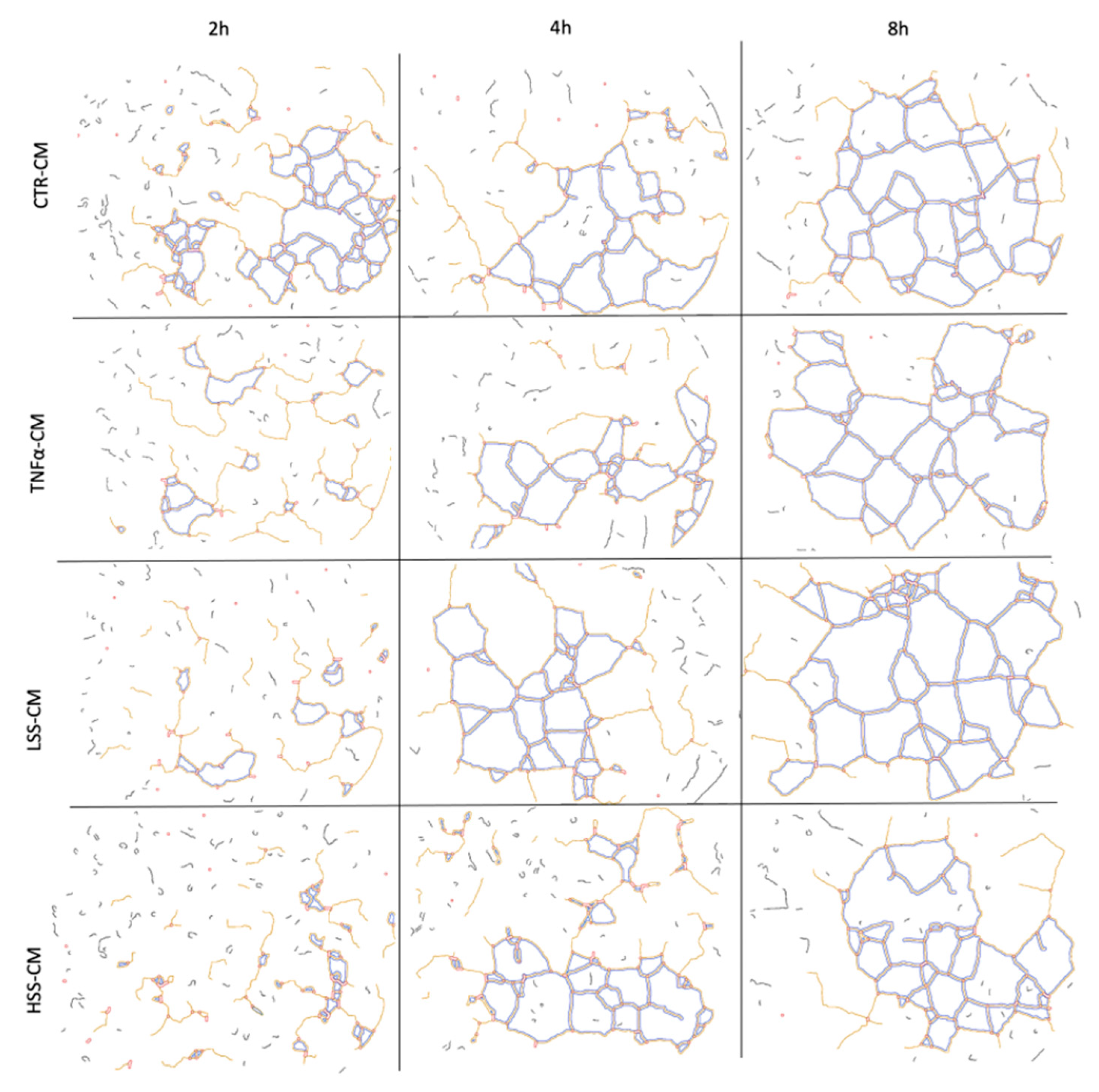
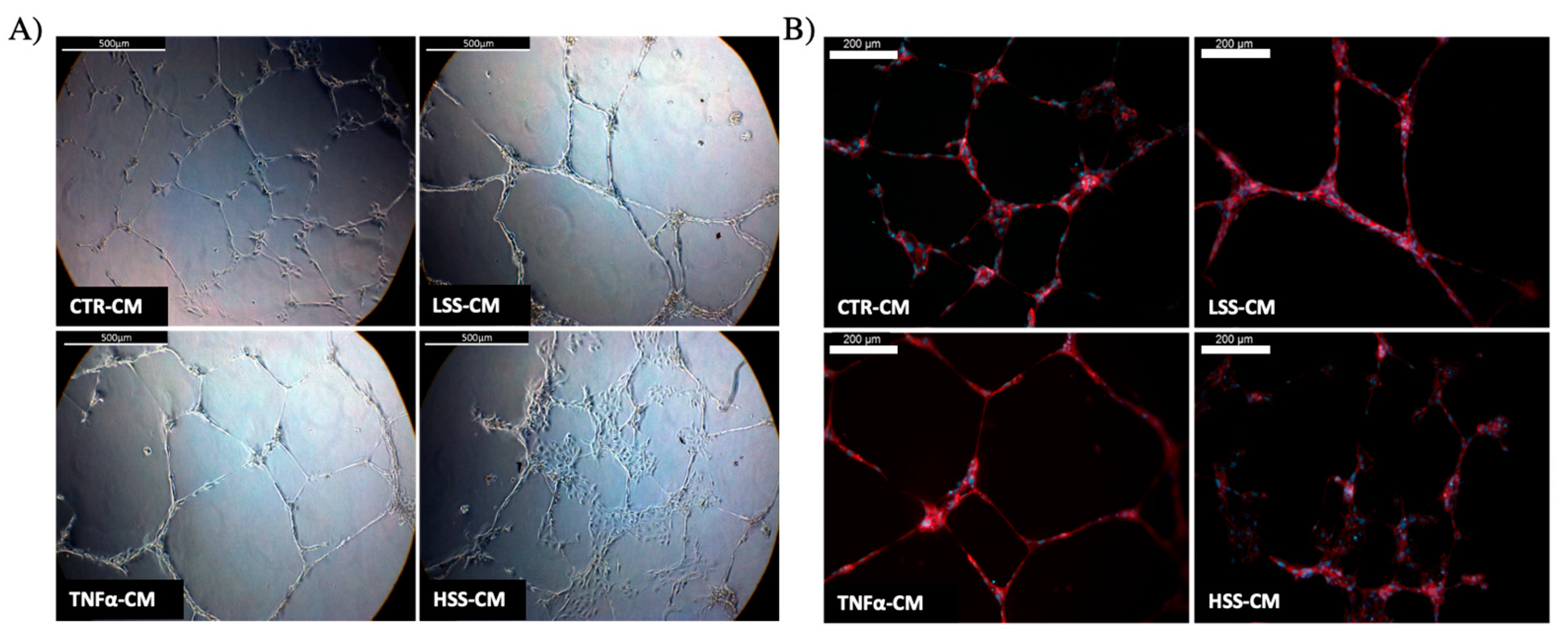
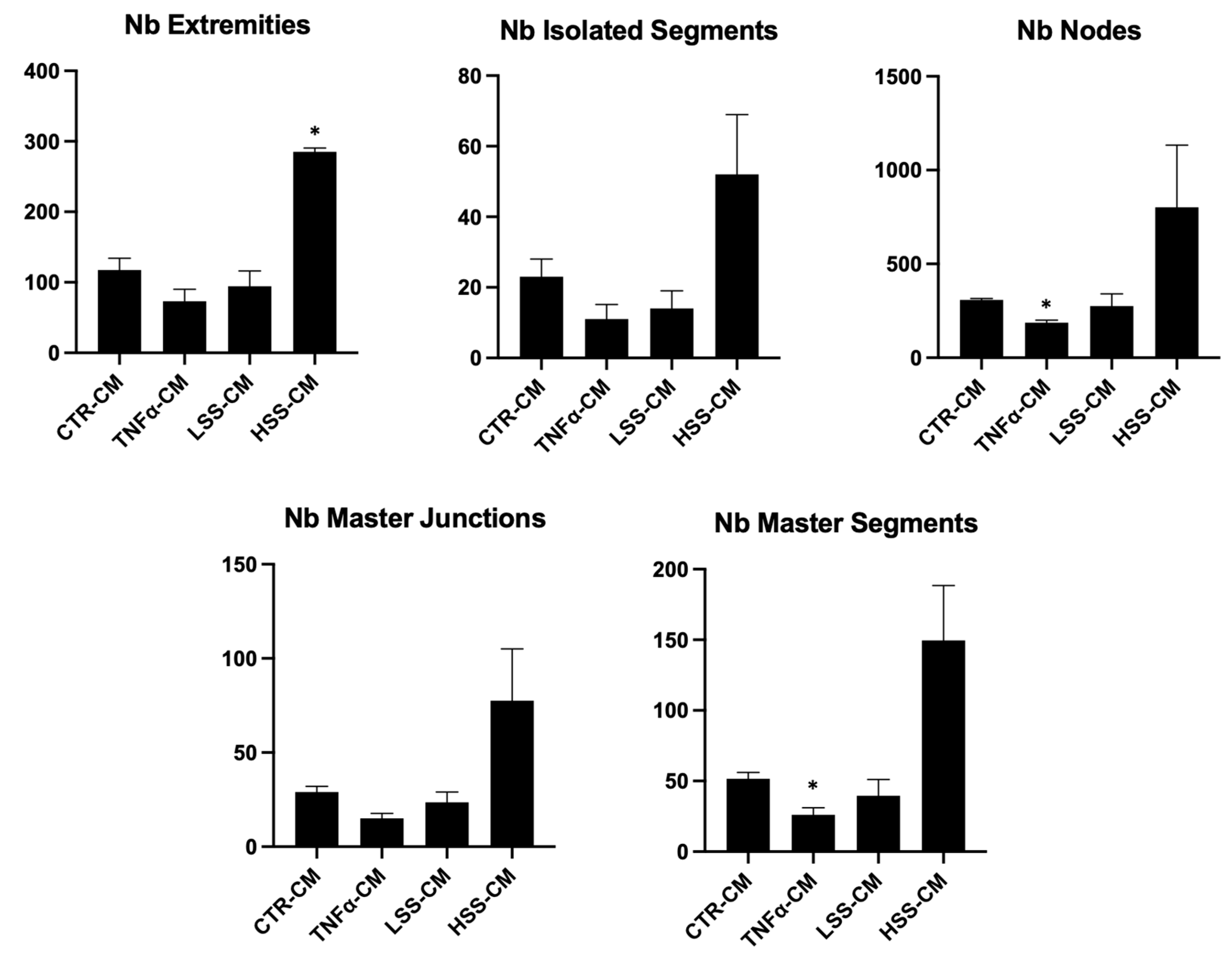
2.2.5. LSS-CM Promotes Tube Formation
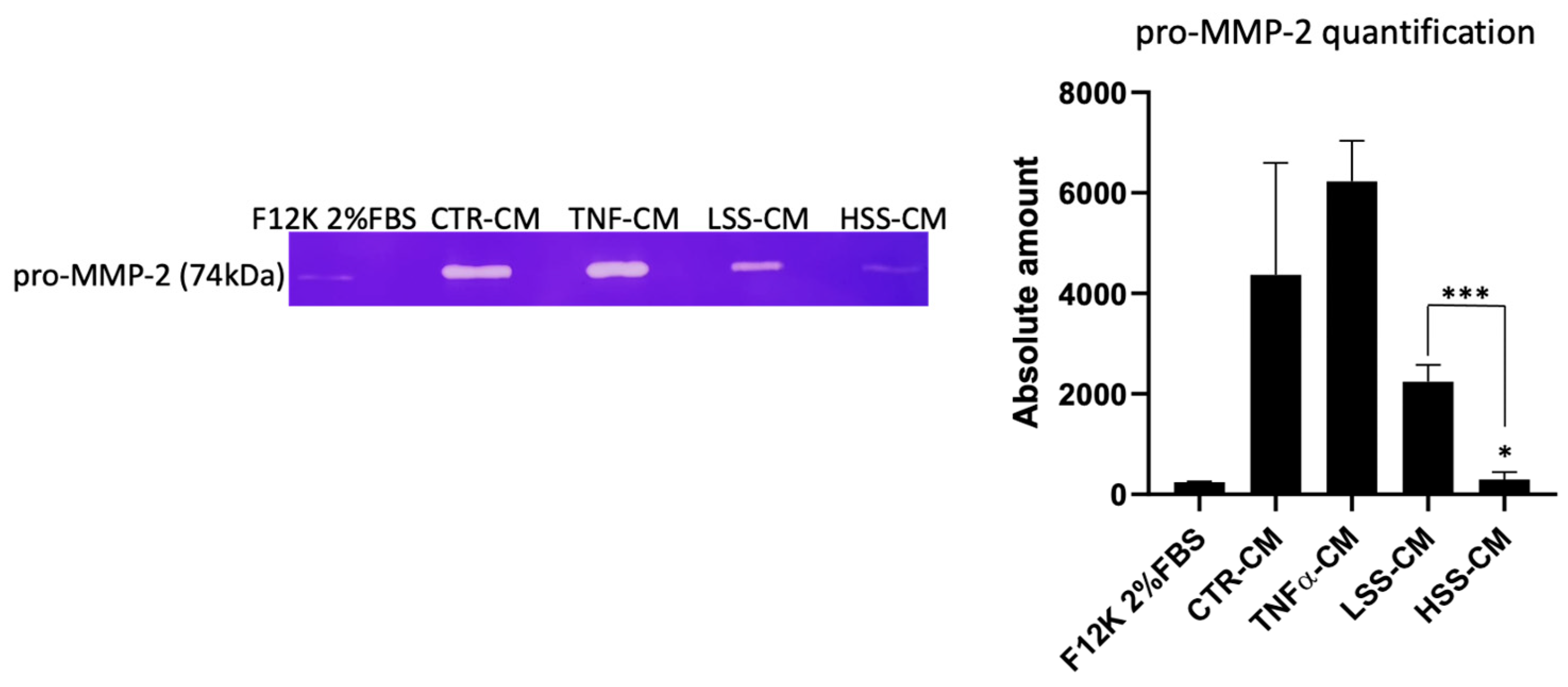
2.2.6. MMP-2 Expression Is Higher in LSS-CM-Cultured HUVECs
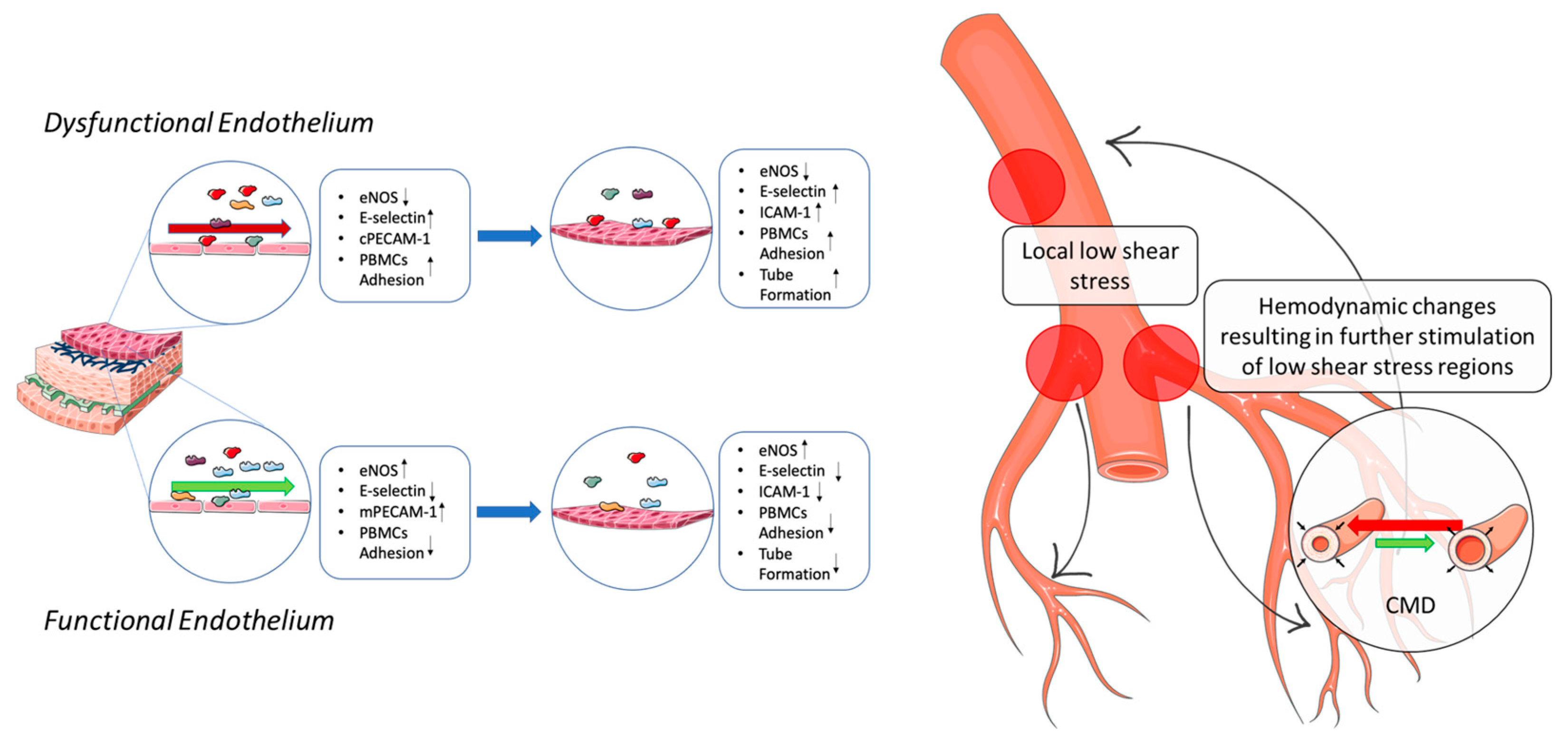
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Microfluidic Culture System
4.3. Morphological Analysis
4.4. Western Blot
4.5. Immunofluorescence Anti-CD31
4.6. Quantitative Real-Time RT-PCR
4.7. Leukocyte Adhesion Assay
4.8. MTT Assay
4.9. Tube Formation Assay
4.10. Gelatin Zymography
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular diseases |
| IHD | Ischemic heart disease |
| ECs | Endothelial cells |
| WSS | Wall shear stress |
| HUVECs | Human umbelical vein endothelial cells |
| HSS | High shear stress |
| HSS-CM | High shear stress conditioned media |
| LSS | Low shear stress |
| LSS-CM | Low shear stress conditioned media |
| eNOS | Endothelial nitric oxide synthase |
| NO | Nitric oxide |
| PBMCs | Peripheral blood mononuclear cells |
| ICAM-1 | Intracellular adhesion molecule 1 |
| TNFα | Tumor necrosis factor alpha |
| TNFα-CM | Tumor necrosis factor alpha conditioned media |
| PECAM-1 | Platelet endothelial cells adhesion molecule 1 |
| VEGFR-2 | Vascular endothelial growth factor receptor 2 |
| CAD | Coronary artery disease |
| E-selectin | Endothelial selectin |
| AJs | Adherens junctions |
| LDL | Low density lipoproteins |
| iNOS | Inducible nitric oxide synthase |
| ROS | Reactive oxygen species |
| HCAECs | Human coronary artery endothelial cells |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet derived growth factor |
| EGF | Epidermal growth factor |
| TGF | Transforming growth factor |
| HRMEC | Human retinal microvascular endothelial cells |
| pro-MMPs | Pro-metalloproteases |
| MMPs | Metalloproteases |
| HMEC-1 | Human microvascular endothelial cells |
| CMD | Coronary microvascular disease |
References
- Loukas, M.; Clarke, P.; Tubbs, R.S.; Kapos, T.; Trotz, M. The His family and their contributions to cardiology. Int. J. Cardiol. 2008, 123, 75–78. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelium and haemostasis. Hamostaseologie 2015, 35, 11–16. [Google Scholar] [CrossRef]
- Félétou, M. The Endothelium, Part I: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators; Morgan & Claypool Life Sciences Publishers: London, UK, 2011; Volume 3. [Google Scholar] [CrossRef]
- Gordon, E.; Schimmel, L.; Frye, M. The Importance of Mechanical Forces for in vitro Endothelial Cell Biology. Front. Physiol. 2020, 11, 684. [Google Scholar] [CrossRef]
- Roux, E.; Bougaran, P.; Dufourcq, P.; Couffinhal, T. Fluid Shear Stress Sensing by the Endothelial Layer. Front. Physiol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; Delisser, H.M.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef]
- Conway, D.E.; Breckenridge, M.T.; Hinde, E.; Gratton, E.; Chen, C.; Schwartz, M.A. Fluid Shear Stress on Endothelial Cells Modulates Mechanical Tension across VE-Cadherin and PECAM-1. Curr. Biol. 2013, 23, 1024–1030. [Google Scholar] [CrossRef] [Green Version]
- Coon, B.G.; Baeyens, N.; Han, J.; Budatha, M.; Ross, T.D.; Fang, J.S.; Yun, S.; Thomas, J.-L.; Schwartz, M.A. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J. Cell Biol. 2015, 208, 975–986. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Effects of Shear Stress on Endothelial Cells: Go with the Flow; Acta Physiologica; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2017; Volume 219, pp. 382–408. [Google Scholar] [CrossRef]
- Lu, T.; Barreuther, M.; Davis, S.; Madri, J.A. Platelet Endothelial Cell Adhesion Molecule-1 Is Phosphorylatable by c-Src, Binds Src-Src homology 2 Domain, and Exhibits Immunoreceptor Tyrosine-based Activation Motif-like Properties. J. Biol. Chem. 1997, 272, 14442–14446. [Google Scholar] [CrossRef] [Green Version]
- Osawa, M.; Masuda, M.; Harada, N.; Lopes, R.B.; Fujiwara, K. Tyrosine phosphorylation of platelet enclothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur. J. Cell Biol. 1997, 72, 229–237. [Google Scholar] [PubMed]
- Xiao, Z.; Zhang, Z.; Diamond, S.L. Shear stress induction of the endothelial nitric oxide synthase gene is calcium-dependent but not calcium-activated. J. Cell. Physiol. 1997, 171, 205–211. [Google Scholar] [CrossRef]
- Davis, M.E.; Grumbach, I.; Fukai, T.; Cutchins, A.; Harrison, D.G. Shear Stress Regulates Endothelial Nitric-oxide Synthase Promoter Activity through Nuclear Factor κB Binding. J. Biol. Chem. 2004, 279, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, K.; Harrison, D.G.; Navas, J.P.; A Fisher, A.; Dockery, S.P.; Uematsu, M.; Nerem, R.M.; Alexander, R.W.; Murphy, T.J. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J. Clin. Investig. 1992, 90, 2092–2096. [Google Scholar] [CrossRef] [Green Version]
- Marsden, P.; Heng, H.; Scherer, S.; Stewart, R.; Hall, A.; Shi, X.; Tsui, L.; Schappert, K. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J. Biol. Chem. 1993, 268, 17478–17488. [Google Scholar] [CrossRef]
- Chatterjee, S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front. Physiol. 2018, 9, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.; Yurdagul, A.; McInnis, M.C.; Albert, P.; Orr, A.W. Flow patterns regulate hyperglycemia-induced subendothelial matrix remodeling during early atherogenesis. Atherosclerosis 2014, 232, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-X.; Qu, X.-L.; Chu, P.; Xie, D.-J.; Zhu, L.-L.; Chao, Y.-L.; Li, L.; Zhang, J.-L.; Chen, S.-L. Low shear stress induces vascular eNOS uncoupling via autophagy-mediated eNOS phosphorylation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.I.; Fulton, D.; Babbitt, R.; Fleming, I.; Busse, R.; Pritchard, K.A., Jr.; Sessa, W.C. Phosphorylation of Threonine 497 in Endothelial Nitric-oxide Synthase Coordinates the Coupling of l-Arginine Metabolism to Efficient Nitric Oxide Production. J. Biol. Chem. 2003, 278, 44719–44726. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.; Remuzzi, A.; Gordon, E.J.; Dewey, C.F.; Gimbrone, M.A. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 2114–2117. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Tempel, D.; Van Haperen, R.; Van Der Baan, A.; Grosveld, F.; Daemen, M.; Krams, R.; De Crom, R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, B.R.; Bäck, M.; Bochaton-Piallat, M.-L.; Caligiuri, G.; Daemen, M.; Davies, P.; Hoefer, I.E.; Holvoet, P.; Jo, H.; Krams, R.; et al. Biomechanical factors in atherosclerosis: Mechanisms and clinical implications. Eur. Hear. J. 2014, 35, 3013–3020. [Google Scholar] [CrossRef]
- Pedrigi, R.M.; Mehta, V.V.; Bovens, S.M.; Mohri, Z.; Poulsen, C.B.; Gsell, W.; Tremoleda, J.L.; Towhidi, L.; de Silva, R.; Petretto, E.; et al. Influence of shear stress magnitude and direction on atherosclerotic plaque composition. R. Soc. Open Sci. 2016, 3, 160588. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.; Qiu, H.; Kim, S.; Jjingo, D.; Hoffman, R.; Kim, C.W.; Jang, I.; Son, D.J.; Kim, D.; Pan, C.; et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J. Clin. Investig. 2014, 124, 3187–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, R.; Qiao, J.; Madhavan, S.; O’Neil, G.L.; Ritchie, B.; Kulkarni, P.; Sridhar, S.; Van De Ven, A.L.; Kemmerling, E.M.C.; Ferris, C.; et al. The comparative effects of high fat diet or disturbed blood flow on glycocalyx integrity and vascular inflammation. Transl. Med. Commun. 2018, 3, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, D.; Ni, C.-W.; Rezvan, A.; Suo, J.; Budzyn, K.; Llanos, A.; Harrison, D.; Giddens, D.; Jo, H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1535–H1543. [Google Scholar] [CrossRef]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial Responses to Shear Stress in Atherosclerosis: A Novel Role for Developmental Genes; Nature Reviews Cardiology; Nature Research: Berlin, Germany, 2020; Volume 17, pp. 52–63. [Google Scholar] [CrossRef]
- Glagov, S.; Zarins, C.; Giddens, D.P.; Ku, D.N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch. Pathol. Lab. Med. 1988, 112, 1018–1031. [Google Scholar] [PubMed]
- Carallo, C.; Tripolino, C.; De Franceschi, M.S.; Irace, C.; Xu, X.Y.; Gnasso, A. Carotid endothelial shear stress reduction with aging is associated with plaque development in twelve years. Atherosclerosis 2016, 251, 63–69. [Google Scholar] [CrossRef]
- Gnasso, A.; Irace, C.; Carallo, C.; De Franceschi, M.S.; Motti, C.; Mattioli, P.L.; Pujia, A. In vivo association between low wall shear stress and plaque in subjects with asymmetrical carotid atherosclerosis. Stroke 1997, 28, 993–998. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, L.M.; Muir, G.; Piterina, A.V.; McGloughlin, T. Vascular Cell Adhesion Molecule-1 Expression in Endothelial Cells Exposed to Physiological Coronary Wall Shear Stresses. J. Biomech. Eng. 2009, 131, 081003. [Google Scholar] [CrossRef]
- Zarins, C.K.; Giddens, D.P.; Bharadvaj, B.K.; Sottiurai, V.S.; Mabon, R.F.; Glagov, S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 1983, 53, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Asakura, T.; Karino, T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ. Res. 1990, 66, 1045–1066. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M.H.; Brinkman, A.M.; Qin, J.J.; Seed, W. Relation between coronary artery geometry and the distribution of early sudanophilic lesions. Atherosclerosis 1993, 98, 193–199. [Google Scholar] [CrossRef]
- Ku, K.H.; Subramaniam, N.; Marsden, P.A. Epigenetic Determinants of Flow-Mediated Vascular Endothelial Gene Expression. Hypertension 2019, 74, 467–476. [Google Scholar] [CrossRef]
- Chiu, J.-J.; Chien, S. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [Green Version]
- Siasos, G.; Tsigkou, V.; Zaromytidou, M.; Sara, J.D.; Varshney, A.; Coskun, A.U.; Lerman, A.; Stone, P.H. Role of Local Coronary Blood Flow Patterns and Shear Stress on the Development of Microvascular and Epicardial Endothelial Dysfunction and Coronary Plaque; Current Opinion in Cardiology; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2018; Volume 33, pp. 638–644. [Google Scholar] [CrossRef]
- Stone, P.H.; Saito, S.; Takahashi, S.; Makita, Y.; Nakamura, S.; Kawasaki, T.; Takahashi, A.; Katsuki, T.; Nakamura, S.; Namiki, A.; et al. Prediction of Progression of Coronary Artery Disease and Clinical Outcomes Using Vascular Profiling of Endothelial Shear Stress and Arterial Plaque Characteristics: The PREDICTION study. Circulation 2012, 126, 172–181. [Google Scholar] [CrossRef]
- Stone, P.H.; Coskun, A.U.; Kinlay, S.; Clark, M.E.; Sonka, M.; Wahle, A.; Ilegbusi, O.J.; Yeghiazarians, Y.; Popma, J.J.; Orav, J.; et al. Effect of Endothelial Shear Stress on the Progression of Coronary Artery Disease, Vascular Remodeling, and In-Stent Restenosis in Humans: In vivo 6-month follow-up study. Circulation 2003, 108, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; De Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Siasos, G.; Sara, J.D.; Zaromytidou, M.; Park, K.H.; Coskun, A.U.; Lerman, L.O.; Oikonomou, E.; Maynard, C.C.; Fotiadis, D.; Stefanou, K.; et al. Local Low Shear Stress and Endothelial Dysfunction in Patients With Nonobstructive Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ren, P.; Li, Q.; Umar, S.A.; Yang, T.; Dong, Y.; Yu, F.; Nie, Y. Low Shear Stress Upregulates CX3CR1 Expression by Inducing VCAM-1 via the NF-κB Pathway in Vascular Endothelial Cells. Cell Biophys. 2020, 78, 383–389. [Google Scholar] [CrossRef]
- Xie, X.; Wang, F.; Zhu, L.; Yang, H.; Pan, D.; Liu, Y.; Qu, X.; Gu, Y.; Li, X.; Chen, S. Low shear stress induces endothelial cell apoptosis and monocyte adhesion by upregulating PECAM-1 expression. Mol. Med. Rep. 2020, 21, 2580–2588. [Google Scholar] [CrossRef]
- Sathanoori, R.; Bryl-Gorecka, P.; Müller, C.E.; Erb, L.; Weisman, G.A.; Olde, B.; Erlinge, D. P2Y2 receptor modulates shear stress-induced cell alignment and actin stress fibers in human umbilical vein endothelial cells. Cell. Mol. Life Sci. 2016, 74, 731–746. [Google Scholar] [CrossRef] [Green Version]
- Levesque, M.J.; Nerem, R.M. The Elongation and Orientation of Cultured Endothelial Cells in Response to Shear Stress. J. Biomech. Eng. 1985, 107, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Steward, R.; Tambe, D.; Hardin, C.C.; Krishnan, R.; Fredberg, J.J. Fluid shear, intercellular stress, and endothelial cell alignment. Am. J. Physiol.-Cell Physiol. 2015, 308, C657–C664. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Cai, H.; Drummond, G.R.; Harrison, D.G. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ. Res. 2001, 89, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zhang, Y.; Zhu, J.; Wang, J.; Jiang, Z.; Zhao, C.; Yang, Q.; Huang, Y.; Yao, W.; Pang, W.; et al. Laminar Flow Protects Vascular Endothelial Tight Junctions and Barrier Function via Maintaining the Expression of Long Non-coding RNA MALAT1. Front. Bioeng. Biotechnol. 2020, 8, 647. [Google Scholar] [CrossRef]
- Seebach, J.; Dieterich, P.; Luo, F.; Schillers, H.; Vestweber, D.; Oberleithner, H.; Galla, H.-J.; Schnittler, H.-J. Endothelial barrier function under laminar fluid shear stress. Lab. Investig. 2000, 80, 1819–1831. [Google Scholar] [CrossRef] [Green Version]
- McCormick, M.E.; Goel, R.; Fulton, D.; Oess, S.; Newman, D.; Tzima, E. PECAM-1 regulates eNOS activity and localization through STAT3-dependent NOSTRIN expression. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 643. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Chatterjee, A.; Catravas, J.D. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vasc. Pharmacol. 2008, 49, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, P.F.; Civelek, M.; Fang, Y.; Fleming, I. The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc. Res. 2013, 99, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, N.; Nawaz, M.; Rezaie, J. Bystander effects of ionizing radiation: Conditioned media from X-ray irradiated MCF-7 cells increases the angiogenic ability of endothelial cells. Cell Commun. Signal. 2019, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Lie, P.; Miao, T.; Tianyu, M.; Lu, Q.; Feng, T.; Li, J.; Zu, T.; Liu, X.; Li, H. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 2015, 12, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Purushothaman, A. Exosomes from Cell Culture-Conditioned Medium: Isolation by Ultracentrifugation and Characterization. In The Extracellular Matrix; Humana Press: New York, NY, USA, 2019; Volume 1952, pp. 233–244. [Google Scholar] [CrossRef]
- Dai, M.; Zhang, Y.; Yu, M.; Tian, W. Therapeutic applications of conditioned medium from adipose tissue. Cell Prolif. 2016, 49, 561–567. [Google Scholar] [CrossRef]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics; Biochemistry (Moscow); Pleiades Publishing: London, UK, 2019; Volume 84, pp. 1375–1389. [Google Scholar] [CrossRef]
- Zhen, J.; Lu, H.; Wang, X.Q.; Vaziri, N.D.; Zhou, X.J. Upregulation of Endothelial and Inducible Nitric Oxide Synthase Expression by Reactive Oxygen Species. Am. J. Hypertens. 2008, 21, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Aljofan, M.; Triggle, C. Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J. Cell. Physiol. 2007, 212, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Ono, S.; Yoshida, A. Low shear stress up-regulation of proinflammatory gene expression in human retinal microvascular endothelial cells. Exp. Eye Res. 2013, 116, 308–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wung, B.S.; Ni, C.W.; Wang, D.L. ICAM-1 induction by TNFα and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J. Biomed. Sci. 2005, 12, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Chico, J.L. Endothelial Function and Shear Stress: Which Came First, the Chicken or the Egg? J. Am. Coll. Cardiol. 2018, 71, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Gloe, T.; Sohn, H.Y.; Meininger, G.A.; Pohl, U. Shear Stress-induced Release of Basic Fibroblast Growth Factor from Endothelial Cells Is Mediated by Matrix Interaction via Integrin αVβ3. J. Biol. Chem. 2002, 277, 23453–23458. [Google Scholar] [CrossRef] [Green Version]
- DeCicco-Skinner, K.L.; Henry, G.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial Cell Tube Formation Assay for the In Vitro Study of Angiogenesis. J. Vis. Exp. 2014, e51312. [Google Scholar] [CrossRef]
- Russo, T.A.; Banuth, A.M.M.; Nader, H.B.; Dreyfuss, J.L. Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis. PLoS ONE 2020, 15, e0241040. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Rojiani, M.V.; Alidina, J.; Esposito, N.; Rojiani, A.M. Expression of MMP-2 correlates with increased angiogenesis in CNS metastasis of lung carcinoma. Int. J. Clin. Exp. Pathol. 2010, 3, 775–781. [Google Scholar]
- Linder, S. The matrix corroded: Podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007, 17, 107–117. [Google Scholar] [CrossRef]
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low Diagnostic Yield of Elective Coronary Angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbaek, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Hear. J. 2012, 33, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, I.; Tuzcu, E.M.; Schoenhagen, P.; Nicholls, S.J.; Chen, M.S.; Crowe, T.; Loyd, A.B.; Kapadia, S.; Nissen, S.E. Paradoxical increase in lumen size during progression of coronary atherosclerosis: Observations from the REVERSAL trial. Atherosclerosis 2006, 189, 229–235. [Google Scholar] [CrossRef]
- Epstein, S.E.; Cannon, R.O. Site of increased resistance to coronary flow in patients with angina pectoris and normal epicardial coronary arteries. J. Am. Coll. Cardiol. 1986, 8, 459–461. [Google Scholar] [CrossRef] [Green Version]
- Camici, P.G.; Rimoldi, O.E. The Clinical Value of Myocardial Blood Flow Measurement. J. Nucl. Med. 2009, 50, 1076–1087. [Google Scholar] [CrossRef] [Green Version]
- Crea, F.; Camici, P.G.; Merz, C.N.B. Coronary microvascular dysfunction: An update. Eur. Hear. J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [Green Version]
- Tarbell, J.M. Shear stress and the endothelial transport barrier. Cardiovasc. Res. 2010, 87, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Ing, M.H.; Salazar, A.; Lassègue, B.; Griendling, K.; Navab, M.; Sevanian, A.; Hsiai, T.K. Pulsatile Versus Oscillatory Shear Stress Regulates NADPH Oxidase Subunit Expression: Implication for Native LDL Oxidation. Circ. Res. 2003, 93, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Ai, L.; Rouhanizadeh, M.; Wu, J.C.; Takabe, W.; Yu, H.; Alavi, M.; Li, R.; Chu, Y.; Miller, J.; Heistad, D.D.; et al. Shear stress influences spatial variations in vascular Mn-SOD expression: Implication for LDL nitration. Am. J. Physiol.-Cell Physiol. 2008, 294, C1576–C1585. [Google Scholar] [CrossRef] [Green Version]
- Schoenhagen, P.; Ziada, K.M.; Kapadia, S.R.; Crowe, T.D.; Nissen, S.E.; Tuzcu, E.M. Extent and Direction of Arterial Remodeling in Stable Versus Unstable Coronary Syndromes: An intravascular ultrasound study. Circulation 2000, 101, 598–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Rouhanizadeh, M.; Hamilton, R.T.; Lin, T.C.; Eiserich, J.P.; Hodis, H.N.; Hsiai, T.K. 17β-Estradiol reverses shear-stress-mediated low density lipoprotein modifications. Free. Radic. Biol. Med. 2006, 41, 568–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertani, F.; Di Francesco, D.; Corrado, M.D.; Talmon, M.; Fresu, L.G.; Boccafoschi, F. Paracrine Shear-Stress-Dependent Signaling from Endothelial Cells Affects Downstream Endothelial Function and Inflammation. Int. J. Mol. Sci. 2021, 22, 13300. https://doi.org/10.3390/ijms222413300
Bertani F, Di Francesco D, Corrado MD, Talmon M, Fresu LG, Boccafoschi F. Paracrine Shear-Stress-Dependent Signaling from Endothelial Cells Affects Downstream Endothelial Function and Inflammation. International Journal of Molecular Sciences. 2021; 22(24):13300. https://doi.org/10.3390/ijms222413300
Chicago/Turabian StyleBertani, Fabio, Dalila Di Francesco, Maria Dolores Corrado, Maria Talmon, Luigia Grazia Fresu, and Francesca Boccafoschi. 2021. "Paracrine Shear-Stress-Dependent Signaling from Endothelial Cells Affects Downstream Endothelial Function and Inflammation" International Journal of Molecular Sciences 22, no. 24: 13300. https://doi.org/10.3390/ijms222413300
APA StyleBertani, F., Di Francesco, D., Corrado, M. D., Talmon, M., Fresu, L. G., & Boccafoschi, F. (2021). Paracrine Shear-Stress-Dependent Signaling from Endothelial Cells Affects Downstream Endothelial Function and Inflammation. International Journal of Molecular Sciences, 22(24), 13300. https://doi.org/10.3390/ijms222413300





