Fucosylation in Urological Cancers
Abstract
:1. Introduction
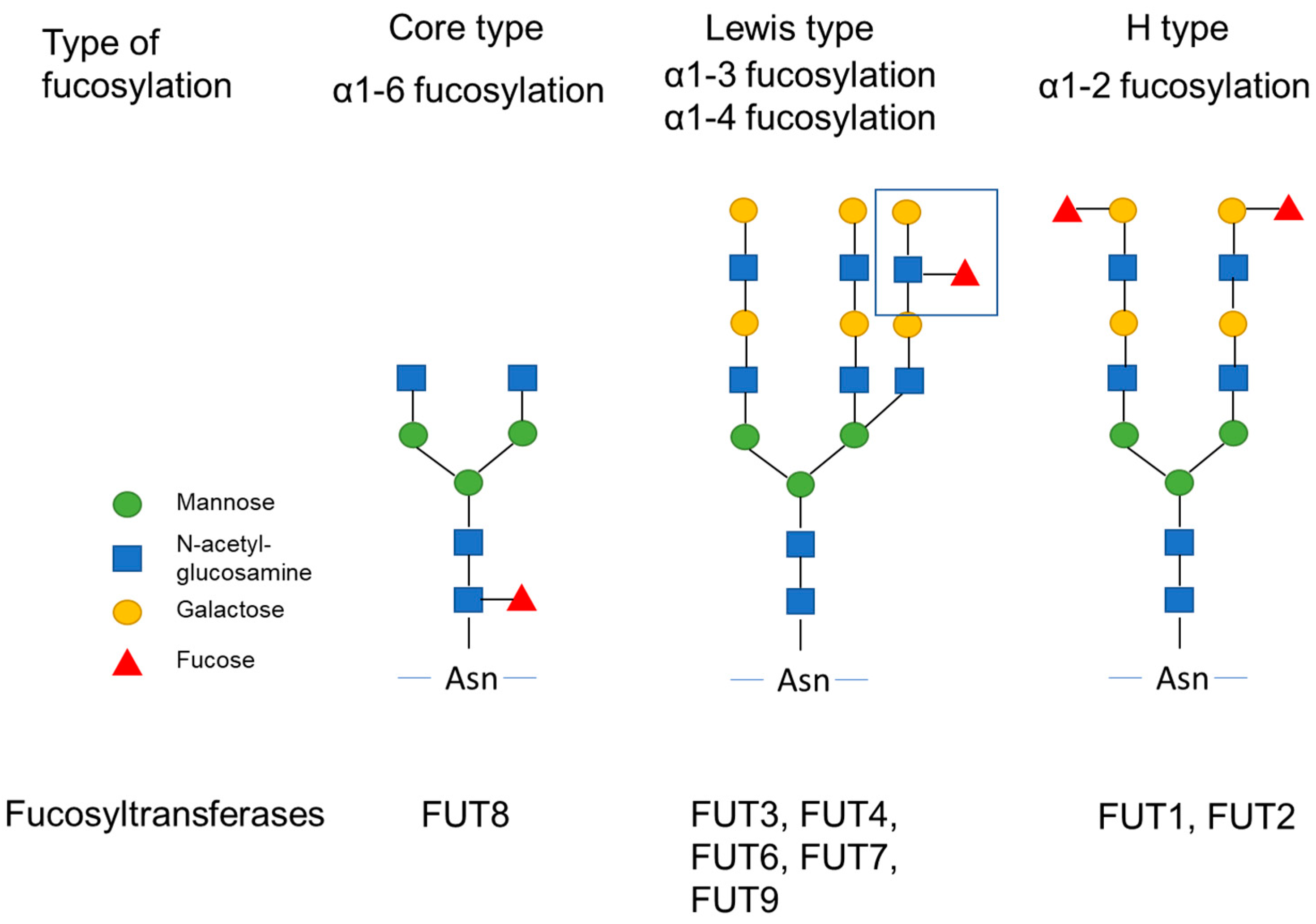
2. Biological Function of Fucosyltransferases and GDP-Fucose Synthetic Enzyme
GDP-Fucose Synthetic Enzyme
3. Fucosylation in Cancer Immunology
4. Fucosylation in Renal Cell Carcinoma
5. Fucosylation in Urothelial Carcinoma
6. Fucosylation in Prostate Cancer
7. Therapeutic Target of Fucosylation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aoyagi, Y.; Isemura, M.; Suzuki, Y.; Sekine, C.; Soga, K.; Ozaki, T.; Ichida, F. Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet 1985, 2, 1353–1354. [Google Scholar] [CrossRef]
- Connor, J.A. Making sense of the Food and Drug Administration. Semin. Pediatr. Surg. 2006, 15, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Szymendera, J.J. Clinical usefulness of three monoclonal antibody-defined tumor markers: CA 19-9, CA 50, and CA 125. Tumour Biol. 1986, 7, 333–342. [Google Scholar] [PubMed]
- Miyoshi, E.; Moriwaki, K.; Terao, N.; Tan, C.C.; Terao, M.; Nakagawa, T.; Matsumoto, H.; Shinzaki, S.; Kamada, Y. Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules 2012, 2, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, E.; Moriwaki, K.; Nakagawa, T. Biological function of fucosylation in cancer biology. J. Biochem. 2008, 143, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Weston, B.W.; Nair, R.P.; Larsen, R.D.; Lowe, J.B. Isolation of a novel human α(1,3)fucosyltransferase gene and molecular comparison to the human lewis blood group α(1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J. Biol. Chem. 1992, 267, 4152–4160. [Google Scholar] [CrossRef]
- Larsen, R.D.; Ernst, L.K.; Nair, R.P.; Lowe, J.B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:β-D-galactoside 2-α-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc. Natl. Acad. Sci. USA 1990, 87, 6674–6678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagidani, S.; Uozumi, N.; Ihara, Y.; Miyoshi, E.; Yamaguchi, N.; Taniguchi, N. Purification and cDNA cloning of GDP-L-Fuc: N-acetyl-β-D-glucosaminide: α1-6 fucosyltransferase (α1-6 FucT) from human gastric cancer MKN45 cells. J. Biochem. 1997, 121, 626–632. [Google Scholar] [CrossRef]
- Lai, T.Y.; Chen, I.J.; Lin, R.J.; Liao, G.S.; Yeo, H.L.; Ho, C.L.; Wu, J.C.; Chang, N.C.; Lee, A.C.L.; Yu, A.L. Fucosyltransferase 1 and 2 play pivotal roles in breast cancer cells. Cell Death Discov. 2019, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, M.; Qi, Y.; Wang, H.; Liu, M.; Liu, Q.; Lin, B. Lewis(y) antigen-mediated positive feedback loop induces and promotes chemotherapeutic resistance in ovarian cancer. Int. J. Oncol. 2018, 53, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Sha, D.; Qin, X.Y.; Li, C.Z.; Lin, B. Alpha1,2-fucosyl transferase gene, the key enzyme of Lewis y synthesis, promotes taxol resistance of ovarian carcinoma through apoptosis-related proteins. Neoplasma 2018, 65, 515–522. [Google Scholar] [CrossRef]
- do Nascimento, J.C.F.; Beltrão, E.I.C.; Rocha, C.R.C. High FUT3 expression is a marker of lower overall survival of breast cancer patients. Glycoconj. J. 2020, 37, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Bassagañas, S.; Allende, H.; Cobler, L.; Ortiz, M.R.; Llop, E.; de Bolós, C.; Peracaula, R. Inflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell lines. Cytokine 2015, 75, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mare, L.; Caretti, A.; Albertini, R.; Trinchera, M. CA19.9 antigen circulating in the serum of colon cancer patients: Where is it from? Int. J. Biochem. Cell Biol. 2013, 45, 792–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasky, L.A. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem. 1995, 64, 113–140. [Google Scholar] [CrossRef]
- Burdick, M.M.; Henson, K.A.; Delgadillo, L.F.; Choi, Y.E.; Goetz, D.J.; Tees, D.F.J.; Benencia, F. Expression of E-selectin ligands on circulating tumor cells: Cross-regulation with cancer stem cell regulatory pathways? Front. Oncol. 2012, 2, 103. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.J.; Zheng, X.F.; Lu, C.H.; Jiang, Q.; Liu, Q.; Xin, Y.H. Effect of FUT3 gene silencing with miRNA on proliferation, invasion and migration abilities of human KATO-III gastric cancer cell line. Cell. Mol. Biol. 2016, 62, 15–20. [Google Scholar] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Gao, W.; Liang, J.; Ye, Y.; Lu, J.; Lin, T.; Wang, N.; Dong, J.; Pan, J. FUT4siRNA augments the chemosensitivity of non-small cell lung cancer to cisplatin through activation of FOXO1-induced apoptosis. BMC Cancer 2020, 20, 895. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Lin, S.Y.; Weng, R.R.; Juan, Y.H.; Chen, Y.W.; Hou, H.H.; Hung, Z.C.; Oswita, G.A.; Huang, Y.J.; Guu, S.Y.; et al. Fucosyltransferase 4 shapes oncogenic glycoproteome to drive metastasis of lung adenocarcinoma. EBioMedicine 2020, 57, 102846. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, Y.; Liu, B.; Pan, S.; Liu, Q.; Shan, Y.; Li, S.; Qi, Y.; Huang, Y.; Jia, L. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2020, 39, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Gao, C.; Li, Y.; Sun, M.; Xu, J.; Li, H.; Jia, L.; Zhao, Y. MiR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017, 8, e2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, H.; Liu, J.; Yu, M.; Wang, H.; Thomas, A.M.; Li, S.; Yan, Q.; Wang, L. FUT7 promotes the malignant transformation of follicular thyroid carcinoma through α1,3-fucosylation of EGF receptor. Exp. Cell Res. 2020, 393, 112095. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, Q.; Chen, S.; Liu, J.; Li, S. Fut7 promotes the epithelial–mesenchymal transition and immune infiltration in bladder urothelial carcinoma. J. Inflamm. Res. 2021, 14, 1069–1084. [Google Scholar] [CrossRef]
- Bastian, K.; Scott, E.; Elliott, D.J.; Munkley, J. Fut8 alpha-(1,6)-fucosyltransferase in cancer. Int. J. Mol. Sci. 2021, 22, 455. [Google Scholar] [CrossRef]
- Tu, C.F.; Wu, M.Y.; Lin, Y.C.; Kannagi, R.; Yang, R.B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017, 19, 111. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gu, J.; Miyoshi, E.; Honke, K.; Taniguchi, N. Phenotype Changes of Fut8 Knockout Mouse: Core Fucosylation Is Crucial for the Function of Growth Factor Receptor(s). Methods Enzymol. 2006, 417, 11–22. [Google Scholar] [PubMed]
- Lin, H.; Wang, D.; Wu, T.; Dong, C.; Shen, N.; Sun, Y.; Sun, Y.; Xie, H.; Wang, N.; Shan, L. Blocking core fucosylation of TGF-β1 receptors downregulates their functions and attenuates the epithelial-mesenchymal transition of renal tubular cells. Am. J. Physiol.-Ren. Physiol. 2011, 300, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, S.; Cui, Y.; Guo, T.; Qiang, J.; Xie, Q.; Yu, W.; Guo, W.; Deng, W.; Gu, C.; et al. α1,6-Fucosyltransferase (FUT8) regulates the cancer-promoting capacity of cancer-associated fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in non-small cell lung cancer (NSCLC). Am. J. Cancer Res. 2020, 10, 816–837. [Google Scholar] [PubMed]
- Clark, D.J.; Schnaubelt, M.; Hoti, N.; Hu, Y.; Zhou, Y.; Gooya, M.; Zhang, H. Impact of Increased FUT8 Expression on the Extracellular Vesicle Proteome in Prostate Cancer Cells. J. Proteome Res. 2020, 19, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Blanas, A.; Zaal, A.; van der Haar Àvila, I.; Kempers, M.; Kruijssen, L.; de Kok, M.; Popovic, M.A.; van der Horst, J.C.; van Vliet, S.J. Fut9-driven programming of colon cancer cells towards a stem cell-like state. Cancers 2020, 12, 2580. [Google Scholar] [CrossRef]
- Zodro, E.; Jaroszewski, M.; Ida, A.; Wrzesiński, T.; Kwias, Z.; Bluyssen, H.; Wesoly, J. FUT11 as a potential biomarker of clear cell renal cell carcinoma progression based on meta-analysis of gene expression data. Tumor Biol. 2014, 35, 2607–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Gao, Y.; Zhang, X.; Guo, M.; Yang, J.; Cui, H.; Kong, P.; Niu, X.; Bi, Y.; Xu, J.; et al. TSTA3 facilitates esophageal squamous cell carcinoma progression through regulating fucosylation of LAMP2 and ERBB2. Theranostics 2020, 10, 11339–11358. [Google Scholar] [CrossRef]
- Arriagada, C.; Cavieres, V.A.; Luchsinger, C.; González, A.E.; Muñoz, V.C.; Cancino, J.; Burgos, P.V.; Mardones, G.A. GOLPH3 regulates EGFR in T98G glioblastoma cells by modulating its glycosylation and ubiquitylation. Int. J. Mol. Sci. 2020, 21, 8880. [Google Scholar] [CrossRef]
- Doud, E.H.; Shetty, T.; Abt, M.; Mosley, A.L.; Corson, T.W.; Mehta, A.; Yeh, E.S. Nf-κb signaling is regulated by fucosylation in metastatic breast cancer cells. Biomedicines 2020, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Righetto, M.; Noessner, E. Checkpoint Inhibition in Bladder Cancer: Clinical Expectations, Current Evidence, and Proposal of Future Strategies Based on a Tumor-Specific Immunobiological Approach. Cancers 2021, 13, 6016. [Google Scholar] [CrossRef]
- Kirwan, A.; Utratna, M.; O’Dwyer, M.E.; Joshi, L.; Kilcoyne, M. Glycosylation-Based Serum Biomarkers for Cancer Diagnostics and Prognostics. Biomed Res. Int. 2015, 2015, 490531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef] [Green Version]
- Veillon, L.; Fakih, C.; Abou-El-Hassan, H.; Kobeissy, F.; Mechref, Y. Glycosylation Changes in Brain Cancer. ACS Chem. Neurosci. 2018, 9, 51–72. [Google Scholar] [CrossRef]
- Okada, M.; Chikuma, S.; Kondo, T.; Hibino, S.; Machiyama, H.; Yokosuka, T.; Nakano, M.; Yoshimura, A. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep. 2017, 20, 1017–1028. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Mao, S.; Sun, S.; Li, M.; Li, Z.; Yu, R.; Ma, T.; Gu, J.; Zhang, J.; Taniguchi, N.; et al. Core fucosylation of the T cell receptor is required for T cell activation. Front. Immunol. 2018, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Fujii, H.; Shinzaki, S.; Iijima, H.; Wakamatsu, K.; Iwamoto, C.; Sobajima, T.; Kuwahara, R.; Hiyama, S.; Hayashi, Y.; Takamatsu, S.; et al. Core Fucosylation on T Cells, Required for Activation of T-Cell Receptor Signaling and Induction of Colitis in Mice, Is Increased in Patients with Inflammatory Bowel Disease. Gastroenterology 2016, 150, 1620–1632. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Mao, S.; Li, M.; Zhang, N.; Sun, S.; Fang, H.; Zhang, J.; Gu, J.; Wang, J.; Li, W. Ablation of core fucosylation attenuates the signal transduction via T cell receptor to suppress the T cell development. Mol. Immunol. 2019, 112, 312–321. [Google Scholar] [CrossRef]
- Zhang, N.; Li, M.; Xu, X.; Zhang, Y.; Liu, Y.; Zhao, M.; Li, P.; Chen, J.; Fukuda, T.; Gu, J.; et al. Loss of core fucosylation enhances the anticancer activity of cytotoxic T lymphocytes by increasing PD-1 degradation. Eur. J. Immunol. 2020, 50, 1820–1833. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Wakamatsu, K.; Fujii, H.; Shinzaki, S.; Takamatsu, S.; Kitazume, S.; Kamada, Y.; Takehara, T.; Taniguchi, N.; Miyoshi, E. Core fucose is essential glycosylation for CD14-dependent Toll-like receptor 4 and Toll-like receptor 2 signalling in macrophages. J. Biochem. 2019, 165, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, T.; Yazawa, S.; Asao, T.; Nakazawa, N.; Mogi, A.; Sano, R.; Kuwano, H.; Kaira, K.; Shirabe, K. Fucosylated α1-acid glycoprotein as a biomarker to predict prognosis following tumor immunotherapy of patients with lung cancer. Sci. Rep. 2019, 9, 14503. [Google Scholar] [CrossRef] [Green Version]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Gloria Meng, Y.; Weikert, S.H.A.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, C.; Grau, S.; Jäger, C.; Sondermann, P.; Brünker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef] [Green Version]
- Pantuck, A.J.; Zisman, A.; Belldegrun, A.S. The changing natural history of renal cell carcinoma. J. Urol. 2001, 166, 1611–1623. [Google Scholar] [CrossRef]
- von Roemeling, C.A.; Marlow, L.A.; Radisky, D.C.; Rohl, A.; Larsen, H.E.; Wei, J.; Sasinowska, H.; Zhu, H.; Drake, R.; Sasinowski, M.; et al. Functional genomics identifies novel genes essential for clear cell renal cell carcinoma tumor cell proliferation and migration. Oncotarget 2014, 5, 5320–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, R.R.; McDowell, C.; West, C.; David, F.; Powers, T.W.; Nowling, T.; Bruner, E.; Mehta, A.S.; Angel, P.M.; Marlow, L.A.; et al. Defining the human kidney N-glycome in normal and cancer tissues using MALDI imaging mass spectrometry. J. Mass Spectrom. 2020, 55, e4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tousi, F.; Bones, J.; Iliopoulos, O.; Hancock, W.S.; Hincapie, M. Multidimensional liquid chromatography platform for profiling alterations of clusterin N-glycosylation in the plasma of patients with renal cell carcinoma. J. Chromatogr. A 2012, 1256, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gbormittah, F.O.; Bones, J.; Hincapie, M.; Tousi, F.; Hancock, W.S.; Iliopoulos, O. Clusterin glycopeptide variant characterization reveals significant site-specific glycan changes in the plasma of clear cell renal cell carcinoma. J. Proteome Res. 2015, 14, 2425–2436. [Google Scholar] [CrossRef]
- Meng, L.; Xu, L.; Yang, Y.; Zhou, L.; Chang, Y.; Shi, T.; Tan, C.; An, H.; Zhu, Y.; Xu, J. High expression of FUT3 is linked to poor prognosis in clear cell renal cell carcinoma. Oncotarget 2017, 8, 61036–61047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, J.; Nishiyama, H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017, 24, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rhijn, B.W.G.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and Progression of Disease in Non-Muscle-Invasive Bladder Cancer: From Epidemiology to Treatment Strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Roehrborn, C.G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: Results of a comprehensive literature review and meta-analyses. Urology 2003, 61, 109–118. [Google Scholar] [CrossRef]
- Mancini, M.; Righetto, M.; Zumerle, S.; Montopoli, M.; Zattoni, F. The Bladder EpiCheck Test as a Non-Invasive Tool Based on the Identification of DNA Methylation in Bladder Cancer Cells in the Urine: A Review of Published Evidence. Int. J. Mol. Sci. 2020, 21, 6542. [Google Scholar] [CrossRef]
- Yang, G.; Tan, Z.; Lu, W.; Guo, J.; Yu, H.; Yu, J.; Sun, C.; Qi, X.; Li, Z.; Guan, F. Quantitative glycome analysis of N-glycan patterns in bladder cancer vs. normal bladder cells using an integrated strategy. J. Proteome Res. 2015, 14, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Chen, C.N.; Chu, C.Y.; Lu, J.H.; Wang, B.J.; Chen, C.H.; Huang, M.C.; Lin, T.H.; Pan, C.C.; Chen, S.S.A.; et al. Calreticulin activates β1 integrin via fucosylation by fucosyltransferase 1 in J82 human bladder cancer cells. Biochem. J. 2014, 460, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Sheinfeld, J.; Reuter, V.E.; Melamed, M.R.; Fair, W.R.; Morse, M.; Sogani, P.C.; Herr, H.W.; Whitmore, W.F.; Cordon-Cardo, C. Enhanced bladder cancer detection with the Lewis X antigen as a marker of neoplastic transformation. J. Urol. 1990, 143, 285–288. [Google Scholar] [CrossRef]
- Blanas, A.; Sahasrabudhe, N.M.; Rodríguez, E.; van Kooyk, Y.; van Vliet, S.J. Fucosylated antigens in cancer: An alliance toward tumor progression, metastasis, and resistance to chemotherapy. Front. Oncol. 2018, 8, 39. [Google Scholar] [CrossRef]
- Pal, S.K.; Pham, A.; Vuong, W.; Liu, X.; Lin, Y.; Ruel, N.; Yuh, B.E.; Chan, K.; Wilson, T.; Lerner, S.P.; et al. Prognostic Significance of Neutrophilic Infiltration in Benign Lymph Nodes in Patients with Muscle-invasive Bladder Cancer. Eur. Urol. Focus 2017, 3, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.J.; Iyengar, P.V.; Lama, D.; Lui, S.K.L.; Ng, H.C.; Haviv-Shapira, L.; Domany, E.; Kappei, D.; Tan, T.Z.; Saie, A.; et al. c-Met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression. Nat. Commun. 2019, 10, 4349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Zhang, M.; Chen, G.; Wang, W.; Zhang, P.; Yue, Y.; Guan, Z.; Wang, X.; Fan, J. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int. J. Oncol. 2019, 54, 1555–1566. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, X.; Tan, Z.; Lu, W.; Yang, G.; Guan, F. Alteration of N-glycans and expression of their related glycogenes in the epithelial-mesenchymal transition of HCV29 bladder epithelial cells. Molecules 2014, 19, 20073–20090. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.K.; Syed, P.; Dhondt, B.; Gidwani, K.; Pettersson, K.; Lamminmäki, U.; Leivo, J. Detection of bladder cancer with aberrantly fucosylated ITGA3. Anal. Biochem. 2021, 628, 114283. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkley, J.; Mills, I.G.; Elliott, D.J. The role of glycans in the development and progression of prostate cancer. Nat. Rev. Urol. 2016, 13, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Munkley, J. Glycans as biomarkers in prostate cancer. Int. J. Mol. Sci. 2019, 20, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Sugiyama, T.; Shimomura, M.; Kamada, Y.; Fujita, K.; Nonomura, N.; Miyoshi, E.; Nakano, M. Site-specific and linkage analyses of fucosylated N-glycans on haptoglobin in sera of patients with various types of cancer: Possible implication for the differential diagnosis of cancer. Glycoconj. J. 2016, 33, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Saldova, R.; Fan, Y.; Fitzpatrick, J.M.; Watson, R.W.G.; Rudd, P.M. Core fucosylation and α2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology 2011, 21, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totten, S.M.; Adusumilli, R.; Kullolli, M.; Tanimoto, C.; Brooks, J.D.; Mallick, P.; Pitteri, S.J. Multi-lectin Affinity Chromatography and Quantitative Proteomic Analysis Reveal Differential Glycoform Levels between Prostate Cancer and Benign Prostatic Hyperplasia Sera. Sci. Rep. 2018, 8, 6509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyselova, Z.; Mechref, Y.; Al Bataineh, M.M.; Dobrolecki, L.E.; Hickey, R.J.; Vinson, J.; Sweeney, C.J.; Novotny, M.V. Alterations in the serum glycome due to metastatic prostate cancer. J. Proteome Res. 2007, 6, 1822–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthel, S.R.; Hays, D.L.; Yazawa, E.M.; Opperman, M.; Walley, K.C.; Nimrichter, L.; Burdick, M.M.; Gillard, B.M.; Moser, M.T.; Pantel, K.; et al. Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 2013, 73, 942–952. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.; Wang, X.; Yang, W.; Eshghi, S.T.; Sun, S.; Hoti, N.; Chen, L.; Yang, S.; Pasay, J.; Rubin, A.; et al. Integrated proteomic and glycoproteomic analyses of prostate cancer cells reveal glycoprotein alteration in protein abundance and glycosylation. Mol. Cell. Proteom. 2015, 14, 2753–2763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yang, W.; Hu, Y.; Höti, N.; Liu, Y.; Shah, P.; Sun, S.; Clark, D.; Thomas, S.; Zhang, H. Site-Specific Fucosylation Analysis Identifying Glycoproteins Associated with Aggressive Prostate Cancer Cell Lines Using Tandem Affinity Enrichments of Intact Glycopeptides Followed by Mass Spectrometry. Anal. Chem. 2017, 89, 7623–7630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Inoue, S.; Gu, J.; Miyoshi, E.; Noda, K.; Li, W.; Mizuno-Horikawa, Y.; Nakano, M.; Asahi, M.; Takahashi, M.; et al. Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA 2005, 102, 15791–15796. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Shimomura, M.; Uemura, M.; Nakata, W.; Sato, M.; Nagahara, A.; Nakai, Y.; Takamatsu, S.; Miyoshi, E.; Nonomura, N. Serum fucosylated haptoglobin as a novel prognostic biomarker predicting high-Gleason prostate cancer. Prostate 2014, 74, 1052–1058. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Li, Q.K.; Peskoe, S.B.; Zhang, B.; Choi, C.; Platz, E.A.; Zhang, H. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology 2014, 24, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Höti, N.; Yang, S.; Hu, Y.; Shah, P.; Haffner, M.C.; Zhang, H. Overexpression of α (1,6) fucosyltransferase in the development of castration-resistant prostate cancer cells. Prostate Cancer Prostatic Dis. 2018, 21, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilgunn, S.; Conroy, P.J.; Saldova, R.; Rudd, P.M.; O’Kennedy, R.J. Aberrant PSA glycosylation—A sweet predictor of prostate cancer. Nat. Rev. Urol. 2013, 10, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, C.; Hosono, M.; Nitta, K.; Oh-eda, M.; Yoshikawa, K.; Habuchi, T.; Arai, Y.; Fukuda, M. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology 2004, 14, 671–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, K.; Satoh, T.; Baba, S.; Yamashita, K. α1,2-Fucosylated and β-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostatic cancer. Glycobiology 2009, 20, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Dwek, M.V.; Jenks, A.; Leathem, A.J.C. A sensitive assay to measure biomarker glycosylation demonstrates increased fucosylation of prostate specific antigen (PSA) in patients with prostate cancer compared with benign prostatic hyperplasia. Clin. Chim. Acta 2010, 411, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.K.; Chen, L.; Ao, M.H.; Chiu, J.H.; Zhang, Z.; Zhang, H.; Chan, D.W. Serum fucosylated prostate-specific antigen (PSA) improves the differentiation of aggressive from non-aggressive prostate cancers. Theranostics 2015, 5, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Rubén, L.C.; Laura, M.R.; Almudena, F.B.; Emilio, G.M. Glycan array analysis of Pholiota squarrosa lectin and other fucose-oriented lectins. Glycobiology 2021, 31, 459–476. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yoneyama, T.; Tobisawa, Y.; Hatakeyama, S.; Kurosawa, T.; Nakamura, K.; Narita, S.; Mitsuzuka, K.; Duivenvoorden, W.; Pinthus, J.H.; et al. An Automated Micro-Total Immunoassay System for Measuring Cancer-Associated α2,3-linked Sialyl N-Glycan-Carrying Prostate-Specific Antigen May Improve the Accuracy of Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2017, 18, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, K.; Hatano, K.; Tomiyama, E.; Hayashi, Y.; Matsushita, M.; Tsuchiya, M.; Yoshikawa, T.; Date, M.; Miyoshi, E.; Nonomura, N. Serum core-type fucosylated prostate-specific antigen index for the detection of high-risk prostate cancer. Int. J. Cancer 2021, 148, 3111–3118. [Google Scholar] [CrossRef]
- Hatano, K.; Yoneyama, T.; Hatakeyama, S.; Tomiyama, E.; Tsuchiya, M.; Nishimoto, M.; Yoshimura, K.; Miyoshi, E.; Uemura, H.; Ohyama, C.; et al. Simultaneous analysis of serum α2,3-linked sialylation and core-type fucosylation of prostate-specific antigen for the detection of high-grade prostate cancer. Br. J. Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Hartke, R.; Li, C.; Yang, Q.; Liu, J.O.; Wang, L.X. Synthetic Fluorinated l -Fucose Analogs Inhibit Proliferation of Cancer Cells and Primary Endothelial Cells. ACS Chem. Biol. 2020, 15, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fukuda, T.; Hang, Q.; Hou, S.; Isaji, T.; Kameyama, A.; Gu, J. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci. Rep. 2017, 7, 11563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriwaki, K.; Noda, K.; Furukawa, Y.; Ohshima, K.; Uchiyama, A.; Nakagawa, T.; Taniguchi, N.; Daigo, Y.; Nakamura, Y.; Hayashi, N.; et al. Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology 2009, 137, 188–198.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashkenazi, A.; Herbst, R.S. To kill a tumor cell: The potential of proapoptotic receptor agonists. J. Clin. Investig. 2008, 118, 1979–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koschny, R.; Holland, H.; Sykora, J.; Haas, T.L.; Sprick, M.R.; Ganten, T.M.; Krupp, W.; Bauer, M.; Ahnert, P.; Meixensberger, J.; et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin. Cancer Res. 2007, 13, 3403–3412. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Zhang, X.D.; Hersey, P. Relative Resistance of Fresh Isolates of Melanoma to Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL)-induced Apoptosis. Cancer Res. 2001, 7, 966s–973s. [Google Scholar]
| Fucosyltransferase | Type of Fucosylation | Function in Cancer | References |
|---|---|---|---|
| FUT1 | α1-2 fucosylation | Migration, invasion, epithelial-mesenchymal transition, and drug resistance | [6,7,8] |
| FUT2 | α1-2 fucosylation | Migration, invasion, and epithelial-mesenchymal transition (EMT) | [6] |
| FUT3 | α1-3/α1-4 fucosylation | Activation of TGF-β signaling pathway | [9,10] |
| FUT4 | α1-3/α1-4 fucosylation | Drug resistance, invasion, migration, EMT, and cell adhesion | [11,12,13] |
| FUT5 | α1-3/α1-4 fucosylation | Activation of PI3K/Akt signaling pathway | [14] |
| FUT6 | α1-3/α1-4 fucosylation | Activation of PI3K/Akt signaling pathway | [14] |
| FUT7 | α1-3/α1-4 fucosylation | Proliferation, migration, invasion, and EMT; activation of MAPK and PI3K/Akt signaling pathway via EGFR | [15,16] |
| FUT8 | α1-6 fucosylation | Invasion, migration, and EMT; activation of TGF-β and EGFR signaling pathway | [17,18,19,20,21,22] |
| FUT9 | α1-3/α1-4 fucosylation | Cancer stemness | [23] |
| FUT10 | No fucoysltransferase activity | ||
| FUT11 | No fucoysltransferase activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, K.; Hatano, K.; Hashimoto, M.; Tomiyama, E.; Miyoshi, E.; Nonomura, N.; Uemura, H. Fucosylation in Urological Cancers. Int. J. Mol. Sci. 2021, 22, 13333. https://doi.org/10.3390/ijms222413333
Fujita K, Hatano K, Hashimoto M, Tomiyama E, Miyoshi E, Nonomura N, Uemura H. Fucosylation in Urological Cancers. International Journal of Molecular Sciences. 2021; 22(24):13333. https://doi.org/10.3390/ijms222413333
Chicago/Turabian StyleFujita, Kazutoshi, Koji Hatano, Mamoru Hashimoto, Eisuke Tomiyama, Eiji Miyoshi, Norio Nonomura, and Hirotsugu Uemura. 2021. "Fucosylation in Urological Cancers" International Journal of Molecular Sciences 22, no. 24: 13333. https://doi.org/10.3390/ijms222413333







