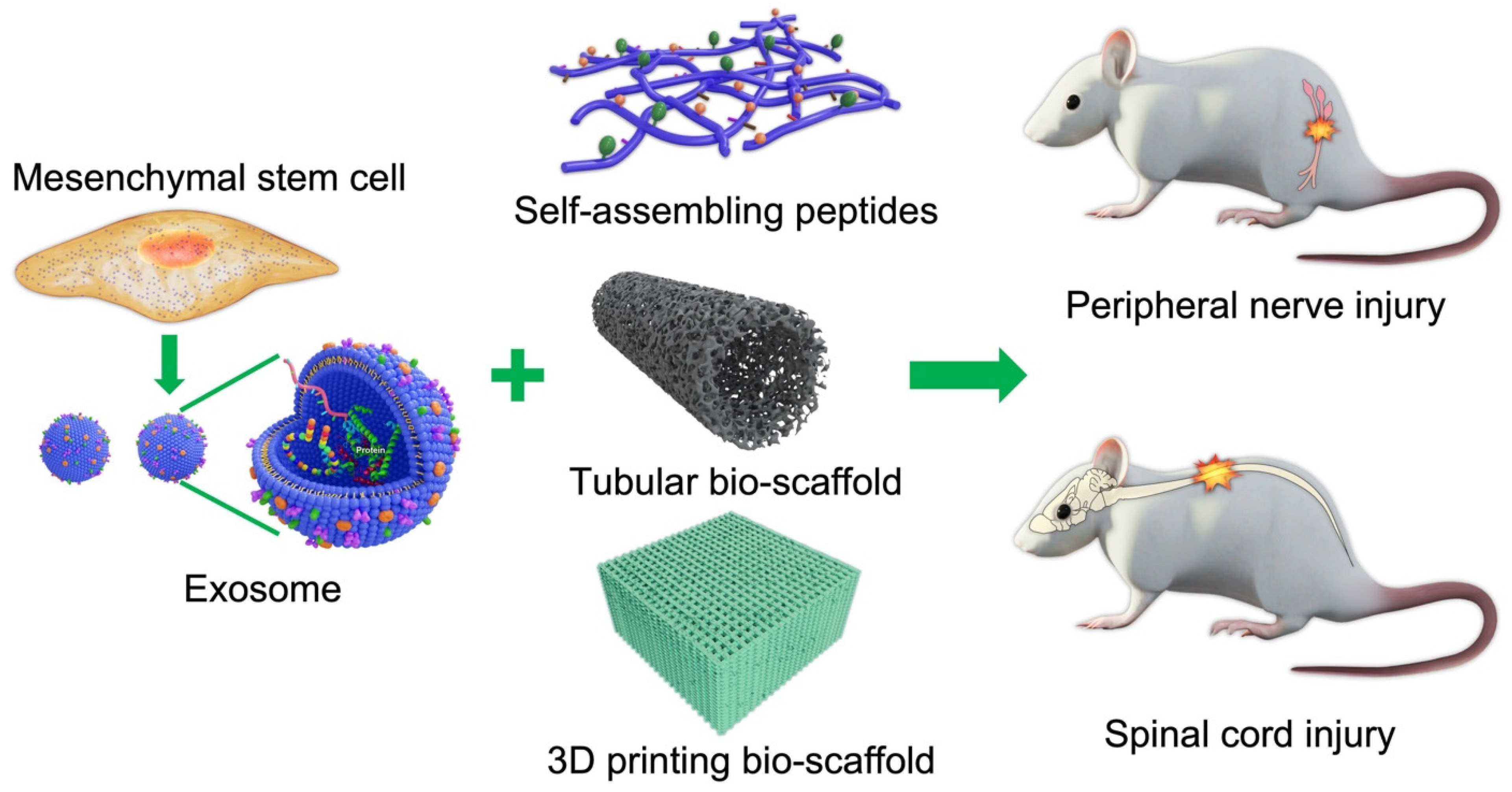
Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair
Abstract
:1. Introduction
2. Mesenchymal Stem Cells for Tissue Replacement
3. Exosomes
4. Natural Polymeric Scaffolds
4.1. Polysaccharide-Based Biomaterials
4.1.1. Hyaluronic Acid
4.1.2. Alginate
4.1.3. Chitosan and Chitin
5. Protein-Based Biomaterials for Nerve Injuries
5.1. Collagen
5.2. Laminin
5.3. Gelatin
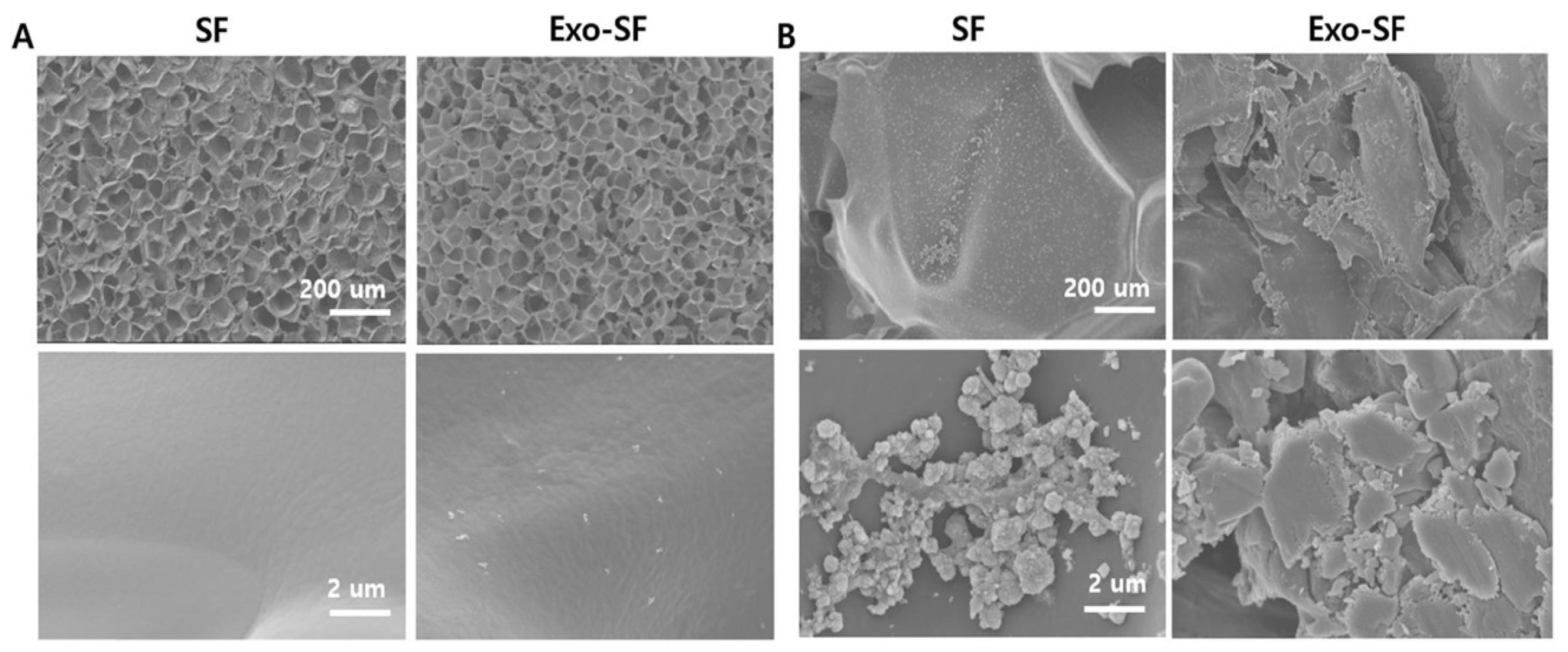
5.4. Silk Fibroin
5.5. Fibrin
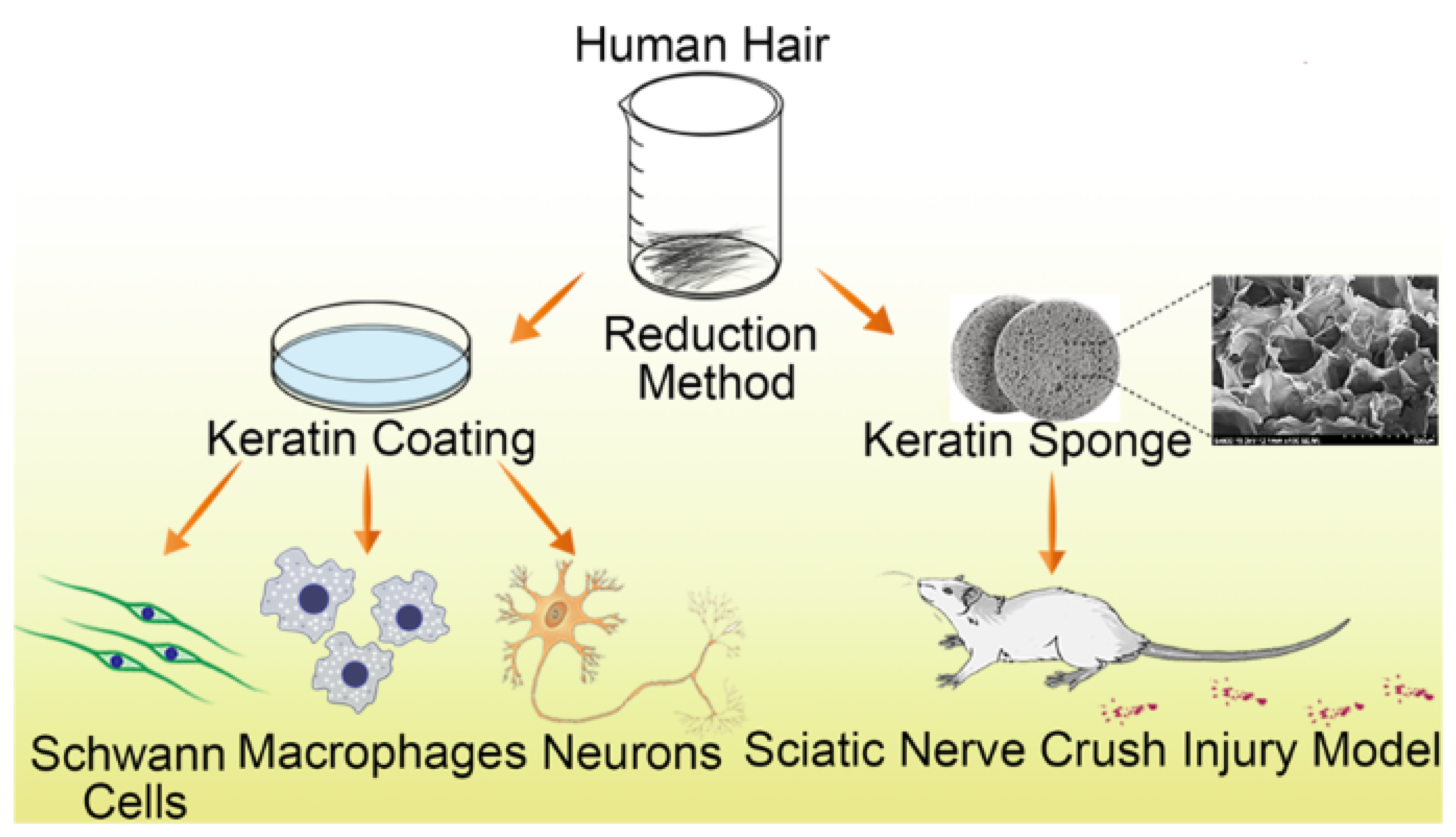
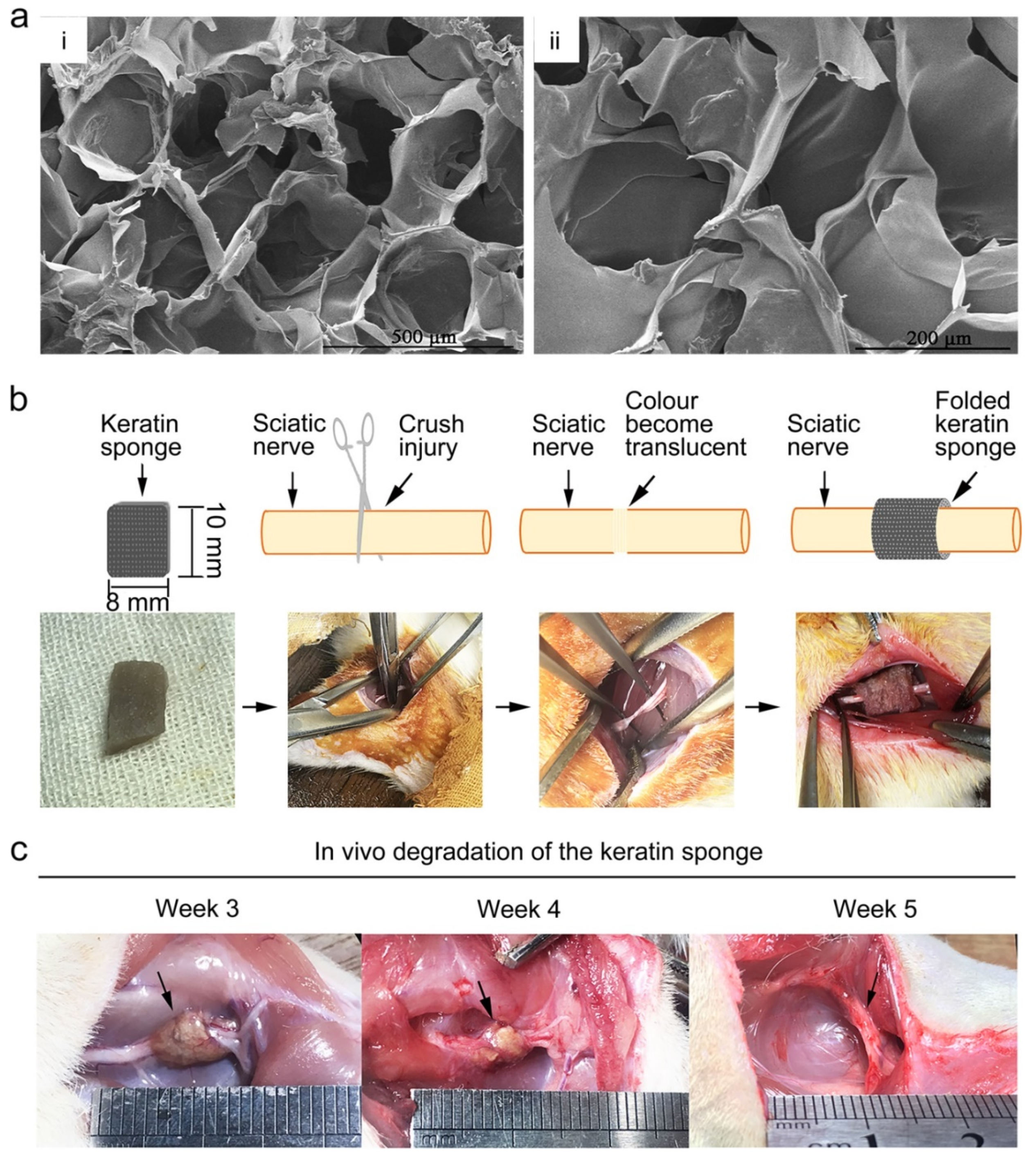
5.6. Keratin
6. Self-Assembling Peptides
7. Three-Dimensional Printed Scaffolds
8. Bio-Scaffolds for Exosomes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L. Tissue Engineering and Regenerative Medicine: New Trends and Directions—A Year in Review. Tissue Eng. Part B Rev. 2017, 23, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Valter, R.M.L. New Challenges in CNS Repair: The Immune and Nervous Connection. Curr. Immunol. Rev. (Discontin.) 2012, 8, 87–93. [Google Scholar] [CrossRef]
- Gathani, K.M.; Raghavendra, S.S. Scaffolds in regenerative endodontics: A review. Dent. Res. J. (Isfahan) 2016, 13, 379–386. [Google Scholar] [CrossRef]
- Huang, L.; Abdalla, A.M.E.; Xiao, L.; Yang, G. Biopolymer-Based Microcarriers for Three-Dimensional Cell Culture and Engineered Tissue Formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.W.; Neufeld, R.J. Modeling the controllable pH-responsive swelling and pore size of networked alginate based biomaterials. Biomaterials 2009, 30, 6119–6129. [Google Scholar] [CrossRef]
- Chansai, P.; Sirivat, A.; Niamlang, S.; Chotpattananont, D.; Viravaidya-Pasuwat, K. Controlled transdermal iontophoresis of sulfosalicylic acid from polypyrrole/poly(acrylic acid) hydrogel. Int. J. Pharm. 2009, 381, 25–33. [Google Scholar] [CrossRef]
- Pérez-Pedroza, R.; Ávila-Ramírez, A.; Khan, Z.; Moretti, M.; Hauser, C.A.E. Supramolecular Biopolymers for Tissue Engineering. Adv. Polym. Technol. 2021, 2021, 8815006. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Mammadov, B.; Sever, M.; Gecer, M.; Zor, F.; Ozturk, S.; Akgun, H.; Ulas, U.H.; Orhan, Z.; Guler, M.O.; Tekinay, A.B. Sciatic nerve regeneration induced by glycosaminoglycan and laminin mimetic peptide nanofiber gels. RSC Adv. 2016, 6, 110535–110547. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. JCDR 2015, 9, ZE21–ZE25. [Google Scholar] [CrossRef]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.-C. Fabrication of Porous Materials from Natural/Synthetic Biopolymers and Their Composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef] [Green Version]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S.; et al. Naturally-Derived Biomaterials for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1077, 421–449. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Current and Future Trends in Adipose Stem Cell Differentiation into Neuroglia. Photomed. Laser Surg. 2018, 36, 230–240. [Google Scholar] [CrossRef]
- Ren, C.; Yin, P.; Ren, N.; Wang, Z.; Wang, J.; Zhang, C.; Ge, W.; Geng, D.; Wang, X. Cerebrospinal fluid-stem cell interactions may pave the path for cell-based therapy in neurological diseases. Stem Cell Res. Ther. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Furno, D.; Mannino, G.; Pellitteri, R.; Zappalà, A.; Parenti, R.; Gili, E.; Vancheri, C.; Giuffrida, R. Conditioned Media From Glial Cells Promote a Neural-Like Connexin Expression in Human Adipose-Derived Mesenchymal Stem Cells. Front. Physiol. 2018, 9, 1742. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Kusuma, G.D.; Carthew, J.; Li, F.; Cloonan, N.; Gomez, G.A.; Cooper-White, J.J. Mechanically-sensitive miRNAs bias human mesenchymal stem cell fate via mTOR signalling. Nat. Commun. 2018, 9, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, N.; Li, D.; Wu, Y.; Wang, H.; Wang, J. Circulating exosomal small RNAs are promising non-invasive diagnostic biomarkers for gastric cancer. J. Cell. Mol. Med. 2020, 24, 14502–14513. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef]
- Boriachek, K.; Islam, M.N.; Möller, A.; Salomon, C.; Nguyen, N.T.; Hossain, M.S.A.; Yamauchi, Y.; Shiddiky, M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2018, 14, 1702153. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. NANOmetric BIO-Banked MSC-Derived Exosome (NANOBIOME) as a Novel Approach to Regenerative Medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef] [Green Version]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med. Devices (Auckl. N. Z.) 2014, 7, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N.u.A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Ortuño-Lizarán, I.; Vilariño-Feltrer, G.; Martínez-Ramos, C.; Pradas, M.M.; Vallés-Lluch, A. Influence of synthesis parameters on hyaluronic acid hydrogels intended as nerve conduits. Biofabrication 2016, 8, 045011. [Google Scholar] [CrossRef] [PubMed]
- Vilariño-Feltrer, G.; Martínez-Ramos, C.; Monleón-de-la-Fuente, A.; Vallés-Lluch, A.; Moratal, D.; Barcia Albacar, J.A.; Monleón Pradas, M. Schwann-cell cylinders grown inside hyaluronic-acid tubular scaffolds with gradient porosity. Acta Biomater. 2016, 30, 199–211. [Google Scholar] [CrossRef]
- Li, R.; Liu, H.; Huang, H.; Bi, W.; Yan, R.; Tan, X.; Wen, W.; Wang, C.; Song, W.; Zhang, Y.; et al. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol. Med. Rep. 2018, 17, 4360–4368. [Google Scholar] [CrossRef] [Green Version]
- Entekhabi, E.; Haghbin Nazarpak, M.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2021, 109, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.C.; Vu, P.; Modi, S.P.; Chung, P.E.; Landis, R.C.; Khaing, Z.Z.; Hardy, J.G.; Schmidt, C.E. Sacrificial Crystal Templated Hyaluronic Acid Hydrogels As Biomimetic 3D Tissue Scaffolds for Nerve Tissue Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Xu, C.; Wang, T.; Wang, Y.; Wang, J.; Quan, D.; Deng, D.Y.B. PTMAc-PEG-PTMAc hydrogel modified by RGDC and hyaluronic acid promotes neural stem cells’ survival and differentiation in vitro. RSC Adv. 2017, 7, 41098–41104. [Google Scholar] [CrossRef] [Green Version]
- Zarei-Kheirabadi, M.; Sadrosadat, H.; Mohammadshirazi, A.; Jaberi, R.; Sorouri, F.; Khayyatan, F.; Kiani, S. Human embryonic stem cell-derived neural stem cells encapsulated in hyaluronic acid promotes regeneration in a contusion spinal cord injured rat. Int. J. Biol. Macromol. 2020, 148, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Song, W.; Zhao, W.; Gao, Y.; Shang, J.; Hao, P.; Yang, Z.; Duan, H.; Li, X. Application of the sodium hyaluronate-CNTF scaffolds in repairing adult rat spinal cord injury and facilitating neural network formation. Sci. China Life Sci. 2018, 61, 559–568. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, G. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 2014, 114, 213–221. [Google Scholar] [CrossRef]
- Dranseikiene, D.; Schrüfer, S.; Schubert, D.W.; Reakasame, S.; Boccaccini, A.R. Cell-laden alginate dialdehyde–gelatin hydrogels formed in 3D printed sacrificial gel. J. Mater. Sci. Mater. Med. 2020, 31, 31. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.-Y.; Young, T.-H.; Yang, H.J.; Ji, Y.-R. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydr. Polym. 2019, 224, 115112. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Y.; Yan, X.; Zhang, L.; Li, G.; Yang, Y. PAM/GO/gel/SA composite hydrogel conduit with bioactivity for repairing peripheral nerve injury. J. Biomed. Mater. Res. Part A 2019, 107, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Askarzadeh, N.; Nazarpak, M.H.; Mansoori, K.; Farokhi, M.; Gholami, M.; Mohammadi, J.; Mottaghitalab, F. Bilayer Cylindrical Conduit Consisting of Electrospun Polycaprolactone Nanofibers and DSC Cross-Linked Sodium Alginate Hydrogel to Bridge Peripheral Nerve Gaps. Macromol. Biosci. 2020, 20, 2000149. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-M.; Shiue, S.-J.; Yang, K.D.; Shiue, H.-S.; Hung, Y.-W.; Pannuru, P.; Poongodi, R.; Lin, H.-Y.; Cheng, J.-K. Locally Applied Stem Cell Exosome-Scaffold Attenuates Nerve Injury-Induced Pain in Rats. J. Pain Res. 2020, 13, 3257–3268. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Stenberg, L.; Kodama, A.; Lindwall-Blom, C.; Dahlin, L.B. Nerve regeneration in chitosan conduits and in autologous nerve grafts in healthy and in type 2 diabetic Goto–Kakizaki rats. Eur. J. Neurosci. 2016, 43, 463–473. [Google Scholar] [CrossRef]
- Neubrech, F.; Sauerbier, M.; Moll, W.; Seegmüller, J.; Heider, S.; Harhaus, L.; Bickert, B.; Kneser, U.; Kremer, T. Enhancing the Outcome of Traumatic Sensory Nerve Lesions of the Hand by Additional Use of a Chitosan Nerve Tube in Primary Nerve Repair: A Randomized Controlled Bicentric Trial. Plast. Reconstr. Surg. 2018, 142, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Gong, J.; Yang, L.; Niu, C.; Ni, X.; Wang, Y.; Peng, S.; Gu, X.; Sun, C.; et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 2017, 134, 64–77. [Google Scholar] [CrossRef]
- Boido, M.; Ghibaudi, M.; Gentile, P.; Favaro, E.; Fusaro, R.; Tonda-Turo, C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci. Rep. 2019, 9, 6402. [Google Scholar] [CrossRef] [Green Version]
- Tonda-Turo, C.; Carmagnola, I.; Chiappone, A.; Feng, Z.; Ciardelli, G.; Hakkarainen, M.; Sangermano, M. Photocurable chitosan as bioink for cellularized therapies towards personalized scaffold architecture. Bioprinting 2020, 18, e00082. [Google Scholar] [CrossRef]
- Ronchi, G.; Fornasari, B.E.; Crosio, A.; Budau, C.A.; Tos, P.; Perroteau, I.; Battiston, B.; Geuna, S.; Raimondo, S.; Gambarotta, G. Chitosan Tubes Enriched with Fresh Skeletal Muscle Fibers for Primary Nerve Repair. BioMed Res. Int. 2018, 2018, 9175248. [Google Scholar] [CrossRef]
- Shapira, Y.; Tolmasov, M.; Nissan, M.; Reider, E.; Koren, A.; Biron, T.; Bitan, Y.; Livnat, M.; Ronchi, G.; Geuna, S.; et al. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery 2016, 36, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Divakar, P.; Hong, J.; Moodie, K.L.; Rosen, J.M.; Sundback, C.A.; Matthew, M.K.; Wegst, U.G.K. Freeze-cast Porous Chitosan Conduit for Peripheral Nerve Repair. MRS Adv. 2018, 3, 1677–1683. [Google Scholar] [CrossRef]
- Crosio, A.; Fornasari, B.E.; Gambarotta, G.; Geuna, S.; Raimondo, S.; Battiston, B.; Tos, P.; Ronchi, G. Chitosan tubes enriched with fresh skeletal muscle fibers for delayed repair of peripheral nerve defects. Neural Regen. Res. 2019, 14, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hong, F.F.; Cao, Z.; Zhao, S.-Y.; Chen, L. In Situ Fabrication of Nerve Growth Factor Encapsulated Chitosan Nanoparticles in Oxidized Bacterial Nanocellulose for Rat Sciatic Nerve Regeneration. Biomacromolecules 2021. [Google Scholar] [CrossRef]
- Muheremu, A.; Chen, L.; Wang, X.; Wei, Y.; Gong, K.; Ao, Q. Chitosan nerve conduits seeded with autologous bone marrow mononuclear cells for 30 mm goat peroneal nerve defect. Sci. Rep. 2017, 7, 44002. [Google Scholar] [CrossRef] [Green Version]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Lu, C.F.; Liu, Z.Y.; Han, S.; Wei, P.; Zhang, D.Y.; Kou, Y.H.; Jiang, B.G. Chitin scaffold combined with autologous small nerve repairs sciatic nerve defects. Neural Regen. Res. 2022, 17, 1106–1114. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A. Advances in peripheral nervous system regenerative therapeutic strategies: A biomaterials approach. Mater. Sci. Eng. C 2016, 65, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.B.; Haynl, C.; Salehi, S.; O’Connor, A.; Scheibel, T. Nerve guidance conduit design based on self-rolling tubes. Mater. Today Bio 2020, 5, 100042. [Google Scholar] [CrossRef]
- Drobnik, J.; Pietrucha, K.; Kudzin, M.; Mader, K.; Szymański, J.; Szczepanowska, A. Comparison of various types of collagenous scaffolds applied for embryonic nerve cell culture. Biologicals 2017, 46, 74–80. [Google Scholar] [CrossRef]
- Saltzman, E.B.; Villa, J.C.; Doty, S.B.; Feinberg, J.H.; Lee, S.K.; Wolfe, S.W. A Comparison Between Two Collagen Nerve Conduits and Nerve Autograft: A Rat Model of Motor Nerve Regeneration. J. Hand Surg. 2019, 44, 700.e1–700.e9. [Google Scholar] [CrossRef]
- Saeki, M.; Tanaka, K.; Imatani, J.; Okamoto, H.; Watanabe, K.; Nakamura, T.; Gotani, H.; Ohi, H.; Nakamura, R.; Hirata, H. Efficacy and safety of novel collagen conduits filled with collagen filaments to treat patients with peripheral nerve injury: A multicenter, controlled, open-label clinical trial. Injury 2018, 49, 766–774. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Y.-Y.; Wang, L.-D.; Tai, C.-X.; Chen, D.; Mu, D.; Cui, Y.-Y.; Wang, B. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural Regen. Res. 2021, 16, 2284–2292. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Ma, K.; Chen, M.; Xu, H.; Niu, X.; Gu, H.; Wang, R.; Chen, X.; Sun, H. Collagen/heparin scaffold combined with vascular endothelial growth factor promotes the repair of neurological function in rats with traumatic brain injury. Biomater. Sci. 2021, 9, 745–764. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71B, 343–354. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.; Toledo Enrique, M.; Gyllborg, D.; Saltó, C.; Carlos Villaescusa, J.; Arenas, E. Niche-derived laminin-511 promotes midbrain dopaminergic neuron survival and differentiation through YAP. Sci. Signal. 2017, 10, eaal4165. [Google Scholar] [CrossRef] [Green Version]
- Tran, K.A.; Partyka, P.P.; Jin, Y.; Bouyer, J.; Fischer, I.; Galie, P.A. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 2020, 104, 76–84. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Andrews, M.R.; Verhaagen, J.; Fawcett, J.W. Integrins promote axonal regeneration after injury of the nervous system. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1339–1362. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Shah, M.B.; Zhou, G.; Walsh, K.; Rudraiah, S.; Kumbar, S.G.; Yu, X. Polymeric nanofibrous nerve conduits coupled with laminin for peripheral nerve regeneration. Biomed. Mater. 2020, 15, 035003. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, A.E.; Bening, M.R.; Pherribo, G.; Dauer, E.A.; Oudega, M. Laminin polymer treatment accelerates repair of the crushed peripheral nerve in adult rats. Acta Biomater. 2019, 86, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, H.-X.; Ortiz, L.S.; Xiao, Z.-D.; Huang, N.-P. Laminin-modified and aligned poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polyethylene oxide nanofibrous nerve conduits promote peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e627–e636. [Google Scholar] [CrossRef]
- Lindberg, D.; Kristoffersen, K.A.; Wubshet, S.G.; Hunnes, L.M.G.; Dalsnes, M.; Dankel, K.R.; Host, V.; Afseth, N.K. Exploring Effects of Protease Choice and Protease Combinations in Enzymatic Protein Hydrolysis of Poultry By-Products. Molecules 2021, 26, 5280. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Jeong, S.-J.; Lee, W.-K. Fabrication of porous scaffold by ternary combination of chitosan, gelatin, and calcium phosphate for tissue engineering. J. Ind. Eng. Chem. 2019, 80, 862–869. [Google Scholar] [CrossRef]
- De la Vega, L.; Abelseth, L.; Sharma, R.; Triviño-Paredes, J.; Restan, M.; Willerth, S.M. 3D Bioprinting Human-Induced Pluripotent Stem Cells and Drug-Releasing Microspheres to Produce Responsive Neural Tissues. Adv. NanoBiomed Res. 2021, 1, 2000077. [Google Scholar] [CrossRef]
- Shin, J.H.; Kang, H.-W. The Development of Gelatin-Based Bio-Ink for Use in 3D Hybrid Bioprinting. Int. J. Precis. Eng. Manuf. 2018, 19, 767–771. [Google Scholar] [CrossRef]
- Echave, C.M.; Saenz del Burgo, L.; Pedraz, L.J.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Yao, M.; Li, J.; Zhang, J.; Ma, S.; Wang, L.; Gao, F.; Guan, F. Dual-enzymatically cross-linked gelatin hydrogel enhances neural differentiation of human umbilical cord mesenchymal stem cells and functional recovery in experimental murine spinal cord injury. J. Mater. Chem. B 2021, 9, 440–452. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Ma, S.; Zhang, Y.; Zhang, J.; Guan, F.; Yao, M. Control the fate of human umbilical cord mesenchymal stem cells with dual-enzymatically cross-linked gelatin hydrogels for potential applications in nerve regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Zhang, H.; Yu, Z.; Yuan, H.; Wang, X.; Zhang, Y. Fabrication of high performance silk fibroin fibers via stable jet electrospinning for potential use in anisotropic tissue regeneration. J. Mater. Chem. B 2018, 6, 3934–3945. [Google Scholar] [CrossRef]
- Luan, C.; Liu, P.; Chen, R.; Chen, B. Hydrogel based 3D carriers in the application of stem cell therapy by direct injection. Nanotechnol. Rev. 2017, 6, 435–448. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E.; Ortega, Z. Enzymatic degradation study of PLA-based composite scaffolds. Rev. Adv. Mater. Sci. 2020, 59, 170–175. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Q.; Li, H.; Wei, Q.; Zhao, X.; Chen, F. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioact. Mater. 2021, 6, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive Silk-Based Nerve Guidance Conduits for Augmenting Peripheral Nerve Repair. Adv. Healthc. Mater. 2018, 7, 1800308. [Google Scholar] [CrossRef] [Green Version]
- Xuan, H.; Tang, X.; Zhu, Y.; Ling, J.; Yang, Y. Freestanding Hyaluronic Acid/Silk-Based Self-healing Coating toward Tissue Repair with Antibacterial Surface. ACS Appl. Bio Mater. 2020, 3, 1628–1635. [Google Scholar] [CrossRef]
- Chen, S.; Liu, S.; Zhang, L.; Han, Q.; Liu, H.; Shen, J.; Li, G.; Zhang, L.; Yang, Y. Construction of injectable silk fibroin/polydopamine hydrogel for treatment of spinal cord injury. Chem. Eng. J. 2020, 399, 125795. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; Li, G.; Yang, Y. Fabrication of high-strength mecobalamin loaded aligned silk fibroin scaffolds for guiding neuronal orientation. Colloids Surf. B Biointerfaces 2019, 173, 689–697. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [Green Version]
- Tang-Schomer, M.D.; Kaplan, D.L.; Whalen, M.J. Film interface for drug testing for delivery to cells in culture and in the brain. Acta Biomater. 2019, 94, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Moisenovich, M.M.; Plotnikov, E.Y.; Moysenovich, A.M.; Silachev, D.N.; Danilina, T.I.; Savchenko, E.S.; Bobrova, M.M.; Safonova, L.A.; Tatarskiy, V.V.; Kotliarova, M.S.; et al. Effect of Silk Fibroin on Neuroregeneration After Traumatic Brain Injury. Neurochem. Res. 2019, 44, 2261–2272. [Google Scholar] [CrossRef]
- Kyung Kim, D.; Lee, S.; Kim, M.; Jeong, Y.; Lee, S. Exosome-coated silk fibroin 3D-scaffold for inducing osteogenic differentiation of bone marrow derived mesenchymal stem cells. Chem. Eng. J. 2021, 406, 127080. [Google Scholar] [CrossRef]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. In Biomimetic Medical Materials. Advances in Experimental Medicine and Biology; Noh, I., Ed.; Springer: Singapore, 2018; Volume 1064. [Google Scholar] [CrossRef]
- Karimi, A.; Shojaei, A.; Tehrani, P. Mechanical properties of the human spinal cord under the compressive loading. J. Chem. Neuroanat. 2017, 86, 15–18. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Wang, K.; Yang, X.; Qi, Z. Application of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Bruekers, S.M.C.; Jaspers, M.; Hendriks, J.M.A.; Kurniawan, N.A.; Koenderink, G.H.; Kouwer, P.H.J.; Rowan, A.E.; Huck, W.T.S. Fibrin-fiber architecture influences cell spreading and differentiation. Cell Adhes. Migr. 2016, 10, 495–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Degrugillier, L.; Tremp, M.; Prautsch, K.; Sottaz, L.; Schaefer, D.J.; Madduri, S.; Kalbermatten, D. Nerve Repair With Fibrin Nerve Conduit and Modified Suture Placement. Anat. Rec. 2018, 301, 1690–1696. [Google Scholar] [CrossRef] [Green Version]
- McGrath, A.M.; Brohlin, M.; Wiberg, R.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Novikova, L.N. Long-Term Effects of Fibrin Conduit with Human Mesenchymal Stem Cells and Immunosuppression after Peripheral Nerve Repair in a Xenogenic Model. Cell Med. 2018, 10, 2155179018760327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; Yu, S.; Cao, Z.; Yang, Y.; Yu, X.; Mao, H.-Q.; Wang, L.-N.; Sun, X.; Zhao, L.; Wang, X. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int. J. Nanomed. 2018, 13, 2883–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-J.; Chen, X.-F.; Zhou, L.-P.; Rao, F.; Zhang, D.-Y.; Wang, Y.-H. A nerve conduit filled with Wnt5a-loaded fibrin hydrogels promotes peripheral nerve regeneration. CNS Neurosci. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef]
- Gupta, P.; Nayak, K.K. Optimization of keratin/alginate scaffold using RSM and its characterization for tissue engineering. Int. J. Biol. Macromol. 2016, 85, 141–149. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, L.; Wei, Y.; Chen, T.; Ji, X.; Ye, K.; Yu, J.; Tang, B.; Sun, X.; Hu, J. Human hair keratins promote the regeneration of peripheral nerves in a rat sciatic nerve crush model. J. Mater. Sci. Mater. Med. 2019, 30, 82. [Google Scholar] [CrossRef] [Green Version]
- Zabarsky, Z.K.; Dean, G.M.; Luo, T.D.; Marquez-Lara, A.; Jinnah, A.H.; Van Dyke, M.; Smith, T.L. Keratin Biomaterials Improve Functional Recovery in a Rat Spinal Cord Injury Model. Spine 2021, 46, 1055–1062. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Fathollahi, A.; Salehi, M.; Gholizadeh, S.; Derakhshankhah, H.; Allahyari, Z.; Jaymand, M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. [Google Scholar] [CrossRef]
- Araújo, M.R.; Kyrylenko, S.; Spejo, A.B.; Castro, M.V.; Ferreira Junior, R.S.; Barraviera, B.; Oliveira, A.L.R. Transgenic human embryonic stem cells overexpressing FGF2 stimulate neuroprotection following spinal cord ventral root avulsion. Exp. Neurol. 2017, 294, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef]
- Fan, C.; Li, X.; Xiao, Z.; Zhao, Y.; Liang, H.; Wang, B.; Han, S.; Li, X.; Xu, B.; Wang, N.; et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017, 51, 304–316. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, Z.; Guo, Y.; Li, H.; Chen, Z. Design of a RADA16-based self-assembling peptide nanofiber scaffold for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 713–736. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Zhu, J.; Lu, C.; Li, H.; Chen, F.; Lu, J.; Zhang, Z.; Yan, X.; Zhao, H.; et al. Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics 2020, 10, 8227–8249. [Google Scholar] [CrossRef]
- Wu, X.; He, L.; Li, W.; Li, H.; Wong, W.-M.; Ramakrishna, S.; Wu, W. Functional self-assembling peptide nanofiber hydrogel for peripheral nerve regeneration. Regen. Biomater. 2017, 4, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanaugh, M.; Silantyeva, E.; Pylypiv Koh, G.; Malekzadeh, E.; Lanzinger, W.D.; Willits, R.K.; Becker, M.L. RGD-Modified Nanofibers Enhance Outcomes in Rats after Sciatic Nerve Injury. J. Funct. Biomater. 2019, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, K.; Ma, T.; Huang, L.; Xia, B.; Zhu, S.; Yang, Y.; Liu, Z.; Quan, X.; Luo, K.; et al. Noncovalent Bonding of RGD and YIGSR to an Electrospun Poly(ε-Caprolactone) Conduit through Peptide Self-Assembly to Synergistically Promote Sciatic Nerve Regeneration in Rats. Adv. Healthc. Mater. 2017, 6, 1600860. [Google Scholar] [CrossRef] [PubMed]
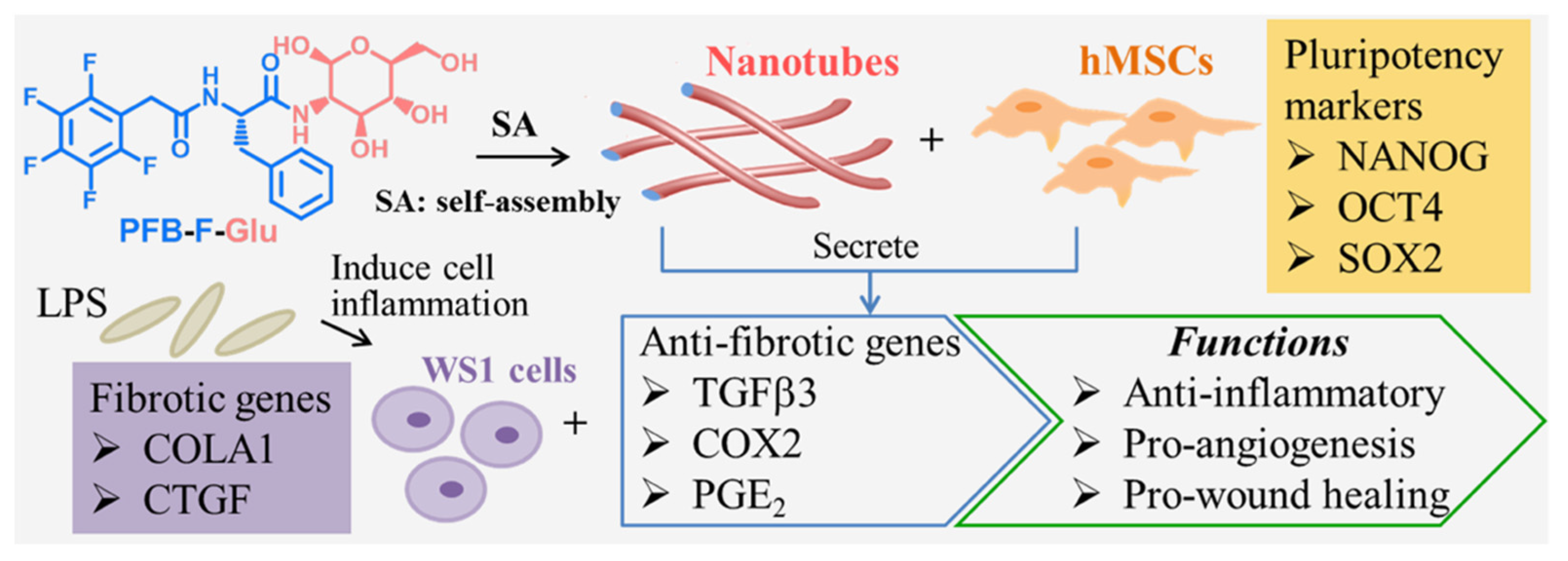
- Talloj, S.K.; Cheng, B.; Weng, J.-P.; Lin, H.-C. Glucosamine-Based Supramolecular Nanotubes for Human Mesenchymal Cell Therapy. ACS Appl. Mater. Interfaces 2018, 10, 15079–15087. [Google Scholar] [CrossRef] [PubMed]
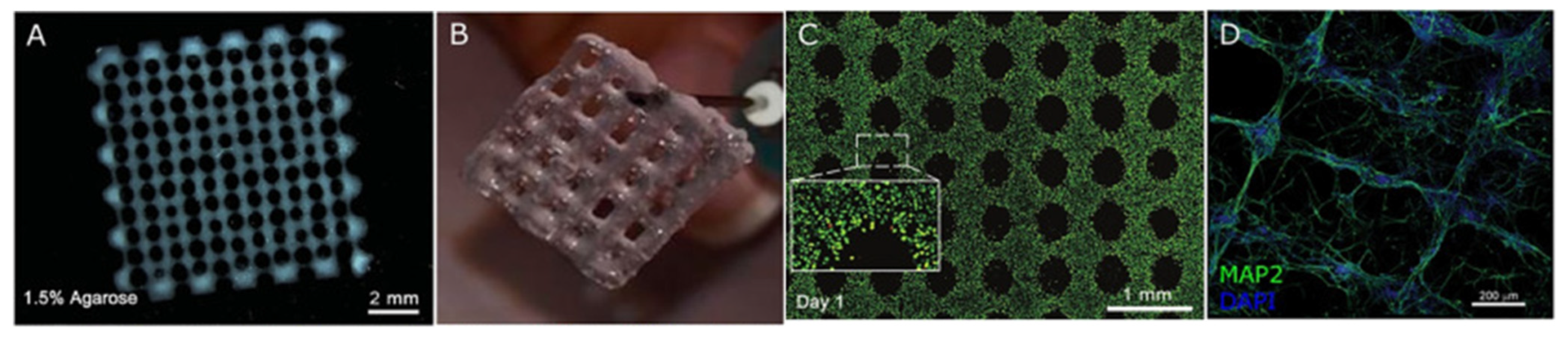
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium Alginate/Gelatine Hydrogels for Direct Bioprinting—The Effect of Composition Selection and Applied Solvents on the Bioink Properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Smits, I.P.M.; De La Vega, L.; Lee, C.; Willerth, S.M. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, D.N.; Pinto, G.B.A.; Cartarozzi, L.P.; de Oliveira, A.L.R.; Bovolato, A.L.C.; de Carvalho, M.; da Silva, J.V.L.; de Andréa Dernowsek, J.; Golim, M.; Barraviera, B.; et al. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res. Ther. 2021, 12, 303. [Google Scholar] [CrossRef]
- Fantini, V.; Bordoni, M.; Scocozza, F.; Conti, M.; Scarian, E.; Carelli, S.; Di Giulio, A.M.; Marconi, S.; Pansarasa, O.; Auricchio, F.; et al. Bioink Composition and Printing Parameters for 3D Modeling Neural Tissue. Cells 2019, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef] [Green Version]
- Salaris, F.; Colosi, C.; Brighi, C.; Soloperto, A.; de Turris, V.; Benedetti, M.C.; Ghirga, S.; Rosito, M.; Di Angelantonio, S.; Rosa, A. 3D Bioprinted Human Cortical Neural Constructs Derived from Induced Pluripotent Stem Cells. J. Clin. Med. 2019, 8, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xu, M.-L.; Wang, C.-N.; Zhang, L.-Z.; Zhao, Y.-H.; Zhu, C.-L.; Chen, Y.; Wu, J.; Yang, Y.-M.; Wang, X.-D. A partition-type tubular scaffold loaded with PDGF-releasing microspheres for spinal cord repair facilitates the directional migration and growth of cells. Neural Regen. Res. 2018, 13, 1231–1240. [Google Scholar] [CrossRef]
- Jia, W.; Jiang, X.; Liu, W.; Wang, L.; Zhu, B.; Zhu, H.; Liu, X.; Zhong, M.; Xie, D.; Huang, W.; et al. Effects of three-dimensional collagen scaffolds on the expression profiles and biological functions of glioma cells. Int. J. Oncol. 2018, 52, 1787–1800. [Google Scholar] [CrossRef]
- Arulmoli, J.; Wright, H.J.; Phan, D.T.T.; Sheth, U.; Que, R.A.; Botten, G.A.; Keating, M.; Botvinick, E.L.; Pathak, M.M.; Zarembinski, T.I.; et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016, 43, 122–138. [Google Scholar] [CrossRef] [Green Version]
- Skop, N.B.; Calderon, F.; Cho, C.H.; Gandhi, C.D.; Levison, S.W. Optimizing a multifunctional microsphere scaffold to improve neural precursor cell transplantation for traumatic brain injury repair. J. Tissue Eng. Regen. Med. 2016, 10, E419–E432. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, X.; Xiao, Z.; Yao, Y.; Chu, Y.; Farkas, B.; Romano, I.; Brandi, F.; Dai, J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Healthc. Mater. 2018, 7, 1800315. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.; Nolan, J.C.; Conlon, R.; Deneweth, L.; Gallagher, C.; Tan, Y.J.; Cavanagh, B.L.; Asraf, A.Z.; Harvey, H.; Miller-Delaney, S.; et al. A physiologically relevant 3D collagen-based scaffold–neuroblastoma cell system exhibits chemosensitivity similar to orthotopic xenograft models. Acta Biomater. 2018, 70, 84–97. [Google Scholar] [CrossRef]
- Shi, W.; Huang, C.J.; Xu, X.D.; Jin, G.H.; Huang, R.Q.; Huang, J.F.; Chen, Y.N.; Ju, S.Q.; Wang, Y.; Shi, Y.W.; et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016, 45, 247–261. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part A 2020, 108, 545–556. [Google Scholar] [CrossRef]
- Rao, F.; Zhang, D.; Fang, T.; Lu, C.; Wang, B.; Ding, X.; Wei, S.; Zhang, Y.; Pi, W.; Xu, H.; et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019, 2019, 2546367. [Google Scholar] [CrossRef]
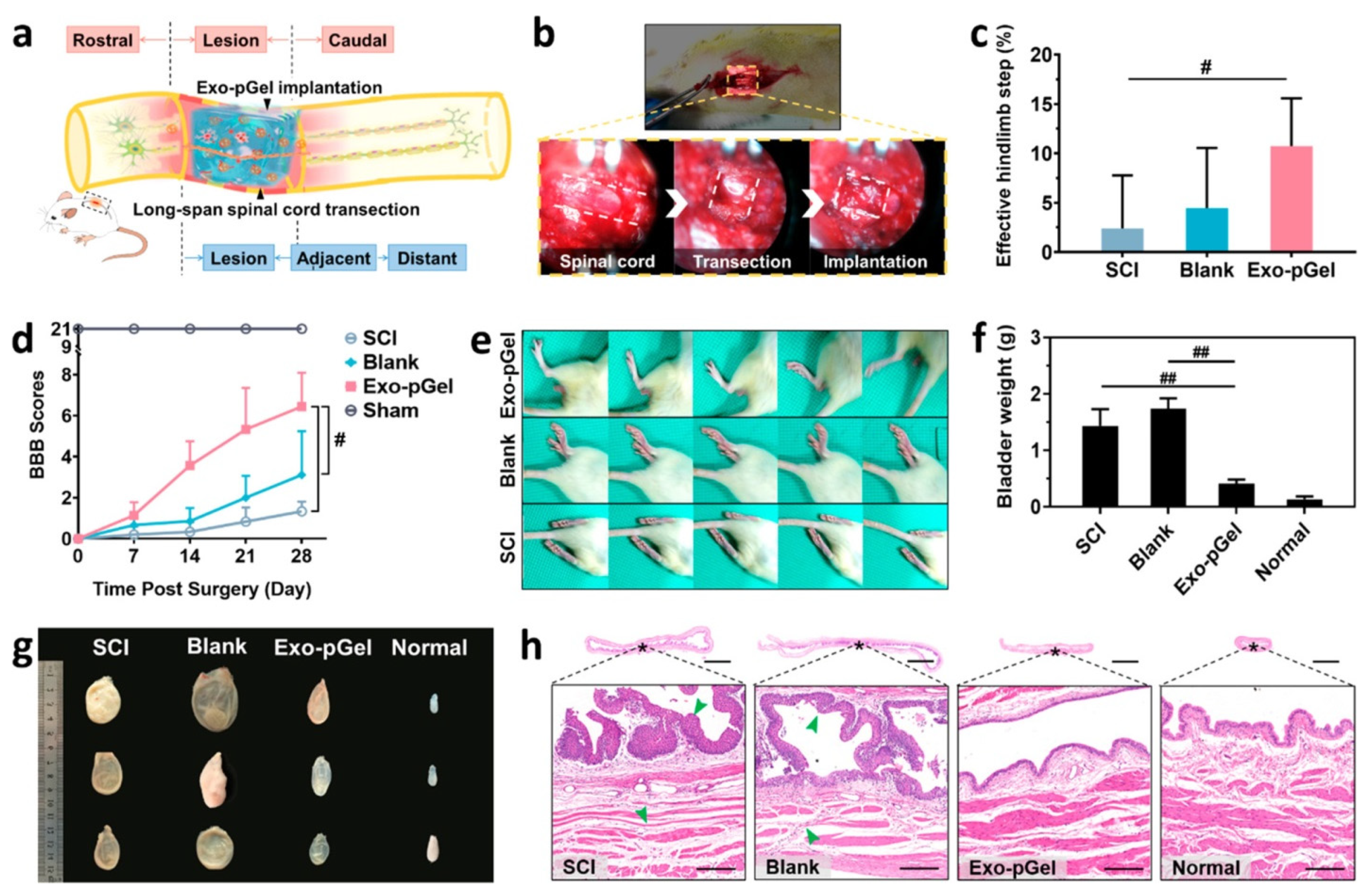
- Li, L.; Zhang, Y.; Mu, J.; Chen, J.; Zhang, C.; Cao, H.; Gao, J. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020, 20, 4298–4305. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Guo, M.; Dong, X.; Liao, M.; Du, M.; Wang, X.; Yin, H.; Yan, H. Exosome-Mediated Delivery of the Neuroprotective Peptide PACAP38 Promotes Retinal Ganglion Cell Survival and Axon Regeneration in Rats With Traumatic Optic Neuropathy. Front. Cell Dev. Biol. 2021, 9, 734. [Google Scholar] [CrossRef]
- Zheng, Y.; Hong, X.; Wang, J.; Feng, L.; Fan, T.; Guo, R.; Zhang, H. 2D Nanomaterials for Tissue Engineering and Regenerative Nanomedicines: Recent Advances and Future Challenges. Adv. Healthc. Mater. 2021, 10, e2001743. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gaihre, B.; George, M.N.; Li, Y.; Tilton, M.; Yaszemski, M.J.; Lu, L. 2D phosphorene nanosheets, quantum dots, nanoribbons: Synthesis and biomedical applications. Biomater. Sci. 2021, 9, 2768–2803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, C.; Akakuru, O.U.; Su, Z.; Wu, A.; Wei, G. The design and biomedical applications of self-assembled two-dimensional organic biomaterials. Chem. Soc. Rev. 2019, 48, 5564–5595. [Google Scholar] [CrossRef] [PubMed]
| Bio-Scaffold | Cell Type | Disease | Results | Reference |
|---|---|---|---|---|
| PDGF-MS-containing tubular scaffold | Neural progenitor | Spinal cord injury | Promoted both growth and migration of MUSE-NPCs | [129] |
| 3D collagen scaffold | Glioma | Glioma | Good biocompatibility with glioma cells and able to influence gene expression and biological functions | [130] |
| Scaffold incorporating salmon fibrin, HA, and laminin | Human neural stem cells | Neurovascular niche | Enhanced vasculogenesis from human endothelial colony-forming cell-derived endothelial cells for cellular therapeutics | [131] |
| Chitosan-based scaffold | Radial glia | Traumatic brain injury | Effective cellular and growth factor delivery vehicle for cell transplantation | [132] |
| Collagen scaffold | Neural stem cells | Spinal cord injury | Promoted nerve regeneration and locomotor function | [71] |
| Bio-Scaffold | Species | Disease | Results | Reference |
|---|---|---|---|---|
| Poly (propylene fumarate) polymer with collagen biomaterial | Rat | Spinal cord injury | Promoted neurotrophy, neuroprotection, myelination, and synapse formation, and reduced CSPG deposits and fibrotic scarring | [133] |
| 3D collagen-based scaffold | Mouse | Neuroblastoma | Promoted microenvironment within scaffold and helps in cell transplantation and drug delivery | [134] |
| Collagen nerve conduit | Rat | Sciatic defect | Promoted motor nerve regeneration | [69] |
| Chitosan hydrogel scaffold | Mouse | Ischemic brain injury | Improved tissue regeneration following hind-limb ischemia | [51] |
| 3D fibrin hydrogel scaffold | Rat | Spinal cord injury | Promoted aligned axonal regrowth and locomotor function | [105] |
| Collagen/heparin/VEGF scaffold | Rat | Traumatic brain injury | Provided an excellent microenvironment for nerve regeneration | [72] |
| Collagen scaffold | Rat | Spinal cord injury | Improved locomotor function and nerve regeneration | [71] |
| Silk fibroin scaffold | Rat | Traumatic brain injury | Neuroprotection | [97] |
| RADA16-BDNF self-assembling peptide hydrogel scaffold | Rat | Traumatic brain injury | Enhanced the growth, survival, and differentiation of MSCs by providing a favorable microenvironment | [135] |
| Chitin scaffold | Rat | Sciatic nerve injury | Improved sciatic nerve regeneration, myelin sheath formation, and functional recovery | [64] |
| Keratin sponge | Rat | Sciatic nerve injury | Regulated inflammatory cytokine release from macrophages, axon extension, and nerve regeneration | [109] |
| Fibrin hydrogel | Rat | Sciatic nerve defect | Promoted regeneration as well as the secretion and signaling of multiple neurotrophic factors | [106] |
| Keratin sponge | Rat | Spinal cord injury | Improved functional recovery and inhibition of inflammatory response through macrophage polarization | [110] |
| Bio-Scaffold | Exosome Source | Disease | Results | Reference |
|---|---|---|---|---|
| Peptide-modified adhesive hydrogel | Human MSC-derived | Spinal cord injury | Promoted nerve regeneration and protected urinary tissue by easing oxidative stress and inflammation | [138] |
| Alginate scaffold | Human umbilical cord MSC-derived | Nerve injury-induced pain | Anti-nociceptive, anti-inflammatory, and neurotrophic effects | [48] |
| Chitin conduit | Human gingiva MSC-derived | Rat sciatic nerve defect | Increased the number and diameter of nerve fibers and promoted myelin formation | [137] |
| Chitosan hydrogel | Human placental MSC-derived | Hind-limb ischemia | Enhanced angiogenesis and tissue regeneration | [51] |
| Pituitary adenylate cyclase-activating polypeptide 38 | Retinal ganglion cell (RGC)-derived | Traumatic optic neuropathy | Promoted retinal ganglion cell survival and axon regeneration | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poongodi, R.; Chen, Y.-L.; Yang, T.-H.; Huang, Y.-H.; Yang, K.D.; Lin, H.-C.; Cheng, J.-K. Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. Int. J. Mol. Sci. 2021, 22, 13347. https://doi.org/10.3390/ijms222413347
Poongodi R, Chen Y-L, Yang T-H, Huang Y-H, Yang KD, Lin H-C, Cheng J-K. Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. International Journal of Molecular Sciences. 2021; 22(24):13347. https://doi.org/10.3390/ijms222413347
Chicago/Turabian StylePoongodi, Raju, Ying-Lun Chen, Tao-Hsiang Yang, Ya-Hsien Huang, Kuender D. Yang, Hsin-Chieh Lin, and Jen-Kun Cheng. 2021. "Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair" International Journal of Molecular Sciences 22, no. 24: 13347. https://doi.org/10.3390/ijms222413347
APA StylePoongodi, R., Chen, Y.-L., Yang, T.-H., Huang, Y.-H., Yang, K. D., Lin, H.-C., & Cheng, J.-K. (2021). Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. International Journal of Molecular Sciences, 22(24), 13347. https://doi.org/10.3390/ijms222413347






