Opioid Receptors and Protonation-Coupled Binding of Opioid Drugs
Abstract
1. Introduction
2. Opioid Receptor Function
3. Architecture of the MOR, and Opioid Binding to the MOR
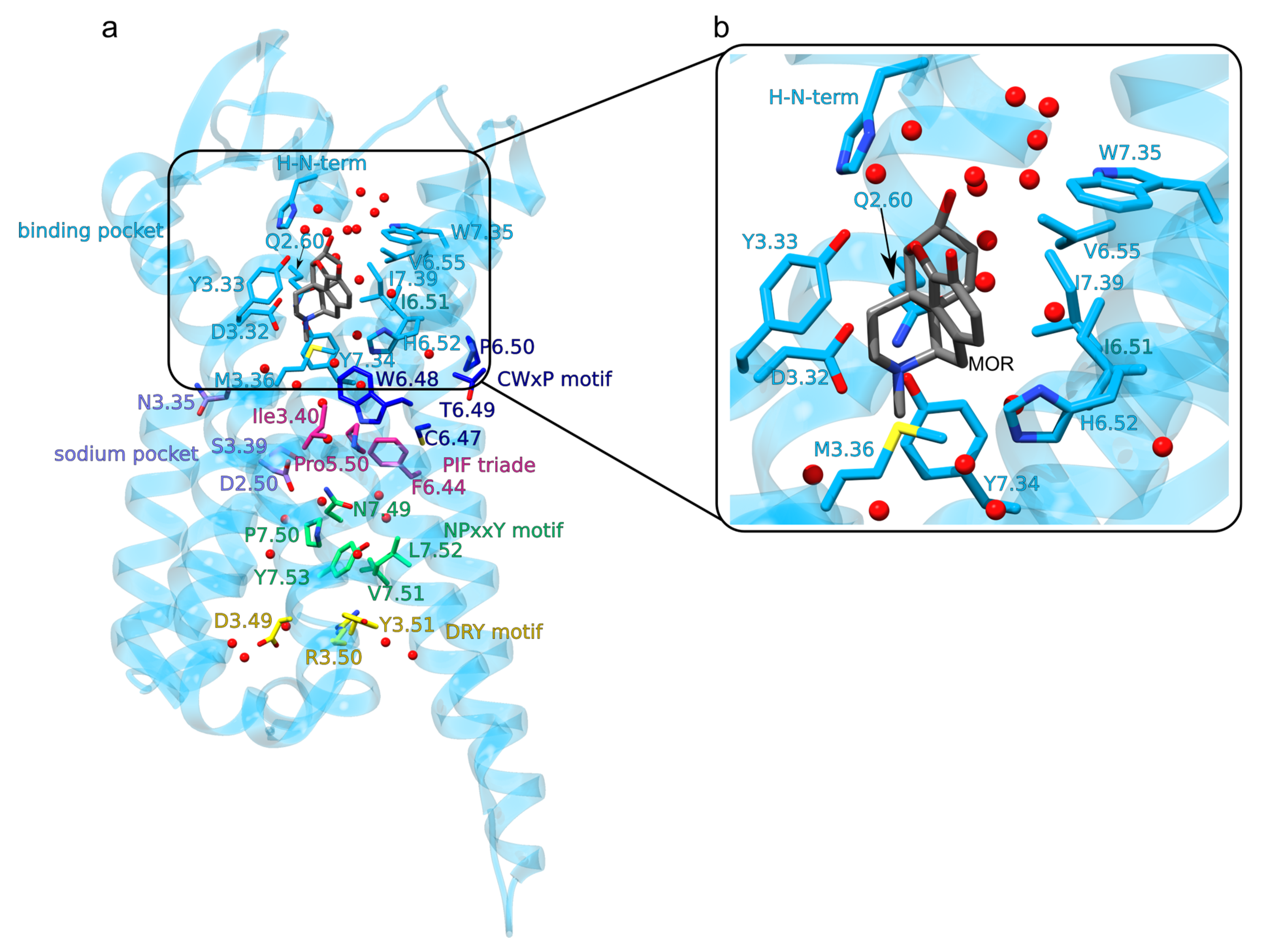
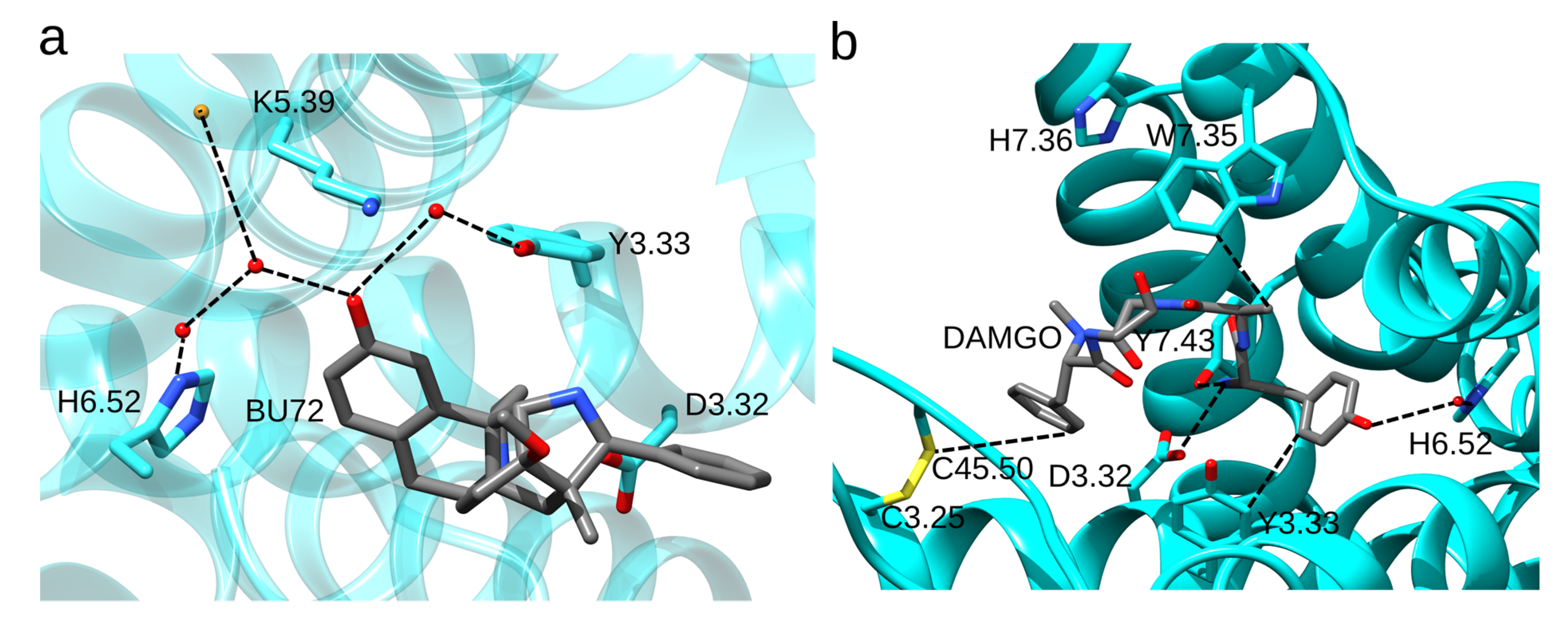
The Opioid Binding Site of the MOR
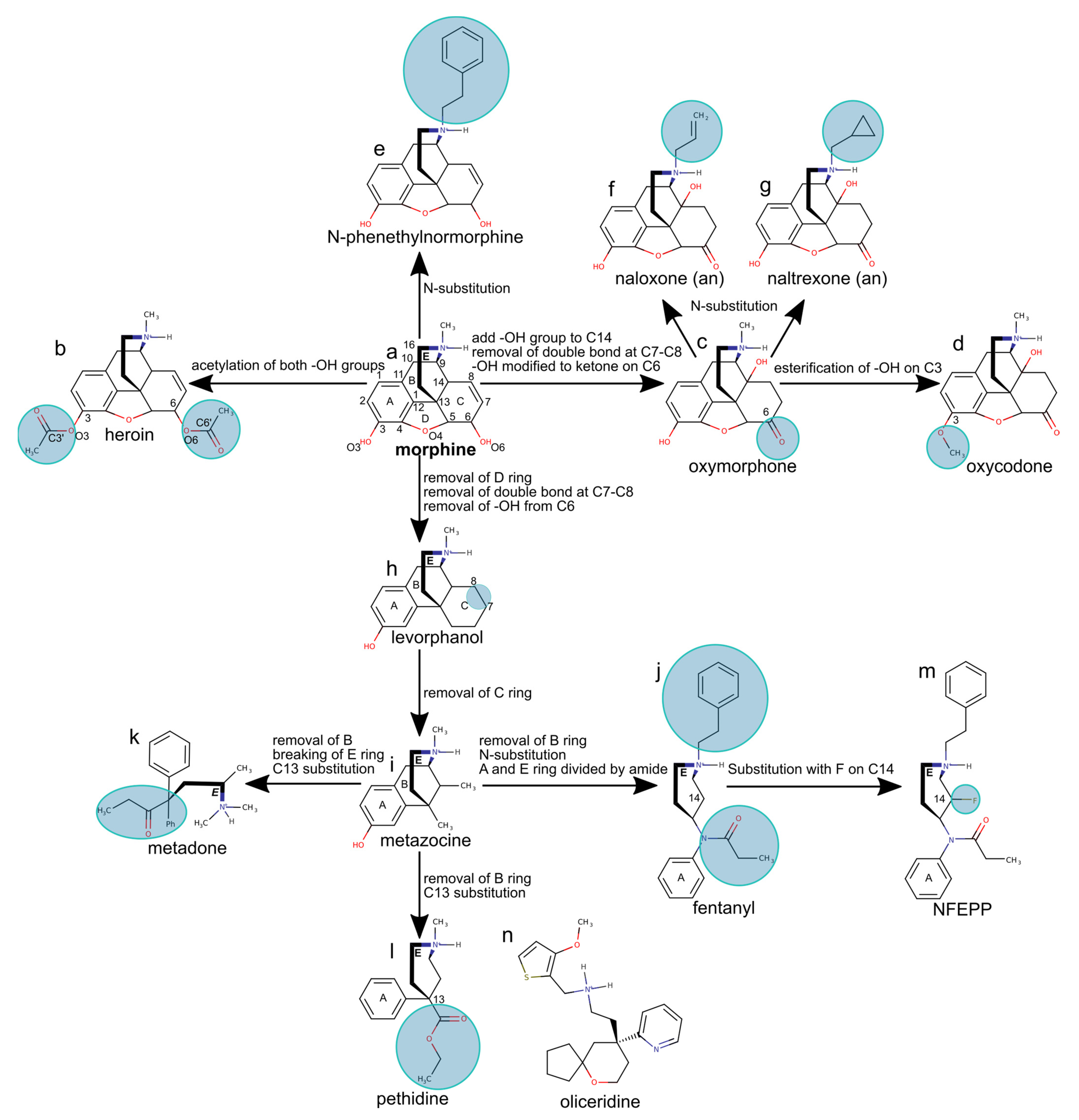
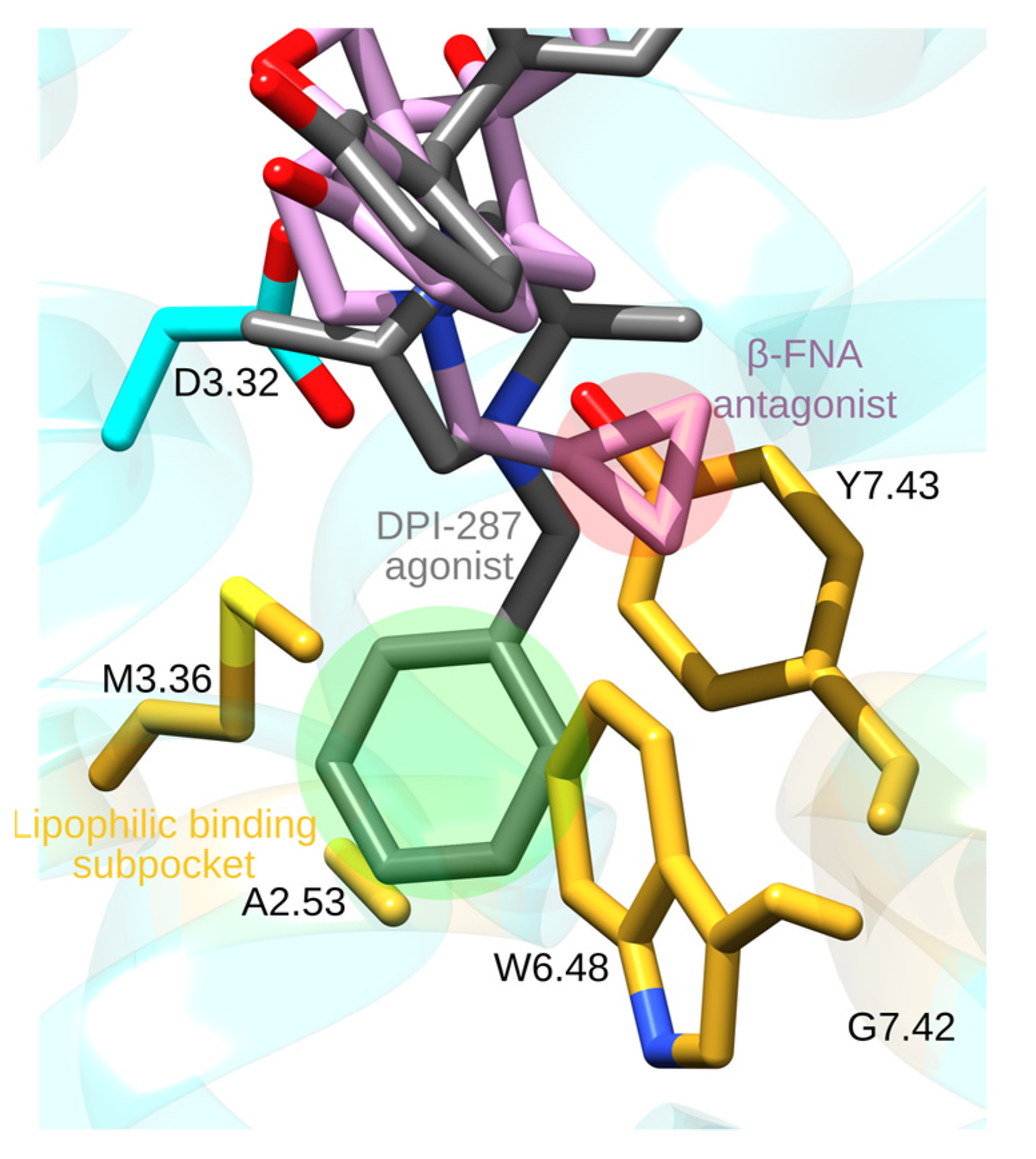
4. Opioid Drugs and How They Bind to the MOR
5. pH-Dependent Binding of a Fluorinated Fentanyl Derivative
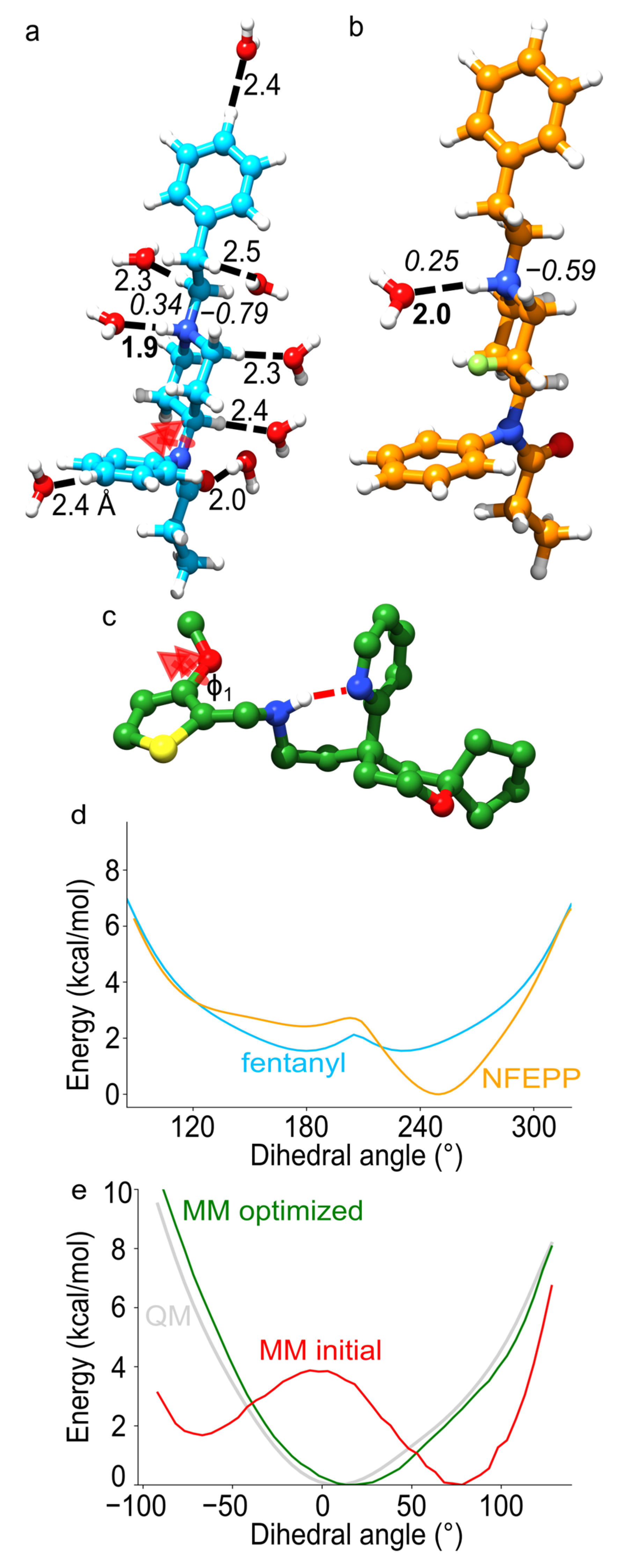
6. Force-Field Parametrization of Opioid Drugs
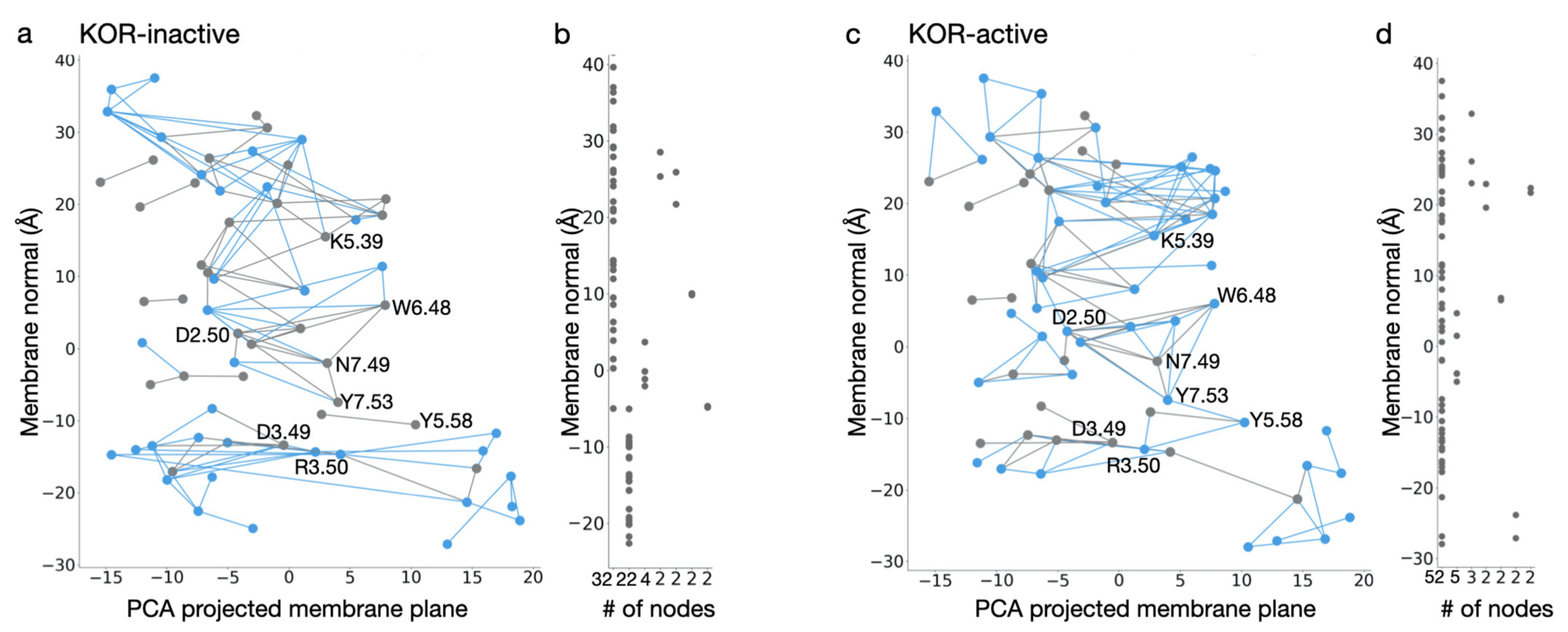
7. Classical Mechanical Computations of Opioid Receptors
8. Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Stein, C. New Concepts in Opioid Analgesia. Expert Opin. Investig. Drugs 2018, 27, 765–775. [Google Scholar] [CrossRef]
- Matthes, H.W.D.; Maldonado, R.; Simonin, F.; Valverde, O.; Slowe, S.; Kitchen, I.; Befort, K.; Dierich, A.; Meur, M.L.; Dollé, P.; et al. Loss of Morphine-Induced Analgesia, Reward Effect and Withdrawal Symptoms in Mice Lacking the µ-Opioid-Receptor Gene. Nature 1996, 383, 819–823. [Google Scholar] [CrossRef]
- Ciccarone, D. The Triple Wave Epidemic: Supply and Demand Drivers of the US Opioid Overdose Crisis. Int. J. Drug Policy 2019, 71, 183–188. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2017: Trends and Developments; Office for Official Publications of the European Communities: Lisbon, Portugal, 2017; ISBN 978-92-9497-074-9. [Google Scholar]
- Johnson, S.M.; Smith, J.C.; Feldman, J.L. Modulation of Respiratory Rhythm in Vitro: Role of Gi/o Protein-Mediated Mechanisms. J. Appl. Physiol. 1996, 80, 2120–2133. [Google Scholar] [CrossRef]
- Hill, R.; Disney, A.; Conibear, A.; Sutcliffe, K.; Dewey, W.; Husbands, S.; Bailey, C.; Kelly, E.; Henderson, G. The Novel μ-Opioid Receptor Agonist PZM21 Depresses Respiration and Induces Tolerance to Antinociception. Br. J. Pharmacol. 2018, 175, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Viswanath, O.; Orhurhu, V.; Gress, K.; Charipova, K.; Kaye, A.D.; Ngo, A. The Utilization of Mu-Opioid Receptor Biased Agonists: Oliceridine, an Opioid Analgesic with Reduced Adverse Effects. Curr. Pain Headache Rep. 2019, 23, 31. [Google Scholar] [CrossRef]
- Gudin, J.; Fudin, J. A Narrative Pharmacological Review of Buprenorphine: A Unique Opioid for the Treatment of Chronic Pain. Pain Ther. 2020, 9, 41–54. [Google Scholar] [CrossRef]
- Gillis, A.; Gondin, A.B.; Kliewer, A.; Sanchez, J.; Lim, H.D.; Alamein, C.; Manandhar, P.; Santiago, M.; Fritzwanker, S.; Schmiedel, F.; et al. Low Intrinsic Efficacy for G Protein Activation Can Explain the Improved Side Effect Profiles of New Opioid Agonists. Sci. Signal. 2020, 13, 1–18. [Google Scholar] [CrossRef]
- Kandasamy, R.; Hillhouse, T.M.; Livingston, K.E.; Kochan, K.E.; Meurice, C.; Eans, S.O.; Li, M.-H.; White, A.D.; Roques, B.P.; McLaughlin, J.P.; et al. Positive Allosteric Modulation of the Mu-Opioid Receptor Produces Analgesia with Reduced Side Effects. Proc. Natl. Acad. Sci. USA 2021, 118, 1–10. [Google Scholar] [CrossRef]
- Livingston, K.E.; Traynor, J.R. Allostery at Opioid Receptors: Modulation with Small Molecule Ligands. Br. J. Pharmacol. 2018, 175, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Burford, N.T.; Traynor, J.R.; Alt, A. Positive Allosteric Modulators of the μ-Opioid Receptor: A Novel Approach for Future Pain Medications. Br. J. Pharmacol. 2015, 172, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Jagla, C.; Martus, P.; Stein, C. Peripheral Opioid Receptor Blockade Increases Postoperative Morphine Demands—A Randomized, Double-Blind, Placebo-Controlled Trial. Pain 2014, 155, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. Opioid Analgesia: Recent Developments. Curr. Opin. Support. Palliat. Care 2020, 14, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Küchler, S. Targeting Inflammation and Wound Healing by Opioids. Trends Pharmacol. Sci. 2013, 34, 303–312. [Google Scholar] [CrossRef]
- Spahn, V.; Del Vecchio, G.; Labuz, D.; Rodriguez-Gaztelumendi, A.; Massaly, N.; Temp, J.; Durmaz, V.; Sabri, P.; Reidelbach, M.; Machelska, H.; et al. A Nontoxic Pain Killer Designed by Modeling of Pathological Receptor Conformations. Science 2017, 355, 966. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Schäfer, M.; Machelska, H. Attacking Pain at Its Source: New Perspectives on Opioids. Nat. Med. 2003, 9, 1003–1008. [Google Scholar] [CrossRef]
- Baamonde, A.; Menéndez, L.; González-Rodríguez, S.; Lastra, A.; Seitz, V.; Stein, C.; Machelska, H. A Low PKa Ligand Inhibits Cancer-Associated Pain in Mice by Activating Peripheral Mu-Opioid Receptors. Sci. Rep. 2020, 10, 18599. [Google Scholar] [CrossRef] [PubMed]
- Munk, C.; Isberg, V.; Mordalski, S.; Harpsøe, K.; Rataj, K.; Hauser, A.S.; Kolb, P.; Bojarski, A.J.; Vriend, G.; Gloriam, D.E. GPCRdb: The G Protein-coupled Receptor Database—An Introduction. Br. J. Pharmacol. 2016, 173, 2195–2207. [Google Scholar] [CrossRef]
- Rang, P.; Humphrey, R. Dale’s Pharmacology, 7th ed.; Churchill Livingstone: Edinburgh, UK, 2011; p. 106. [Google Scholar]
- Stein, C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Cheng, W.; Peiris, M.; Wyse, B.D.; Roberts-Thomson, S.J.; Zheng, J.; Monteith, G.R.; Cabot, P.J. Rapid, Opioid-Sensitive Mechanisms Involved in Transient Receptor Potential Vanilloid 1 Sensitization. J. Biol. Chem. 2008, 283, 19540–19550. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Miess, E.; Gondin, A.B.; Yousuf, A.; Steinborn, R.; Mösslein, N.; Yang, Y.; Göldner, M.; Ruland, J.G.; Bünemann, M.; Krasel, C.; et al. Multisite Phosphorylation Is Required for Sustained Interaction with GRKs and Arrestins during Rapid μ-Opioid Receptor Desensitization. Sci. Signal. 2018, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cuitavi, J.; Hipólito, L.; Canals, M. The Life Cycle of the Mu-Opioid Receptor. Trends Biochem. Sci. 2020, 46, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Trzaskowski, B.; Latek, D.; Yuan, S.; Ghoshdastider, U.; Debinski, A.; Filipek, S. Action of Molecular Switches in GPCRs—Theoretical and Experimental Studies. Curr. Med. Chem. 2012, 19, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.A.; Weinstein, H. Integrated Methods for the Construction of Three-Dimensional Models and Computational Probing of Structure-Function Relations in G Protein-Coupled Receptors. In Methods in Neurosciences; Sealfon, S.C., Ed.; Receptor Molecular Biology; Academic Press: Cambridge, MA, USA, 1995; Volume 25, pp. 366–428. [Google Scholar]
- Deupi, X.; Edwards, P.; Singhal, A.; Nickle, B.; Oprian, D.; Schertler, G.; Standfuss, J. Stabilized G Protein Binding Site in the Structure of Constitutively Active Metarhodopsin-II. Proc. Natl. Acad. Sci. USA 2012, 109, 119–124. [Google Scholar] [CrossRef]
- Rasmussen, S.G.F.; DeVree, B.T.; Zou, Y.; Kruse, A.C.; Chung, K.Y.; Kobilka, T.S.; Thian, F.S.; Chae, P.S.; Pardon, E.; Calinski, D.; et al. Crystal Structure of the Β2Adrenergic Receptor-Gs Protein Complex. Nature 2011, 477, 549–555. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Ma, A.K.; Fonseca, R.; Latorraca, N.R.; Kelly, B.; Betz, R.M.; Asawa, C.; Kobilka, B.K.; Dror, R.O. Diverse GPCRs Exhibit Conserved Water Networks for Stabilization and Activation. Proc. Natl. Acad. Sci. USA 2019, 116, 3288–3293. [Google Scholar] [CrossRef]
- Katritch, V.; Fenalti, G.; Abola, E.E.; Roth, B.L.; Cherezov, V.; Stevens, R.C. Allosteric Sodium in Class A GPCR Signaling. Trends Biochem. Sci. 2014, 39, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Manglik, A.; Venkatakrishnan, A.J.; Laeremans, T.; Feinberg, E.N.; Sanborn, A.L.; Kato, H.E.; Livingston, K.E.; Thorsen, T.S.; Kling, R.C.; et al. Structural Insights into Μ-Opioid Receptor Activation. Nature 2015, 524, 315–321. [Google Scholar] [CrossRef]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal Structure of the Μ-Opioid Receptor Bound to a Morphinan Antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef]
- Koehl, A.; Hu, H.; Maeda, S.; Zhang, Y.; Qu, Q.; Paggi, J.M.; Latorraca, N.R.; Hilger, D.; Dawson, R.; Matile, H.; et al. Structure of the Μ-Opioid Receptor–Gi Protein Complex. Nature 2018, 558, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yeliseev, A.; Liu, R. Ligand Interaction, Binding Site and G Protein Activation of the Mu Opioid Receptor. Eur. J. Pharmacol. 2013, 702, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Harding, W.W.; Tidgewell, K.; Byrd, N.; Cobb, H.; Dersch, C.M.; Butelman, E.R.; Rothman, R.B.; Prisinzano, T.E. Neoclerodane Diterpenes as a Novel Scaffold for μ Opioid Receptor Ligands. J. Med. Chem. 2005, 48, 4765–4771. [Google Scholar] [CrossRef] [PubMed]
- Surratt, C.K.; Johnson, P.S.; Moriwaki, A.; Seidleck, B.K.; Blaschak, C.J.; Wang, J.B.; Uhl, G.R. Mu Opiate Receptor. Charged Transmembrane Domain Amino Acids Are Critical for Agonist Recognition and Intrinsic Activity. J. Biol. Chem. 1994, 269, 20548–20553. [Google Scholar] [CrossRef]
- Li, J.; Huang, P.; Chen, C.; de Riel, J.K.; Weinstein, H.; Liu-Chen, L.Y. Constitutive Activation of the Mu Opioid Receptor by Mutation of D3.49(164), but Not D3.32(147): D3.49(164) Is Critical for Stabilization of the Inactive Form of the Receptor and for Its Expression. Biochemistry 2001, 40, 12039–12050. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved Protein–Ligand Docking Using GOLD. Proteins Struct. Funct. Bioinform. 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Dumitrascuta, M.; Bermudez, M.; Ben Haddou, T.; Guerrieri, E.; Schläfer, L.; Ritsch, A.; Hosztafi, S.; Lantero, A.; Kreutz, C.; Massotte, D.; et al. N-Phenethyl Substitution in 14-Methoxy- N-Methylmorphinan-6-Ones Turns Selective µ Opioid Receptor Ligands into Dual µ/δ Opioid Receptor Agonists. Sci. Rep. 2020, 10, 5653. [Google Scholar] [CrossRef]
- Meyer, J.; Vecchio, G.D.; Seitz, V.; Massaly, N.; Stein, C. Modulation of μ-Opioid Receptor Activation by Acidic PH Is Dependent on Ligand Structure and an Ionizable Amino Acid Residue. Br. J. Pharmacol. 2019, 176, 4510–4520. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.N.; Mahinthichaichan, P.; Shen, J.; Ellis, C.R. How μ -Opioid Receptor Recognizes Fentanyl. Nat. Commun. 2021, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, P.F.J.; Jarończyk, M.; Dobrowolski, J.C.; Sadlej, J. Molecular Dynamics of Fentanyl Bound to μ-Opioid Receptor. J. Mol. Model. 2019, 25, 144. [Google Scholar] [CrossRef] [PubMed]
- Ricarte, A.; Dalton, J.A.R.; Giraldo, J. Structural Assessment of Agonist Efficacy in the μ-Opioid Receptor: Morphine and Fentanyl Elicit Different Activation Patterns. J. Chem. Inf. Model. 2021, 61, 1251–1274. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Y.F.; Partilla, J.S.; Zheng, Q.X.; Wang, J.B.; Brine, G.A.; Carroll, F.I.; Rice, K.C.; Chen, K.X.; Chi, Z.Q.; et al. Opioid Peptide Receptor Studies, 11: Involvement of Tyr148, Trp318 and His319 of the Rat Mu-Opioid Receptor in Binding of Mu-Selective Ligands. Synapse 1999, 32, 23–28. [Google Scholar] [CrossRef]
- Ulens, C.; Van Boven, M.; Daenens, P.; Tytgat, J. Interaction of P-Fluorofentanyl on Cloned Human Opioid Receptors and Exploration of the Role of Trp-318 and His-319 in Mu-Opioid Receptor Selectivity. J. Pharmacol. Exp. Ther. 2000, 294, 1024–1033. [Google Scholar] [PubMed]
- Schneider, S.; Provasi, D.; Filizola, M. How Oliceridine (TRV-130) Binds and Stabilizes a μ-Opioid Receptor Conformational State That Selectively Triggers G Protein Signaling Pathways. Biochemistry 2016, 55, 6456–6466. [Google Scholar] [CrossRef]
- Cong, X.; Campomanes, P.; Kless, A.; Schapitz, I.; Wagener, M.; Koch, T.; Carloni, P. Structural Determinants for the Binding of Morphinan Agonists to the μ-Opioid Receptor. PLoS ONE 2015, 10, e0135998. [Google Scholar] [CrossRef]
- Bonner, G.; Meng, F.; Akil, H. Selectivity of μ-Opioid Receptor Determined by Interfacial Residues near Third Extracellular Loop. Eur. J. Pharmacol. 2000, 403, 37–44. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Nagarajan, S.; Wolfrum, K.M.; Reed, J.F.; Nilsen, A.; Torralva, R.; Janowsky, A. Affinity, Potency, Efficacy, Selectivity, and Molecular Modeling of Substituted Fentanyls at Opioid Receptors. Biochem. Pharmacol. 2020, 182, 114293. [Google Scholar] [CrossRef]
- Kaserer, T.; Lantero, A.; Schmidhammer, H.; Spetea, M.; Schuster, D. μ Opioid Receptor: Novel Antagonists and Structural Modeling. Sci. Rep. 2016, 6, 21548. [Google Scholar] [CrossRef]
- Mansour, A.; Taylor, L.P.; Fine, J.L.; Thompson, R.C.; Hoversten, M.T.; Mosberg, H.I.; Watson, S.J.; Akil, H. Key Residues Defining the Mu-Opioid Receptor Binding Pocket: A Site-Directed Mutagenesis Study. J. Neurochem. 1997, 68, 344–353. [Google Scholar] [CrossRef]
- Zhang, P.; Johnson, P.S.; Zöllner, C.; Wang, W.; Wang, Z.; Montes, A.E.; Seidleck, B.K.; Blaschak, C.J.; Surratt, C.K. Mutation of Human μ Opioid Receptor Extracellular “Disulfide Cysteine” Residues Alters Ligand Binding but Does Not Prevent Receptor Targeting to the Cell Plasma Membrane. Mol. Brain Res. 1999, 72, 195–204. [Google Scholar] [CrossRef]
- Møllendal, H.; Balcells, D.; Eisenstein, O.; Syversen, L.; Suissa, M.R. Conformational Complexity of Morphine and Morphinum in the Gas Phase and in Water. A DFT and MP2 Study. RSC Adv. 2014, 4, 24729–24735. [Google Scholar] [CrossRef]
- Dosen-Micovic, L.; Ivanovic, M.; Micovic, V. Steric Interactions and the Activity of Fentanyl Analogs at the μ-Opioid Receptor. Bioorg. Med. Chem. 2006, 14, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- de Waal, P.W.; Shi, J.; You, E.; Wang, X.; Melcher, K.; Jiang, Y.; Xu, H.E.; Dickson, B.M. Molecular Mechanisms of Fentanyl Mediated β-Arrestin Biased Signaling. PLoS Comput. Biol. 2020, 16, e1007394. [Google Scholar] [CrossRef]
- Ellis, C.R.; Kruhlak, N.L.; Kim, M.T.; Hawkins, E.G.; Stavitskaya, L. Predicting Opioid Receptor Binding Affinity of Pharmacologically Unclassified Designer Substances Using Molecular Docking. PLoS ONE 2018, 13, e0197734. [Google Scholar] [CrossRef]
- Norn, S.; Kruse, P.R.; Kruse, E. History of opium poppy and morphine. Dan. Med. Arbog 2005, 33, 171–184. [Google Scholar]
- Patrick, G.L. An Introduction to Medicinal Chemistry; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Glare, P.; Walsh, D.; Sheehan, D. The Adverse Effects of Morphine: A Prospective Survey of Common Symptoms during Repeated Dosing for Chronic Cancer Pain. Am. J. Hosp. Palliat. Care 2006, 23, 229–235. [Google Scholar] [CrossRef]
- Yongye, A.B.; Martínez-Mayorga, K.; Racz, G.B.; Noe, C.E. Molecular Aspects of Opioid Receptors and Opioid Receptor Painkillers. In Pain Management—Current Issues and Opinions; Racz, G., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-307-813-7. [Google Scholar]
- Claff, T.; Yu, J.; Blais, V.; Patel, N.; Martin, C.; Wu, L.; Han, G.W.; Holleran, B.J.; der Poorten, O.V.; White, K.L.; et al. Elucidating the Active δ-Opioid Receptor Crystal Structure with Peptide and Small-Molecule Agonists. Sci. Adv. 2019, 5, eaax9115. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Huang, C.C.; Ferrin, T.E. Tools for Integrated Sequence-Structure Analysis with UCSF Chimera. BMC Bioinform. 2006, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Coop, A.; MacKerell, A.D. Molecular Details of the Activation of the μ Opioid Receptor. J. Phys. Chem. B 2013, 117, 7907–7917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Dong, G.; Sheng, C. Structural Simplification of Natural Products. Chem. Rev. 2019, 119, 4180–4220. [Google Scholar] [CrossRef]
- Eguchi, M. Recent Advances in Selective Opioid Receptor Agonists and Antagonists. Med. Res. Rev. 2004, 24, 182–212. [Google Scholar] [CrossRef]
- Muijsers, R.B.; Wagstaff, A.J. Transdermal Fentanyl: An Updated Review of Its Pharmacological Properties and Therapeutic Efficacy in Chronic Cancer Pain Control. Drugs 2001, 61, 2289–2307. [Google Scholar] [CrossRef]
- Volpe, D.A.; McMahon Tobin, G.A.; Mellon, R.D.; Katki, A.G.; Parker, R.J.; Colatsky, T.; Kropp, T.J.; Verbois, S.L. Uniform Assessment and Ranking of Opioid μ Receptor Binding Constants for Selected Opioid Drugs. Regul. Toxicol. Pharmacol. RTP 2011, 59, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Shapira, B.; Rosca, P.; Berkovitz, R.; Gorjaltsan, I.; Neumark, Y. The Switch from One Substance-of-Abuse to Another: Illicit Drug Substitution Behaviors in a Sample of High-Risk Drug Users. PeerJ 2020, 8, e9461. [Google Scholar] [CrossRef] [PubMed]
- Woolmer, R. Analgesic Drugs. BJA Br. J. Anaesth. 1955, 27, 276–285. [Google Scholar] [CrossRef][Green Version]
- Stein, C. Targeting Pain and Inflammation by Peripherally Acting Opioids. Front. Pharmacol. 2013, 4, 123. [Google Scholar] [CrossRef]
- DeWire, S.M.; Yamashita, D.S.; Rominger, D.H.; Liu, G.; Cowan, C.L.; Graczyk, T.M.; Chen, X.-T.; Pitis, P.M.; Gotchev, D.; Yuan, C.; et al. A G Protein-Biased Ligand at the μ-Opioid Receptor Is Potently Analgesic with Reduced Gastrointestinal and Respiratory Dysfunction Compared with Morphine. J. Pharmacol. Exp. Ther. 2013, 344, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Oliceridine: First Approval. Drugs 2020, 80, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Hans, G.; Dickenson, A.H. Peripheral Input and Its Importance for Central Sensitization. Ann. Neurol. 2013, 74, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.; Spahn, V.; Stein, C. Novel Opioid Analgesics and Side Effects. ACS Chem. Neurosci. 2017, 8, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
- Erra Díaz, F.; Dantas, E.; Geffner, J. Unravelling the Interplay between Extracellular Acidosis and Immune Cells. Mediat. Inflamm. 2018, 2018, e1218297. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Clark, J.D.; Oh, U.; Vasko, M.R.; Wilcox, G.L.; Overland, A.C.; Vanderah, T.W.; Spencer, R.H. Peripheral Mechanisms of Pain and Analgesia. Brain Res. Rev. 2009, 60, 90–113. [Google Scholar] [CrossRef]
- Vetter, I.; Kapitzke, D.; Hermanussen, S.; Monteith, G.R.; Cabot, P.J. The Effects of PH on Beta-Endorphin and Morphine Inhibition of Calcium Transients in Dorsal Root Ganglion Neurons. J. Pain 2006, 7, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.; Labuz, D.; Temp, J.; Seitz, V.; Kloner, M.; Negrete, R.; Rodriguez-Gaztelumendi, A.; Weber, M.; Machelska, H.; Stein, C. PK a of Opioid Ligands as a Discriminating Factor for Side Effects. Sci. Rep. 2019, 9, 19344. [Google Scholar] [CrossRef] [PubMed]
- Spahn, V.; Vecchio, G.D.; Rodriguez-Gaztelumendi, A.; Temp, J.; Labuz, D.; Kloner, M.; Reidelbach, M.; Machelska, H.; Weber, M.; Stein, C. Opioid Receptor Signaling, Analgesic and Side Effects Induced by a Computationally Designed PH-Dependent Agonist. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gaztelumendi, A.; Spahn, V.; Labuz, D.; Machelska, H.; Stein, C. Analgesic Effects of a Novel PH-Dependent μ-Opioid Receptor Agonist in Models of Neuropathic and Abdominal Pain. Pain 2018, 159, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Vargas, N.N.; Yu, Y.; Jensen, D.D.; Bok, D.D.; Wisdom, M.; Latorre, R.; Lopez, C.; Jaramillo-Polanco, J.O.; Degro, C.; Guzman-Rodriguez, M.; et al. Agonist That Activates the Μ-Opioid Receptor in Acidified Microenvironments Inhibits Colitis Pain without Side Effects. Gut 2021. [Google Scholar] [CrossRef] [PubMed]
- Lans, I.; Dalton, J.A.R.; Giraldo, J. Selective Protonation of Acidic Residues Triggers Opsin Activation. J. Phys. Chem. B 2015, 119, 9510–9519. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; MacKerell, A.D. Automation of the CHARMM General Force Field (CGenFF) I: Bond Perception and Atom Typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of Bonded Parameters and Partial Atomic Charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef]
- Lešnik, S.; Hodošček, M.; Bren, U.; Stein, C.; Bondar, A.-N. Potential Energy Function for Fentanyl-Based Opioid Pain Killers. J. Chem. Inf. Model. 2020, 60, 3566–3576. [Google Scholar] [CrossRef]
- Giannos, T.; Lešnik, S.; Bren, U.; Hodošček, M.; Domratcheva, T.; Bondar, A.-N. CHARMM Force-Field Parameters for Morphine, Heroin, and Oliceridine, and Conformational Dynamics of Opioid Drugs. J. Chem. Inf. Model. 2021, 61, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Bertalan, É.; Lešnik, S.; Bren, U.; Bondar, A.-N. Protein-Water Hydrogen-Bond Networks of G Protein-Coupled Receptors: Graph-Based Analyses of Static Structures and Molecular Dynamics. J. Struct. Biol. 2020, 212, 107634. [Google Scholar] [CrossRef]
- Bertalan, É.; Lesca, E.; Schertler, G.F.X.; Bondar, A.-N. C-Graphs Tool with Graphical User Interface to Dissect Conserved Hydrogen-Bond Networks: Applications to Visual Rhodopsins. J. Chem. Inf. Model. 2021, 61, 5692–5707. [Google Scholar] [CrossRef]
- Kenakin, T. Biased Receptor Signaling in Drug Discovery. Pharmacol. Rev. 2019, 71, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Neto, J.; Costanzini, A.; De Giorgio, R.; Lambert, D.G.; Ruzza, C.; Calò, G. Biased versus Partial Agonism in the Search for Safer Opioid Analgesics. Molecules 2020, 25, 3870. [Google Scholar] [CrossRef]
- Piekielna-Ciesielska, J.; Wtorek, K.; Janecka, A. Biased Agonism as an Emerging Strategy in the Search for Better Opioid Analgesics. Curr. Med. Chem. 2020, 27, 1562–1575. [Google Scholar] [CrossRef]
- Stanczyk, M.A.; Kandasamy, R. Biased Agonism: The Quest for the Analgesic Holy Grail. PAIN Rep. 2018, 3, e650. [Google Scholar] [CrossRef]
- Thompson, G.L.; Lane, J.R.; Coudrat, T.; Sexton, P.M.; Christopoulos, A.; Canals, M. Biased Agonism of Endogenous Opioid Peptides at the μ-Opioid Receptor. Mol. Pharmacol. 2015, 88, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Wisler, J.W.; Xiao, K.; Thomsen, A.R.; Lefkowitz, R.J. Recent Developments in Biased Agonism. Curr. Opin. Cell Biol. 2014, 27, 18–24. [Google Scholar] [CrossRef]
- Kelly, B.; Hollingsworth, S.A.; Blakemore, D.C.; Owen, R.M.; Storer, R.I.; Swain, N.A.; Aydin, D.; Torella, R.; Warmus, J.S.; Dror, R.O. Delineating the Ligand–Receptor Interactions That Lead to Biased Signaling at the μ-Opioid Receptor. J. Chem. Inf. Model. 2021, 61, 3696–3707. [Google Scholar] [CrossRef]
- Mondal, D.; Kolev, V.; Warshel, A. Exploring the Activation Pathway and Gi-Coupling Specificity of the μ-Opioid Receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 26218–26225. [Google Scholar] [CrossRef]
- Marmolejo-Valencia, A.F.; Martínez-Mayorga, K. Allosteric Modulation Model of the Mu Opioid Receptor by Herkinorin, a Potent Not Alkaloidal Agonist. J. Comput. Aided Mol. Des. 2017, 31, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Partilla, J.S.; Wang, X.; Rutherford, J.M.; Tidgewell, K.; Prisinzano, T.E.; Bohn, L.M.; Rothman, R.B. A Comparison of Noninternalizing (Herkinorin) and Internalizing (DAMGO) μ-Opioid Agonists on Cellular Markers Related to Opioid Tolerance and Dependence. Synapse 2007, 61, 166–175. [Google Scholar] [CrossRef]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking Barriers to Novel Analgesic Drug Development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lešnik, S.; Bertalan, É.; Bren, U.; Bondar, A.-N. Opioid Receptors and Protonation-Coupled Binding of Opioid Drugs. Int. J. Mol. Sci. 2021, 22, 13353. https://doi.org/10.3390/ijms222413353
Lešnik S, Bertalan É, Bren U, Bondar A-N. Opioid Receptors and Protonation-Coupled Binding of Opioid Drugs. International Journal of Molecular Sciences. 2021; 22(24):13353. https://doi.org/10.3390/ijms222413353
Chicago/Turabian StyleLešnik, Samo, Éva Bertalan, Urban Bren, and Ana-Nicoleta Bondar. 2021. "Opioid Receptors and Protonation-Coupled Binding of Opioid Drugs" International Journal of Molecular Sciences 22, no. 24: 13353. https://doi.org/10.3390/ijms222413353
APA StyleLešnik, S., Bertalan, É., Bren, U., & Bondar, A.-N. (2021). Opioid Receptors and Protonation-Coupled Binding of Opioid Drugs. International Journal of Molecular Sciences, 22(24), 13353. https://doi.org/10.3390/ijms222413353






