Apoptosis Pathways Triggered by a Potent Antiproliferative Hybrid Chalcone on Human Melanoma Cells
Abstract
:1. Introduction
2. Results and Discussion
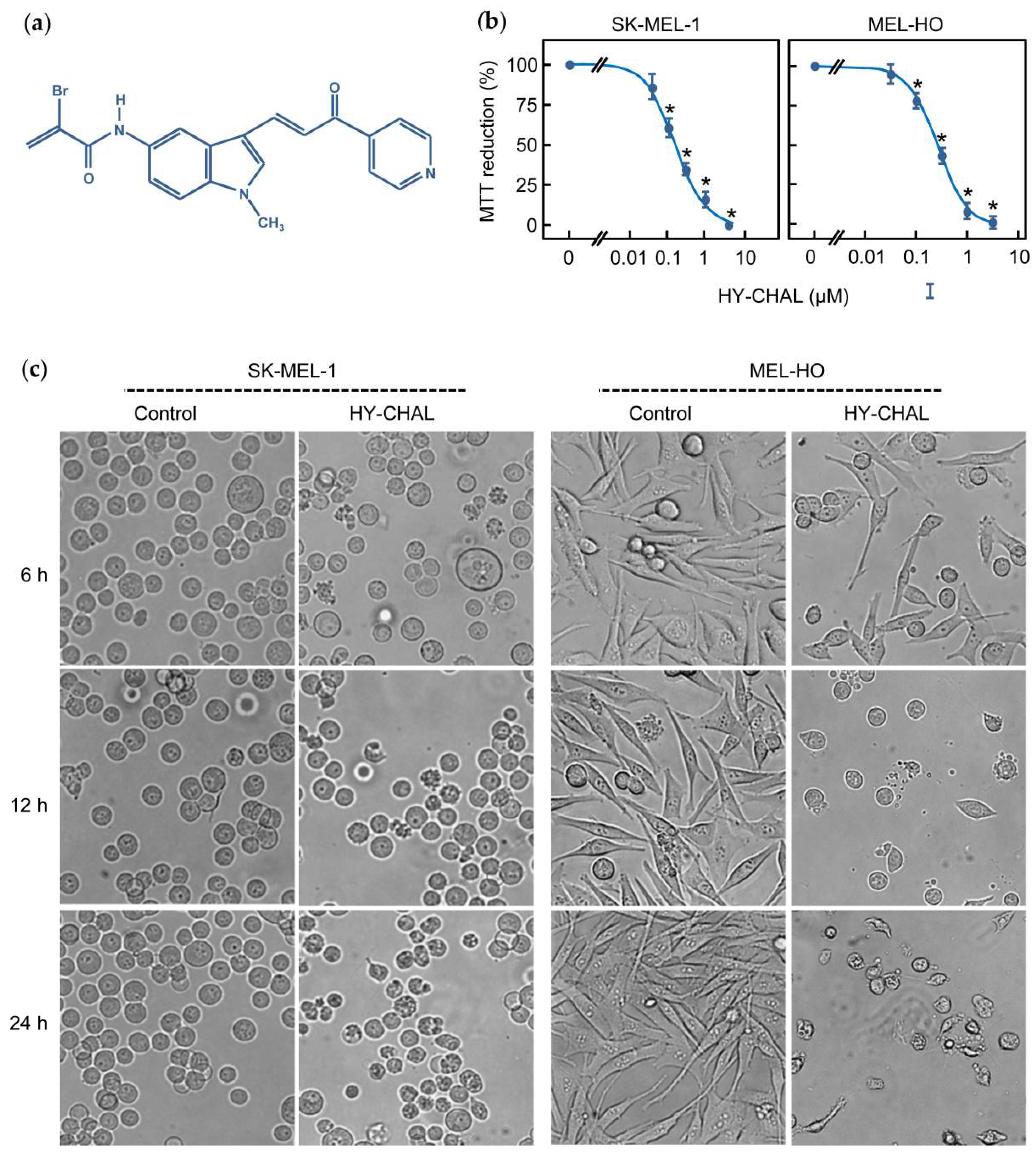
2.1. The Synthetic Hybrid Chalcone HY-CHAL Decreases the Viability of Human Melanoma Cells
2.2. HY-CHAL Induced Cell Death by Apoptosis on Human Melanoma Cells
2.3. HY-CHAL Induces Cell Death by a Caspase-Dependent Pathway in Human Melanoma Cells
2.4. HY-CHAL Down-Regulated the Pro-Survival Bcl-2 Members and Up-Regulated the Pro-Apoptotic Bcl-2 Proteins and Death Receptors and Its Ligand in Human Melanoma SK-MEL-1 Cells
2.5. HY-CHAL Induced Cytochrome c Release and Mitochondrial Membrane Potential Depolarization in Human Melanoma Cells
2.6. HY-CHAL Activated the Mitogen-Activated Protein Kinase Pathway and Inhibited AKT in Human Melanoma SK-MEL-1 Cells
2.7. HY-CHAL Downregulated β-Catenin, c-Myc and p21Cip1/WAF1 and Inhibited NF-κB Pathway in Human Melanoma SK-MEL-1 Cells
2.8. HY-CHAL Increased Reactive Oxygen Species (ROS) Generation and Glutathione Blocked ROS Formation and Cell Death in Human SK-MEL-1 Melanoma Cells
3. Materials and Methods
3.1. Chemicals and Antibodies
3.2. Cell Culture and Viability Assays
3.3. Evaluation and Quantification of Apoptosis
3.4. Western Blot Analysis
3.5. Assay of Caspase Activity
3.6. Analysis of Mitochondrial Membrane Potential ΔΨm and Detection of Intracellular Reactive Oxygen Species (ROS)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2021. American Cancer Society: Atlanta, 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 29 October 2021).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA A Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Malissen, N.; Grob, J.-J. Metastatic Melanoma: Recent Therapeutic Progress and Future Perspectives. Drugs 2018, 78, 1197–1209. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Derakhshan, M.; Darabi, H.; Hedayat, P.; Momeni, M. Melanoma: Where we are and where we go. J. Cell. Physiol. 2019, 234, 3307–3320. [Google Scholar] [CrossRef]
- Fujimura, T.; Hidaka, T.; Kambayashi, Y.; Aiba, S. BRAF kinase inhibitors for treatment of melanoma: Developments from early-stage animal studies to Phase II clinical trials. Expert Opin. Investig. Drugs 2018, 28, 143–148. [Google Scholar] [CrossRef]
- Payandeh, Z.; Yarahmadi, M.; Nariman-Saleh-Fam, Z.; Tarhriz, V.; Islami, M.; Aghdam, A.M.; Eyvazi, S. Immune therapy of melanoma: Overview of therapeutic vaccines. J. Cell. Physiol. 2019, 234, 14612–14621. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Berk-Krauss, J.; Stein, J.; Weber, J.; Polsky, D.; Geller, A.C. New Systematic Therapies and Trends in Cutaneous Melanoma Deaths Among US Whites, 1986–2016. Am. J. Public Health 2020, 110, 731–733. [Google Scholar] [CrossRef]
- Mason, R.; Au, L.; Garces, A.I.R.; Larkin, J. Current and emerging systemic therapies for cutaneous metastatic melanoma. Expert Opin. Pharmacother. 2019, 20, 1135–1152. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef]
- Garnett, M.J.; Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 2004, 6, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Tsao, H.; Yang, G.; Goel, V.; Wu, H.; Haluska, F.G. Genetic Interaction Between NRAS and BRAF Mutations and PTEN/MMAC1 Inactivation in Melanoma. J. Investig. Dermatol. 2004, 122, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Pappalardo, F.; Russo, G.; Candido, S.; Pennisi, M.; Cavalieri, S.; Motta, S.; McCubrey, J.A.; Nicoletti, F.; Libra, M. Computational Modeling of PI3K/AKT and MAPK Signaling Pathways in Melanoma Cancer. PLoS ONE 2016, 11, e0152104. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Shukla, S.; Sood, A.K.; Goyal, K.; Singh, A.; Sharma, V.; Guliya, N.; Gulati, S.; Kumar, S. Chalcone Scaffolds as Anticancer Drugs: A Review on Molecular Insight in Action of Mechanisms and Anticancer Properties. Anti-Cancer Agents Med. Chem. 2021, 21, 1650–1670. [Google Scholar] [CrossRef]
- Jandial, D.; Blair, C.; Zhang, S.; Krill, L.; Zhang, Y.-B.; Zi, X. Molecular targeted approaches to cancer therapy and prevention using chalcones. Curr. Cancer Drug Targets 2014, 14, 181–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquina, S.; Maldonado-Santiago, M.; Sánchez-Carranza, J.N.; Antúnez-Mojica, M.; González-Maya, L.; Razo-Hernández, R.S.; Alvarez, L. Design, synthesis and QSAR study of 2′-hydroxy-4′-alkoxy chalcone derivatives that exert cytotoxic activity by the mitochondrial apoptotic pathway. Bioorganic Med. Chem. 2019, 27, 43–54. [Google Scholar] [CrossRef]
- Romagnoli, R.; Prencipe, F.; Lopez-Cara, L.C.; Oliva, P.; Baraldi, S.; Baraldi, P.G.; Estévez-Sarmiento, F.; Quintana, J.; Estévez, F. Synthesis and biological evaluation of alpha-bromoacryloylamido indolyl pyridinyl propenones as potent apoptotic inducers in human leukaemia cells. J. Enzym. Inhib. Med. Chem. 2018, 33, 727–742. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.J.; Hayward, R.; Viros, A.; Marais, R. Metformin Accelerates the Growth of BRAFV600E-Driven Melanoma by Upregulating VEGF-A. Cancer Discov. 2012, 2, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Margolin, N.; Raybuck, S.A.; Wilson, K.P.; Chen, W.; Fox, T.; Gu, Y.; Livingston, D.J. Substrate and Inhibitor Specificity of Interleukin-1β-converting Enzyme and Related Caspases. J. Biol. Chem. 1997, 272, 7223–7228. [Google Scholar] [CrossRef] [Green Version]
- Eskes, R.; Antonsson, B.; Osen-Sand, A.; Montessuit, S.; Richter, C.; Sadoul, R.; Mazzei, G.; Nichols, A.; Martinou, J.-C. Bax-induced Cytochrome C Release from Mitochondria Is Independent of the Permeability Transition Pore but Highly Dependent on Mg2+ Ions. J. Cell Biol. 1998, 143, 217–224. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, L.; Strasser, A.; O’Reilly, L.A.; Hausmann, G.; Adams, J.; Cory, S.; Huang, D. Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998, 17, 384–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.P.; Pincus, L.B.; Ruben, B.S.; et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.P.; Liao, Y.; Xia, W.; Spohn, B.; Lee, M.-H.; Hung, M.-C. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 2001, 3, 245–252. [Google Scholar] [CrossRef]
- Dajani, R.; Fraser, E.; Roe, M.; Young, N.; Good, V.; Dale, T.; Pearl, L.H. Crystal Structure of Glycogen Synthase Kinase 3β: Structural Basis for Phosphate-Primed Substrate Specificity and Autoinhibition. Cell 2001, 105, 721–732. [Google Scholar] [CrossRef]
- De Herreros, A.G.; Duñach, M.; De Herreros, G. Intracellular Signals Activated by Canonical Wnt Ligands Independent of GSK3 Inhibition and β-Catenin Stabilization. Cells 2019, 8, 1148. [Google Scholar] [CrossRef] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Patel, S.; Doble, B.; Woodgett, J. Glycogen synthase kinase-3 in insulin and Wnt signalling: A double-edged sword? Biochem. Soc. Trans. 2004, 32, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. β-Catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef] [Green Version]
- Brancolini, C.; Sgorbissa, A.; Schneider, C. Proteolytic processing of the adherens junctions components β-catenin and γ-catenin/plakoglobin during apoptosis. Cell Death Differ. 1998, 5, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Guttridge, D.C.; You, Z.; Zhang, Z.; Fribley, A.; Mayo, M.W.; Kitajewski, J.; Wang, C.-Y. WNT-1 Signaling Inhibits Apoptosis by Activating β-Catenin/T Cell Factor–Mediated Transcription. J. Cell Biol. 2001, 152, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.-H.; Zhong, Y.; Pérez-Soler, R. Disruption of Cell Adhesion and Caspase-Mediated Proteolysis of β- and γ-Catenins and APC Protein in Paclitaxel-Induced Apoptosis. Mol. Pharmacol. 2001, 59, 593–603. [Google Scholar] [CrossRef]
- Schmeiser, K.; Grand, R.J. The fate of E- and P-cadherin during the early stages of apoptosis. Cell Death Differ. 1999, 6, 377–386. [Google Scholar] [CrossRef]
- Steinhusen, U.; Weiske, J.; Badock, V.; Tauber, R.; Bommert, K.; Huber, O. Cleavage and Shedding of E-cadherin after Induction of Apoptosis. J. Biol. Chem. 2001, 276, 4972–4980. [Google Scholar] [CrossRef] [Green Version]
- Stasevich, E.; Murashko, M.; Zinevich, L.; Demin, D.; Schwartz, A. The Role of Non-Coding RNAs in the Regulation of the Proto-Oncogene MYC in Different Types of Cancer. Biomedicines 2021, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. MYC on the Path to Cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkat, M.; De Melo, J.; Hickman, K.A.; Lourenco, C.; Redel, C.; Resetca, D.; Tamachi, A.; Tu, W.B.; Penn, L.Z. MYC Deregulation in Primary Human Cancers. Genes 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonetti, C.; Biroccio, A.; Candiloro, A.; Citro, G.; Fornari, C.; Mottolese, M.; Del Bufalo, D.; Zupi, G. Increase of cisplatin sensitivity by c-myc antisense oligodeoxynucleotides in a human metastatic melanoma inherently resistant to cisplatin. Clin. Cancer Res. 1999, 5, 2588–2595. [Google Scholar]
- Gorospe, M.; Shack, S.; Guyton, K.Z.; Samid, D.; Holbrook, N.J. Up-regulation and functional role of p21Waf1/Cip1 during growth arrest of human breast carcinoma MCF-7 cells by phenylacetate. Cell Growth Differ. 1996, 7, 1609–1615. [Google Scholar]
- Wang, Y.A.; Elson, A.; Leder, P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 14590–14595. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fujita, N.; Tsuruo, T. Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells from growth arrest to undergoing apoptosis. Oncogene 1999, 18, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Gartel, A.L.; Tyner, A.L. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol. Cancer Ther. 2002, 1, 639–649. [Google Scholar]
- Melnikova, V.O.; Bar-Eli, M. Transcriptional control of the melanoma malignant phenotype. Cancer Biol. Ther. 2008, 7, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Baud, V.; Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free. Radic. Res. 2018, 52, 507–543. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, E.D.; Wang, J.; Panzhinskiy, E.E.; Tong, C.; Li, W.; Li, J. Natural Antioxidant-Isoliquiritigenin Selectively Inhibits Proliferation of Prostate Cancer Cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 841–847. [Google Scholar] [CrossRef]
- Champelovier, P.; Chauchet, X.; Puch, F.; Vergnaud, S.; Garrel, C.; Laporte, F.; Boutonnat, J.; Boumendjel, A. Cellular and molecular mechanisms activating the cell death processes by chalcones: Critical structural effects. Toxicol. Vitr. 2013, 27, 2305–2315. [Google Scholar] [CrossRef]
- Boumendjel, A.; McLeer-Florin, A.; Champelovier, P.; Allegro, D.; Muhammad, D.; Souard, F.; Derouazi, M.; Peyrot, V.; Toussaint, B.; Boutonnat, J. A novel chalcone derivative which acts as a microtubule depolymerising agent and an inhibitor of P-gp and BCRP in in-vitro and in-vivo glioblastoma models. BMC Cancer 2009, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yang, J.; Jia, Y.; Tian, Y.; Wen, A. Pharmacokinetic properties of hydroxysafflor yellow A in healthy Chinese female volunteers. J. Ethnopharmacol. 2009, 124, 635–638. [Google Scholar] [CrossRef]
- Wu, W.; Ye, H.; Wan, L.; Han, X.; Wang, G.; Hu, J.; Tang, M.; Duan, X.; Fan, Y.; He, S.; et al. Millepachine, a novel chalcone, induces G 2/M arrest by inhibiting CDK1 activity and causing apoptosis via ROS-mitochondrial apoptotic pathway in human hepatocarcinoma cells in vitro and in vivo. Carcinogenesis 2013, 34, 1636–1643. [Google Scholar] [CrossRef]
- Lee, C.K.; Son, S.H.; Park, K.K.; Park, J.H.Y.; Lim, S.S.; Kim, S.-H.; Chung, W.Y. Licochalcone A Inhibits the Growth of Colon Carcinoma and Attenuates Cisplatin-Induced Toxicity without a Loss of Chemotherapeutic Efficacy in Mice. Basic Clin. Pharmacol. Toxicol. 2008, 103, 48–54. [Google Scholar] [CrossRef]
- Lee, C.K.; Son, S.H.; Park, K.K.; Park, J.H.Y.; Lim, S.S.; Chung, W.Y. Isoliquiritigenin Inhibits Tumor Growth and Protects the Kidney and Liver Against Chemotherapy-Induced Toxicity in a Mouse Xenograft Model of Colon Carcinoma. J. Pharmacol. Sci. 2008, 106, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, S.; Morita, T.; Endo, H.; Hamamoto, T.; Baba, M.; Joichi, Y.; Kaneko, S.; Okada, Y.; Okuyama, T.; Nishino, H.; et al. Isoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinoma. Cancer Lett. 2002, 183, 23–30. [Google Scholar] [CrossRef]
- Oettgen, H.F.; Aoki, T.; Old, L.J.; Boyse, E.A.; De Harven, E.; Mills, G.M. Suspension Culture of a Pigment-Producing Cell Line Derived from a Human Malignant Melanoma2. J. Natl. Cancer Inst. 1968, 41, 827–843. [Google Scholar] [CrossRef]
- Perdomo, J.; Quintana, C.; González, I.; Hernández, I.; Rubio, S.; Loro, J.; Reiter, R.; Estévez, F.; Quintana, J. Melatonin Induces Melanogenesis in Human SK-MEL-1 Melanoma Cells Involving Glycogen Synthase Kinase-3 and Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 4970. [Google Scholar] [CrossRef]
- Sarif, Z.; Tolksdorf, B.; Fechner, H.; Eberle, J. Mcl-1 targeting strategies unlock the proapoptotic potential of TRAIL in melanoma cells. Mol. Carcinog. 2020, 59, 1256–1268. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Zhu, G.; Xing, M. Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, E.; Estévez-Sarmiento, F.; Said, M.; Eiroa, J.L.; Rubio, S.; Quintana, J.; Estévez, F. Cytotoxicity of the Sesquiterpene Lactone Spiciformin and Its Acetyl Derivative against the Human Leukemia Cell Lines U-937 and HL-60. Int. J. Mol. Sci. 2020, 21, 2782. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, I.; Saavedra, E.; del Rosario, H.; Perdomo, J.; Quintana, J.; Prencipe, F.; Oliva, P.; Romagnoli, R.; Estévez, F. Apoptosis Pathways Triggered by a Potent Antiproliferative Hybrid Chalcone on Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 13462. https://doi.org/10.3390/ijms222413462
Rodríguez I, Saavedra E, del Rosario H, Perdomo J, Quintana J, Prencipe F, Oliva P, Romagnoli R, Estévez F. Apoptosis Pathways Triggered by a Potent Antiproliferative Hybrid Chalcone on Human Melanoma Cells. International Journal of Molecular Sciences. 2021; 22(24):13462. https://doi.org/10.3390/ijms222413462
Chicago/Turabian StyleRodríguez, Irene, Ester Saavedra, Henoc del Rosario, Juan Perdomo, José Quintana, Filippo Prencipe, Paola Oliva, Romeo Romagnoli, and Francisco Estévez. 2021. "Apoptosis Pathways Triggered by a Potent Antiproliferative Hybrid Chalcone on Human Melanoma Cells" International Journal of Molecular Sciences 22, no. 24: 13462. https://doi.org/10.3390/ijms222413462





