Inflammation in Coronary Microvascular Dysfunction
Abstract
:1. Introduction
2. Multimodality Assessment of CMVD
2.1. Echocardiography
2.2. Cardiac CT Angiography
2.3. Fat Attenuation Index
2.4. Cardiac Positron Emission Tomography
2.5. Cardiac MRI
2.6. Biomarkers
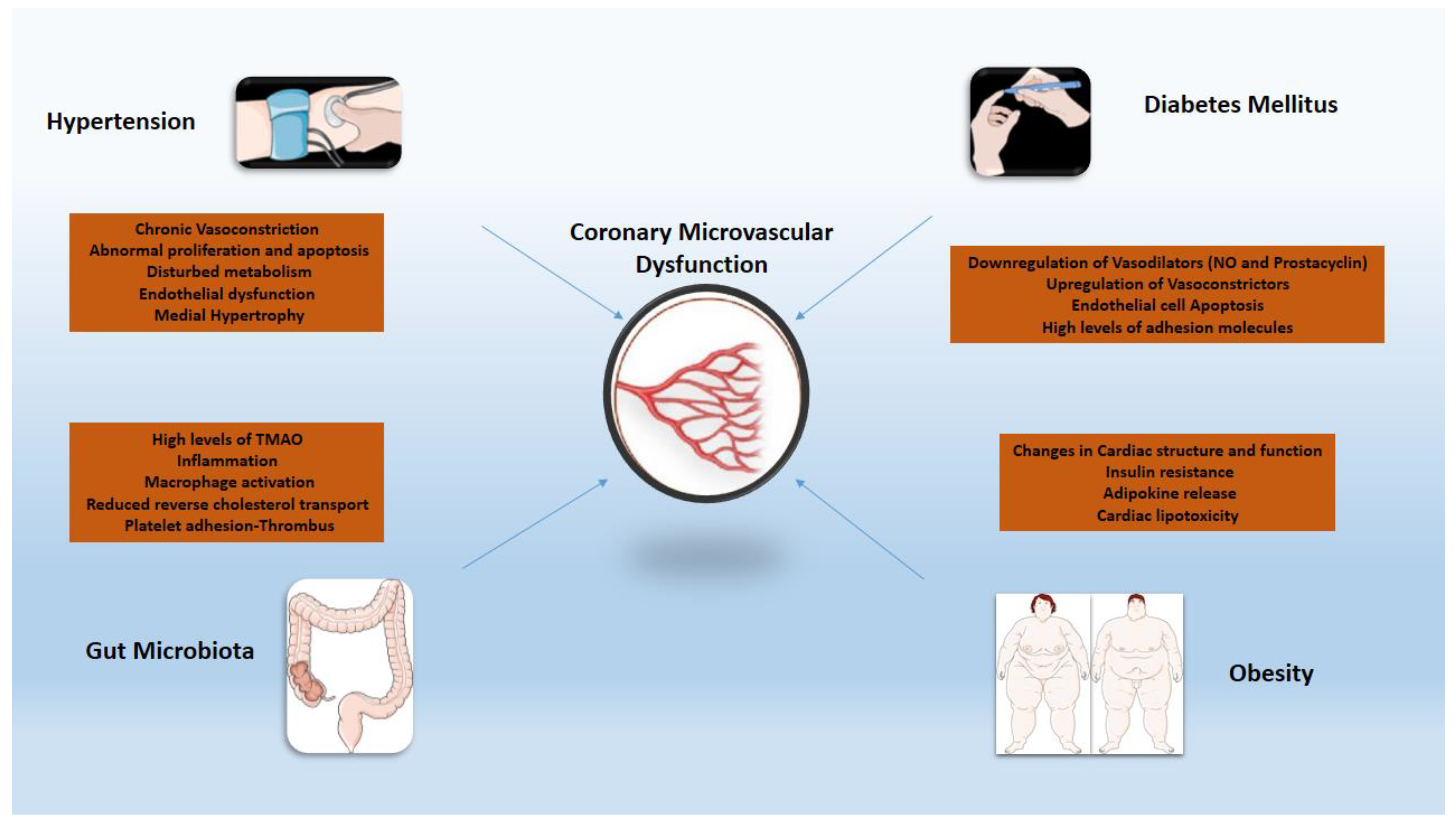
3. The Role of Inflammation in the Pathogenesis of CMVD
3.1. Principal Pathophysiologic Mechanisms
3.2. Hypertension
3.3. Diabetes
3.4. Obesity
3.5. Gut Microbiota
4. Anti-Inflammatory Drugs
4.1. Statins-Aspirin
4.2. Colchicine
4.3. Anakinra–Canakinumab
4.4. Tocilizumab
4.5. Etanercept-Adalinumab
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, B.D.; Shaw, L.J.; Buchthal, S.D.; Bairey Merz, C.N.; Kim, H.W.; Scott, K.N.; Doyle, M.; Olson, M.B.; Pepine, C.J.; den Hollander, J.; et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: Results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004, 109, 2993–2999. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.Y.; Shaw, L.J.; Dunning, A.M.; Labounty, T.M.; Choi, J.H.; Weinsaft, J.W.; Koduru, S.; Gomez, M.J.; Delago, A.J.; Callister, T.Q.; et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: A prospective 2-center study of 2583 patients undergoing 64-detector row coronary computed tomographic angiography. J. Am. Coll. Cardiol. 2011, 58, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Lichtlen, P.R.; Bargheer, K.; Wenzlaff, P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J. Am. Coll. Cardiol. 1995, 25, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Elgendy, I.Y.; Pepine, C.J. Heart Failure With Preserved Ejection Fraction: Is Ischemia Due to Coronary Microvascular Dysfunction a Mechanistic Factor? Am. J. Med. 2019, 132, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with Myocardial Infarction. Cardiovasc. Res. 2021, cvab264. [Google Scholar] [CrossRef]
- Zanatta, E.; Colombo, C.; D’Amico, G.; d’Humieres, T.; Dal Lin, C.; Tona, F. Inflammation and Coronary Microvascular Dysfunction in Autoimmune Rheumatic Diseases. Int. J. Mol. Sci. 2019, 20, 5563. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Jayaweera, A.R.; Firoozan, S.; Linka, A.; Skyba, D.M.; Kaul, S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998, 97, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Montaldo, C.; Zedda, N.; Montisci, R.; Ruscazio, M.; Lai, G.; Cadeddu, M.; Meloni, L.; Iliceto, S. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: Comparison with intracoronary Doppler flow wire. J. Am. Coll. Cardiol. 1999, 34, 1193–1200. [Google Scholar] [CrossRef]
- Lethen, H.; H, P.T.; Kersting, S.; Lambertz, H. Validation of noninvasive assessment of coronary flow velocity reserve in the right coronary artery. A comparison of transthoracic echocardiographic results with intracoronary Doppler flow wire measurements. Eur. Heart J. 2003, 24, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Cortigiani, L.; Rigo, F.; Gherardi, S.; Bovenzi, F.; Picano, E.; Sicari, R. Implication of the continuous prognostic spectrum of Doppler echocardiographic derived coronary flow reserve on left anterior descending artery. Am. J. Cardiol. 2010, 105, 158–162. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Schulman-Marcus, J.; Min, J.K. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, R.T.; Jerosch-Herold, M.; Silva, C.; Kitagawa, K.; Bluemke, D.A.; Lima, J.A.; Lardo, A.C. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Investig. Radiol. 2007, 42, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; d’Amati, G.; Rimoldi, O. Coronary microvascular dysfunction: Mechanisms and functional assessment. Nat. Rev. Cardiol. 2015, 12, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, R.; Shturman, L.D.; Rogers, I.S.; Rocha-Filho, J.A.; Okada, D.R.; Sarwar, A.; Soni, A.V.; Bezerra, H.; Ghoshhajra, B.B.; Petranovic, M.; et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J. Am. Coll. Cardiol. 2009, 54, 1072–1084. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Dai, X.; Deng, J.; Lu, Z.; Shen, C.; Zhang, J. Diagnostic performance of perivascular fat attenuation index to predict hemodynamic significance of coronary stenosis: A preliminary coronary computed tomography angiography study. Eur. Radiol. 2020, 30, 673–681. [Google Scholar] [CrossRef]
- Hoshino, M.; Yang, S.; Sugiyama, T.; Zhang, J.; Kanaji, Y.; Yamaguchi, M.; Hada, M.; Sumino, Y.; Horie, T.; Nogami, K.; et al. Peri-coronary inflammation is associated with findings on coronary computed tomography angiography and fractional flow reserve. J. Cardiovasc. Comput. Tomogr. 2020, 14, 483–489. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Dey, D.; Cadet, S.; Lee, S.E.; Otaki, Y.; Huynh, P.T.; Doris, M.K.; Eisenberg, E.; Yun, M.; Jansen, M.A.; et al. Peri-Coronary Adipose Tissue Density Is Associated With (18)F-Sodium Fluoride Coronary Uptake in Stable Patients With High-Risk Plaques. JACC Cardiovasc. Imaging 2019, 12, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, T.; Kanaji, Y.; Hoshino, M.; Yamaguchi, M.; Hada, M.; Ohya, H.; Sumino, Y.; Hirano, H.; Kanno, Y.; Horie, T.; et al. Determinants of Pericoronary Adipose Tissue Attenuation on Computed Tomography Angiography in Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e016202. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Williams, M.C.; Kotanidis, C.P.; Desai, M.Y.; Marwan, M.; Antonopoulos, A.S.; Thomas, K.E.; Thomas, S.; Akoumianakis, I.; Fan, L.M.; et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur. Heart J. 2019, 40, 3529–3543. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Slomka, P.; Berman, D.S.; Alexanderson, E.; Germano, G. The role of PET quantification in cardiovascular imaging. Clin. Transl. Imaging 2014, 2, 343–358. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R.; et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 2018, 39, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Johnson, N.P. Coronary Physiology beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2642–2662. [Google Scholar] [CrossRef]
- Biglands, J.D.; Magee, D.R.; Sourbron, S.P.; Plein, S.; Greenwood, J.P.; Radjenovic, A. Comparison of the Diagnostic Performance of Four Quantitative Myocardial Perfusion Estimation Methods Used in Cardiac MR Imaging: CE-MARC Substudy. Radiology 2015, 275, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrick, D.; Haig, C.; Ahmed, N.; Carberry, J.; Yue May, V.T.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; Hood, S.; et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment-Elevation Myocardial Infarction. Circulation 2016, 134, 1833–1847. [Google Scholar] [CrossRef] [Green Version]
- Thomson, L.E.; Wei, J.; Agarwal, M.; Haft-Baradaran, A.; Shufelt, C.; Mehta, P.K.; Gill, E.B.; Johnson, B.D.; Kenkre, T.; Handberg, E.M.; et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ. Cardiovasc. Imaging 2015, 8, e002481. [Google Scholar] [CrossRef] [Green Version]
- Indorkar, R.; Kwong, R.Y.; Romano, S.; White, B.E.; Chia, R.C.; Trybula, M.; Evans, K.; Shenoy, C.; Farzaneh-Far, A. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovasc. Imaging 2019, 12, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Mygind, N.D.; Frestad, D.; Michelsen, M.; Suhrs, H.E.; Bove, K.B.; Gustafsson, I.; Kastrup, J.; Prescott, E. Pro-inflammatory biomarkers in women with non-obstructive angina pectoris and coronary microvascular dysfunction. Int. J. Cardiol. Heart Vasc. 2019, 24, 100370. [Google Scholar] [CrossRef]
- Aslan, G.; Polat, V.; Bozcali, E.; Opan, S.; Cetin, N.; Ural, D. Evaluation of serum sST2 and sCD40L values in patients with microvascular angina. Microvasc. Res. 2019, 122, 85–93. [Google Scholar] [CrossRef]
- Dollard, J.; Kearney, P.; Clarke, G.; Moloney, G.; Cryan, J.F.; Dinan, T.G. A prospective study of C-reactive protein as a state marker in Cardiac Syndrome X. Brain Behav. Immun. 2015, 43, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Antonopoulos, A.S.; Oikonomou, E.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Platelet Activation: Focus on Atherosclerosis and COVID-19. Int. J. Mol. Sci. 2021, 22, 11170. [Google Scholar] [CrossRef]
- Safdar, B.; Guo, X.; Johnson, C.; D’Onofrio, G.; Dziura, J.; Sinusas, A.J.; Testani, J.; Rao, V.; Desir, G. Elevated renalase levels in patients with acute coronary microvascular dysfunction - A possible biomarker for ischemia. Int. J. Cardiol. 2019, 279, 155–161. [Google Scholar] [CrossRef]
- Suhrs, H.E.; Schroder, J.; Bove, K.B.; Mygind, N.D.; Frestad, D.; Michelsen, M.M.; Lange, T.; Gustafsson, I.; Kastrup, J.; Prescott, E. Inflammation, non-endothelial dependent coronary microvascular function and diastolic function-Are they linked? PLoS ONE 2020, 15, e0236035. [Google Scholar] [CrossRef]
- Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Oikonomou, E.; Tsigkou, V.; Paschou, S.A.; Vlasis, K.; Marinos, G.; Vavuranakis, M.; Stefanadis, C.; et al. MicroRNAs in cardiovascular disease. Hell. J. Cardiol. HJC = Hell. Kardiol. Ep. 2020, 61, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzi, G.; Oikonomou, E.; Deftereos, S.; Siasos, G.; Tousoulis, D. Peripheral artery disease: A micro-RNA-related condition? Curr. Opin. Pharmacol. 2018, 39, 105–112. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Vogiatzi, G.; Antonopoulos, A.S.; Siasos, G.; Iliopoulos, D.C.; Perrea, D.; Tsioufis, C.; Tousoulis, D. The impact of proangiogenic microRNA modulation on blood flow recovery following hind limb ischemia. A systematic review and meta-analysis of animal studies. Vasc. Pharmacol. 2021, 106906. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Vogiatzi, G.; Oikonomou, E.; Gazouli, M.; Siasos, G.; Katifelis, H.; Perrea, D.; Vavuranakis, M.; Iliopoulos, D.C.; Tsioufis, C.; et al. The Effect of MicroRNA-126 Mimic Administration on Vascular Perfusion Recovery in an Animal Model of Hind Limb Ischemia. Front. Mol. Biosci. 2021, 8, 724465. [Google Scholar] [CrossRef]
- Villeneuve, L.M.; Kato, M.; Reddy, M.A.; Wang, M.; Lanting, L.; Natarajan, R. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes 2010, 59, 2904–2915. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; He, S.; Wara, A.K.M.; Icli, B.; Shvartz, E.; Tesmenitsky, Y.; Belkin, N.; Li, D.; Blackwell, T.S.; Sukhova, G.K.; et al. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ. Res. 2014, 114, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.A.; Jin, W.; Villeneuve, L.; Wang, M.; Lanting, L.; Todorov, I.; Kato, M.; Natarajan, R. Pro-inflammatory role of microrna-200 in vascular smooth muscle cells from diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Cowan, C.; Muraleedharan, C.K.; O’Donnell, J.J., 3rd; Singh, P.K.; Lum, H.; Kumar, A.; Xu, S. MicroRNA-146 inhibits thrombin-induced NF-kappaB activation and subsequent inflammatory responses in human retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4944–4951. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.W.; Sheu, W.H.; Lee, W.J.; Lee, W.L.; Fu, C.P.; Wang, J.S. Differential expression of circulating vascular cell adhesion molecule-1 in subjects with coronary artery disease and cardiac syndrome X without known diabetes mellitus. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2017, 22, 798–804. [Google Scholar] [CrossRef]
- Efe, S.C.; Demirci, K.; Ozturk, S.; Gurbuz, A.S.; Poci, N.; Kilicgedik, A.; Guler, A.; Yilmaz, M.F.; Izgi, I.A.; Kirma, C. Serum endocan levels in patients with cardiac syndrome X. Herz 2018, 43, 359–363. [Google Scholar] [CrossRef]
- Prasad, M.; Matteson, E.L.; Herrmann, J.; Gulati, R.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Uric Acid Is Associated With Inflammation, Coronary Microvascular Dysfunction, and Adverse Outcomes in Postmenopausal Women. Hypertension 2017, 69, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Altiparmak, I.H.; Erkus, M.E.; Sezen, H.; Demirbag, R.; Kaya, Z.; Sezen, Y.; Gunebakmaz, O.; Asoglu, R.; Besli, F.; Neselioglu, S.; et al. Evaluation of thiol levels, thiol/disulfide homeostasis and their relation with inflammation in cardiac syndrome X. Coron. Artery Dis. 2016, 27, 295–301. [Google Scholar] [CrossRef]
- Mekonnen, G.; Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Eapen, D.J.; Al-Kassem, H.; Rasoul-Arzrumly, E.; Gogas, B.D.; McDaniel, M.C.; Pielak, T.; et al. Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis 2015, 239, 55–60. [Google Scholar] [CrossRef]
- Bozcali, E.; Polat, V.; Aciksari, G.; Opan, S.; Bayrak, I.H.; Paker, N.; Karakaya, O. Serum concentrations of galectin-3 in patients with cardiac syndrome X. Atherosclerosis 2014, 237, 259–263. [Google Scholar] [CrossRef]
- Tenekecioglu, E.; Yilmaz, M.; Demir, S.; Bekler, A.; Ozluk, O.A.; Aydin, U.; Goncu, T.; Yontar, O.C. HDL-cholesterol is associated with systemic inflammation in cardiac syndrome X. Minerva Med. 2015, 106, 133–141. [Google Scholar]
- Faccini, A.; Kaski, J.C.; Camici, P.G. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur. Heart J. 2016, 37, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: Clinical and therapeutic implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef]
- Puddu, P.; Puddu, G.M.; Cravero, E.; Muscari, S.; Muscari, A. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can. J. Cardiol. 2010, 26, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Duncker, D.J.; Bache, R.J. Regulation of coronary blood flow during exercise. Physiol. Rev. 2008, 88, 1009–1086. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.G.; Davies, G.J.; Kerwin, R.; Hackett, D.; Larkin, S.; Dawbarn, D.; Lee, Y.; Bloom, S.R.; Yacoub, M.; Maseri, A. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet 1987, 1, 1057–1059. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Bairey Merz, C.N. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, L.; Rosen, S.D.; Patel, D.; Nihoyannopoulos, P.; Camici, P.G. Coronary vasodilator reserve in primary and secondary left ventricular hypertrophy. A study with positron emission tomography. Eur. Heart J. 1997, 18, 108–116. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Tsioufis, C.; Oikonomou, E.; Antoniades, C.; Crea, F.; Kaski, J.C.; Tousoulis, D. Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives. Int. J. Mol. Sci. 2021, 22, 6607. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; Lindsay, M.M.; Davie, A.; et al. Myocardial Hemorrhage After Acute Reperfused ST-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ. Cardiovasc. Imaging 2016, 9, e004148. [Google Scholar] [CrossRef] [Green Version]
- Eitel, I.; Kubusch, K.; Strohm, O.; Desch, S.; Mikami, Y.; de Waha, S.; Gutberlet, M.; Schuler, G.; Friedrich, M.G.; Thiele, H. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ. Cardiovasc. Imaging 2011, 4, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Duprez, D.A. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: A clinical review. J. Hypertens. 2006, 24, 983–991. [Google Scholar] [CrossRef]
- Hao, Y.; Tsuruda, T.; Sekita-Hatakeyama, Y.; Kurogi, S.; Kubo, K.; Sakamoto, S.; Nakamura, M.; Udagawa, N.; Sekimoto, T.; Hatakeyama, K.; et al. Cardiac hypertrophy is exacerbated in aged mice lacking the osteoprotegerin gene. Cardiovasc. Res. 2016, 110, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Siedlinski, M.; Nosalski, R.; Szczepaniak, P.; Ludwig-Galezowska, A.H.; Mikolajczyk, T.; Filip, M.; Osmenda, G.; Wilk, G.; Nowak, M.; Wolkow, P.; et al. Vascular transcriptome profiling identifies Sphingosine kinase 1 as a modulator of angiotensin II-induced vascular dysfunction. Sci. Rep. 2017, 7, 44131. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Kim, S.H.; Monticone, R.E.; Lakatta, E.G. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015, 65, 698–703. [Google Scholar] [CrossRef]
- Camici, P.G.; Olivotto, I.; Rimoldi, O.E. The coronary circulation and blood flow in left ventricular hypertrophy. J. Mol. Cell Cardiol. 2012, 52, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Cortigiani, L.; Rigo, F.; Gherardi, S.; Galderisi, M.; Bovenzi, F.; Sicari, R. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: A transthoracic Doppler echocardiographic study. J. Am. Soc. Echocardiogr. 2014, 27, 742–748. [Google Scholar] [CrossRef]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Gaber, M.; Hainer, J.; Klein, J.; Dorbala, S.; Blankstein, R.; Di Carli, M.F. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012, 126, 1858–1868. [Google Scholar] [CrossRef] [Green Version]
- Sagris, M.; Giannopoulos, S.; Giannopoulos, S.; Tzoumas, A.; Texakalidis, P.; Charisis, N.; Kokkinidis, D.G.; Malgor, R.D.; Mouawad, N.J.; Bakoyiannis, C. Transcervical carotid artery revascularization: A systematic review and meta-analysis of outcomes. J. Vasc. Surg. 2021, 74, 657–665.e612. [Google Scholar] [CrossRef]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.M.; Vita, J.A. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, L.A.; Poot, M.; Gerrity, R.G.; Bornfeldt, K.E. Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis: Lack of direct growth-promoting effects of high glucose levels. Diabetes 2001, 50, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid. Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef]
- Stolen, C.M.; Madanat, R.; Marti, L.; Kari, S.; Yegutkin, G.G.; Sariola, H.; Zorzano, A.; Jalkanen, S. Semicarbazide sensitive amine oxidase overexpression has dual consequences: Insulin mimicry and diabetes-like complications. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 702–704. [Google Scholar] [CrossRef]
- Diavati, S.; Sagris, M.; Terentes-Printzios, D.; Vlachopoulos, C. Anticoagulation Treatment in Venous Thromboembolism: Options and Optimal Duration. Curr. Pharm. Des. 2021. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Sorop, O.; Heinonen, I.; van Kranenburg, M.; van de Wouw, J.; de Beer, V.J.; Nguyen, I.T.N.; Octavia, Y.; van Duin, R.W.B.; Stam, K.; van Geuns, R.J.; et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc. Res. 2018, 114, 954–964. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Tanese, L.; Russo, G.; Pitocco, D.; Lanza, G.A.; et al. Impact of Glycemic Variability on Chromatin Remodeling, Oxidative Stress, and Endothelial Dysfunction in Patients With Type 2 Diabetes and With Target HbA1c Levels. Diabetes 2017, 66, 2472–2482. [Google Scholar] [CrossRef] [Green Version]
- Siasos, G.; Skotsimara, G.; Oikonomou, E.; Sagris, M.; Vasiliki-Chara, M.; Bletsa, E.; Stampouloglou, P.; Theofilis, P.; Charalampous, G.; Tousoulis, D. Antithrombotic Treatment in Diabetes Mellitus: A Review of the Literature about Antiplatelet and Anticoagulation Strategies Used for Diabetic Patients in Primary and Secondary Prevention. Curr. Pharm. Des. 2020, 26, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Despres, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Goossens, G.H.; Blaak, E.E. Adipose Tissue Dysfunction and Impaired Metabolic Health in Human Obesity: A Matter of Oxygen? Front. Endocrinol. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Frayn, K.N.; Karpe, F. Regulation of human subcutaneous adipose tissue blood flow. Int. J. Obes. 2014, 38, 1019–1026. [Google Scholar] [CrossRef]
- Lempesis, I.G.; van Meijel, R.L.J.; Manolopoulos, K.N.; Goossens, G.H. Oxygenation of adipose tissue: A human perspective. Acta Physiol. 2019, 228, e13298. [Google Scholar] [CrossRef]
- Bays, H.E. Adiposopathy is “sick fat” a cardiovascular disease? J. Am. Coll. Cardiol. 2011, 57, 2461–2473. [Google Scholar] [CrossRef] [Green Version]
- Bays, H.E. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: Treating “sick fat” through improving fat function with antidiabetes therapies. Am. J. Cardiol. 2012, 110, 4B–12B. [Google Scholar] [CrossRef]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef] [Green Version]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Sagris, M.; Kokkinidis, D.G.; Lempesis, I.G.; Giannopoulos, S.; Rallidis, L.; Mena-Hurtado, C.; Bakoyiannis, C. Nutrition, dietary habits, and weight management to prevent and treat patients with peripheral artery disease. Rev. Cardiovasc. Med. 2020, 21, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Naoumova, R.P.; Kindler, H.; Leccisotti, L.; Mongillo, M.; Khan, M.T.; Neuwirth, C.; Seed, M.; Holvoet, P.; Betteridge, J.; Camici, P.G. Pioglitazone improves myocardial blood flow and glucose utilization in nondiabetic patients with combined hyperlipidemia: A randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 2007, 50, 2051–2058. [Google Scholar] [CrossRef]
- Lang, D.H.; Yeung, C.K.; Peter, R.M.; Ibarra, C.; Gasser, R.; Itagaki, K.; Philpot, R.M.; Rettie, A.E. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: Selective catalysis by FMO3. Biochem. Pharmacol. 1998, 56, 1005–1012. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [Green Version]
- Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [Green Version]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Richey, J.J.; Zigler, M.C.; Cuevas, L.M.; Gonzalez, A.; Vazquez-Baeza, Y.; Battson, M.L.; Smithson, A.T.; Gilley, A.D.; et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 2019, 597, 2361–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Tian, R.; Wang, H.; Feng, S.; Li, H.; Xiao, Y.; Luan, X.; Zhang, Z.; Shi, N.; Niu, H.; et al. Gut microbiota from coronary artery disease patients contributes to vascular dysfunction in mice by regulating bile acid metabolism and immune activation. J. Transl. Med. 2020, 18, 382. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Bocchino, B.; Novellino, E. Lactofermented Annurca Apple Puree as a Functional Food Indicated for the Control of Plasma Lipid and Oxidative Amine Levels: Results from a Randomised Clinical Trial. Nutrients 2019, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Tardif, J.C.; Waters, D.D.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; Berry, C.; Koenig, W.; Lopez-Sendon, J.; Gamra, H.; et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 2020, 41, 4092–4099. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.N.; Kremastinos, D.T. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008, 117, 2662–2669. [Google Scholar] [CrossRef]
- Holte, E.; Kleveland, O.; Ueland, T.; Kunszt, G.; Bratlie, M.; Broch, K.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Aakhus, S.; et al. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infarction. Heart 2017, 103, 1521–1527. [Google Scholar] [CrossRef]
- Portman, M.A.; Olson, A.; Soriano, B.; Dahdah, N.; Williams, R.; Kirkpatrick, E. Etanercept as adjunctive treatment for acute Kawasaki disease: Study design and rationale. Am. Heart J. 2011, 161, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Sakellarios, A.I.; Fotiadis, D.I. Editorial commentary: The pleiotropic effect of statins on the atherosclerotic plaque and coronary heart disease. Trends Cardiovasc. Med. 2019, 29, 456–457. [Google Scholar] [CrossRef]
- Stumpf, C.; Petzi, S.; Seybold, K.; Wasmeier, G.; Arnold, M.; Raaz, D.; Yilmaz, A.; Daniel, W.G.; Garlichs, C.D. Atorvastatin enhances interleukin-10 levels and improves cardiac function in rats after acute myocardial infarction. Clin. Sci. 2009, 116, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Wu, H.; Wang, C.; Shao, H.; Huang, H.; Jing, H.; Li, D. Simvastatin attenuates cardiopulmonary bypass-induced myocardial inflammatory injury in rats by activating peroxisome proliferator-activated receptor gamma. Eur. J. Pharmacol. 2010, 649, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular Safety of Tocilizumab Versus Etanercept in Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Rheumatol. 2020, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.H.; Guo, Y.; Huang, J.W.; Zhang, P.D. Do Statins Have a Positive Impact on Patients with Coronary Microvascular Dysfunction on Long-Term Clinical Outcome? A Large Retrospective Cohort Study. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetzel, S.; DeMets, D.; Schneider, R.; Borzak, S.; Schneider, W.; Serebruany, V.; Schroder, H.; Hennekens, C.H. Aspirin increases nitric oxide formation in chronic stable coronary disease. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 217–221. [Google Scholar] [CrossRef]
- Yang, Y.; Hwang, E.; Lee, S.A.; Lee, S.; Kim, D.H.; Song, J.M.; Kang, D.H. Effect of Rosuvastatin on Coronary Flow Reserve in Hypertensive Patients at Cardiovascular Risk. J. Cardiovasc. Imaging 2021, 29, 255–262. [Google Scholar] [CrossRef]
- Sun, B.J.; Hwang, E.; Jang, J.Y.; Kim, D.H.; Song, J.M.; Kang, D.H. Effect of rosuvastatin on coronary flow reserve in patients with systemic hypertension. Am. J. Cardiol. 2014, 114, 1234–1237. [Google Scholar] [CrossRef]
- Ishida, K.; Geshi, T.; Nakano, A.; Uzui, H.; Mitsuke, Y.; Okazawa, H.; Ueda, T.; Lee, J.D. Beneficial effects of statin treatment on coronary microvascular dysfunction and left ventricular remodeling in patients with acute myocardial infarction. Int. J. Cardiol. 2012, 155, 442–447. [Google Scholar] [CrossRef]
- Yong, J.; Tian, J.; Yang, X.; Xing, H.; He, Y.; Song, X. Effects of Oral Drugs on Coronary Microvascular Function in Patients Without Significant Stenosis of Epicardial Coronary Arteries: A Systematic Review and Meta-Analysis of Coronary Flow Reserve. Front. Cardiovasc. Med. 2020, 7, 580419. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S. Microvascular Coronary Dysfunction-an Overview. Curr. Atheroscler. Rep. 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, M.; Thompson, P.L. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am. J. Cardiol. 2007, 99, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Thompson, P.L. Why Colchicine Should Be Considered for Secondary Prevention of Atherosclerosis: An Overview. Clin. Ther. 2019, 41, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Castaneda, S.; Martinez-Quintanilla, D.; Martin-Varillas, J.L.; Garcia-Castaneda, N.; Atienza-Mateo, B.; Gonzalez-Gay, M.A. Tocilizumab for the treatment of adult-onset Still’s disease. Expert Opin. Biol. Ther. 2019, 19, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, L.A.; Boothby, A.; Gertner, E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020, 2, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Andreadou, I.; Triantafyllidi, H.; Tsoumani, M.; Varoudi, M.; Vlastos, D.; Makavos, G.; Kostelli, G.; et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2019, 108, 1093–1101. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Cacciapaglia, F.; Anelli, M.G.; Rinaldi, A.; Fornaro, M.; Lopalco, G.; Scioscia, C.; Lapadula, G.; Iannone, F. Lipids and Atherogenic Indices Fluctuation in Rheumatoid Arthritis Patients on Long-Term Tocilizumab Treatment. Mediat. Inflamm. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airo, P.; Bazzani, C.; Beindorf, E.A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 102568. [Google Scholar] [CrossRef]
- Van Kraaij, T.D.; Mostard, R.L.; Ramiro, S.; Magro Checa, C.; van Dongen, C.M.; van Haren, E.H.; Buijs, J.; Landewe, R.B. Tocilizumab in Severe COVID-19 Pneumonia and Concomitant Cytokine Release Syndrome. Eur. J. Case Rep. Intern. Med. 2020, 7, 001675. [Google Scholar] [CrossRef] [PubMed]
- Strang, A.C.; Bisoendial, R.J.; Kootte, R.S.; Schulte, D.M.; Dallinga-Thie, G.M.; Levels, J.H.; Kok, M.; Vos, K.; Tas, S.W.; Tietge, U.J.; et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 2013, 229, 174–181. [Google Scholar] [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tollefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damas, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients With Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Gniadecki, R.; Iversen, L.; Bryld, L.E.; Lasthein, S.; Lindhardsen, J.; Kristensen, S.L.; Torp-Pedersen, C.; et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, L.T.; Turesson, C.; Gulfe, A.; Kapetanovic, M.C.; Petersson, I.F.; Saxne, T.; Geborek, P. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1213–1218. [Google Scholar] [PubMed]
- Bergstrom, U.; Jovinge, S.; Persson, J.; Jacobsson, L.T.H.; Turesson, C. Effects of Treatment with Adalimumab on Blood Lipid Levels and Atherosclerosis in Patients with Rheumatoid Arthritis. Curr. Ther. Res. Clin. Exp. 2018, 89, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Yan, B.X.; Man, X.Y. TNFalpha inhibitor may be effective for severe COVID-19: Learning from toxic epidermal necrolysis. Ther. Adv. Respir. Dis. 2020, 14. [Google Scholar] [CrossRef]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Kroetsch, J.T.; Levy, A.S.; Zhang, H.; Aschar-Sobbi, R.; Lidington, D.; Offermanns, S.; Nedospasov, S.A.; Backx, P.H.; Heximer, S.P.; Bolz, S.S. Constitutive smooth muscle tumour necrosis factor regulates microvascular myogenic responsiveness and systemic blood pressure. Nat. Commun. 2017, 8, 14805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonomidis, I.; Papadavid, E.; Makavos, G.; Andreadou, I.; Varoudi, M.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Moutsatsou, P.; et al. Lowering Interleukin-12 Activity Improves Myocardial and Vascular Function Compared With Tumor Necrosis Factor-a Antagonism or Cyclosporine in Psoriasis. Circulation. Cardiovasc. Imaging 2017, 10, e006283. [Google Scholar] [CrossRef] [Green Version]
- Piaserico, S.; Osto, E.; Famoso, G.; Zanetti, I.; Gregori, D.; Poretto, A.; Iliceto, S.; Peserico, A.; Tona, F. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis 2016, 251, 25–30. [Google Scholar] [CrossRef]
- Dominguez, H.; Storgaard, H.; Rask-Madsen, C.; Steffen Hermann, T.; Ihlemann, N.; Baunbjerg Nielsen, D.; Spohr, C.; Kober, L.; Vaag, A.; Torp-Pedersen, C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 2005, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]

| Study | Biomarker | Outcome |
|---|---|---|
| Aslan et al. [33] | sCD40-L | sCD40-L was related to MVD in regression analysis |
| Dollard et al. [34] | hsCRP | hsCRP levels are proportional to disease severity |
| Safdar et al. [36] | Renalase | Independent predictor of coronary MVD even after adjustment for risk factors |
| Liang et al. [46] | VCAM-1 | VCAM-1 is a significant factor differentiating obstructive CAD with CSX |
| Efe et al. [47] | Endocan | Endocan levels ≥2072 ng/L had a 72% sensitivity and 54% specificity for accurate prediction of CSX |
| Prasad et al. [48] | Uric acid | Uric acid was associated with markers of inflammation and coronary endothelial dysfunction in postmenopausal women |
| Altiparmak et al. [49] | Thiol | Specificity 84% and sensitivity 86% of CSX prediction with total thiol values ≤338.4 µmol/L |
| Schroder et al. [32] | Component of: CCL16 CXCL16 PGLYRP1 TNFR1 GDF15 TNFRSF10C | The 9-biomarker component was associated with MVD but did not provide further diagnostic utility |
| Mekonnen et al. [50] | suPAR | Association of suPAR with CFR |
| Bozcali et al. [51] | Galectin 3 | ↑ Galectin-3 in patients with CSX even after adjustment for risk factors |
| Tenekecioglou et al. [52] | HDL-C | ↓ HDL-C is associated with systemic inflammation in CSX |
| DRUG | ACTION | TRIAL |
|---|---|---|
| Aspirin | Inhibitor of COX | Coronary Microvascular Angina Trial (CorMicA) [102] |
| Colchicine | Inhibition of NLRP3 inflammasome | COLCOT Trial [103] |
| Anakinra | Monoclonal antibody against IL-1 Receptor | Ikonomidis et al. [104] |
| Canakinumab | monoclonal antibody against IL-1-beta | CANTOS Trial [5] |
| Tocilizumab | IL-6 Receptor Inhibitor | Holte et al. [105] |
| Etanercept | TNF-α antagonists | ENTRACTE Trial [106] |
| Adalimumab | TNF-α antagonists | ENTRACTE Trial [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Paschaliori, C.; Galiatsatos, N.; Tsioufis, K.; Tousoulis, D. Inflammation in Coronary Microvascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 13471. https://doi.org/10.3390/ijms222413471
Sagris M, Theofilis P, Antonopoulos AS, Oikonomou E, Paschaliori C, Galiatsatos N, Tsioufis K, Tousoulis D. Inflammation in Coronary Microvascular Dysfunction. International Journal of Molecular Sciences. 2021; 22(24):13471. https://doi.org/10.3390/ijms222413471
Chicago/Turabian StyleSagris, Marios, Panagiotis Theofilis, Alexios S. Antonopoulos, Evangelos Oikonomou, Christina Paschaliori, Nikolaos Galiatsatos, Kostas Tsioufis, and Dimitris Tousoulis. 2021. "Inflammation in Coronary Microvascular Dysfunction" International Journal of Molecular Sciences 22, no. 24: 13471. https://doi.org/10.3390/ijms222413471
APA StyleSagris, M., Theofilis, P., Antonopoulos, A. S., Oikonomou, E., Paschaliori, C., Galiatsatos, N., Tsioufis, K., & Tousoulis, D. (2021). Inflammation in Coronary Microvascular Dysfunction. International Journal of Molecular Sciences, 22(24), 13471. https://doi.org/10.3390/ijms222413471









