The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin
Abstract
1. Introduction
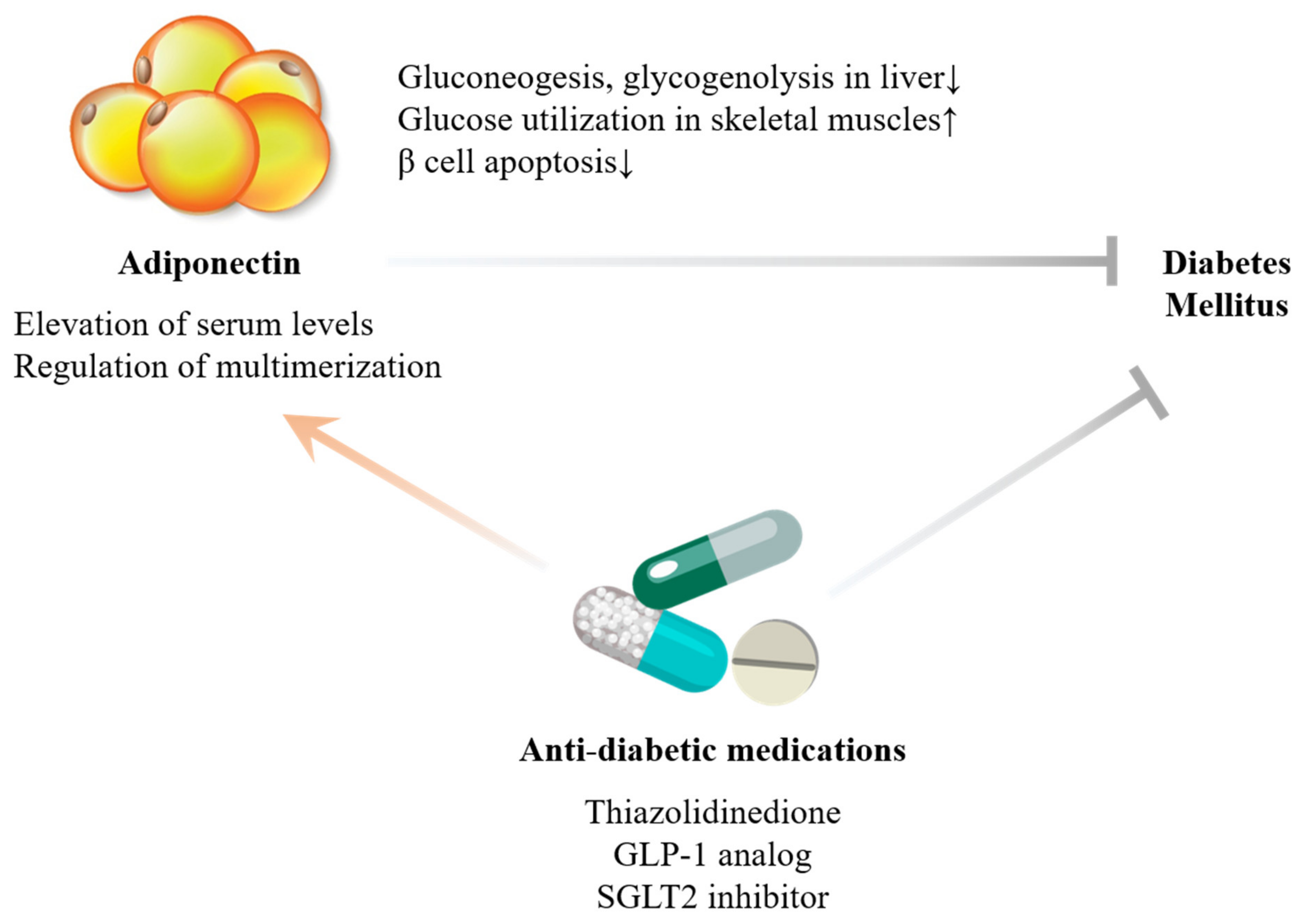
2. Adiponectin
2.1. Biological Actions
2.2. Human Relevance
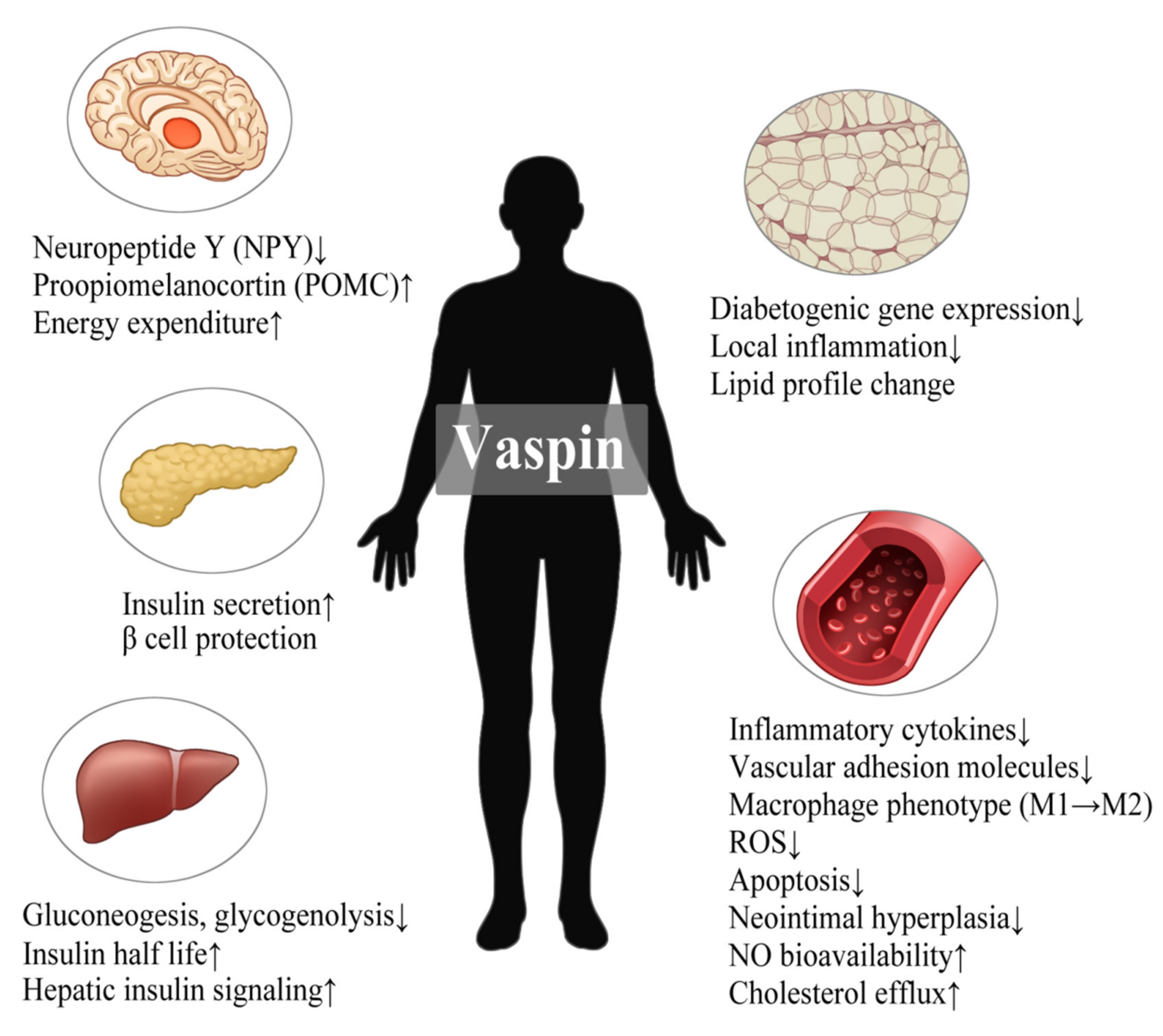
3. Vaspin
3.1. Biological Actions
3.2. Human Relevance
4. C1q/TNF-Related Protein (CTRP) Family
4.1. CTRP9
4.1.1. Biological Actions
4.1.2. Human Relevance
4.2. Other Members of the CTRP Family with Anti-Inflammatory Effects
4.2.1. Biological Actions
4.2.2. Human Relevance
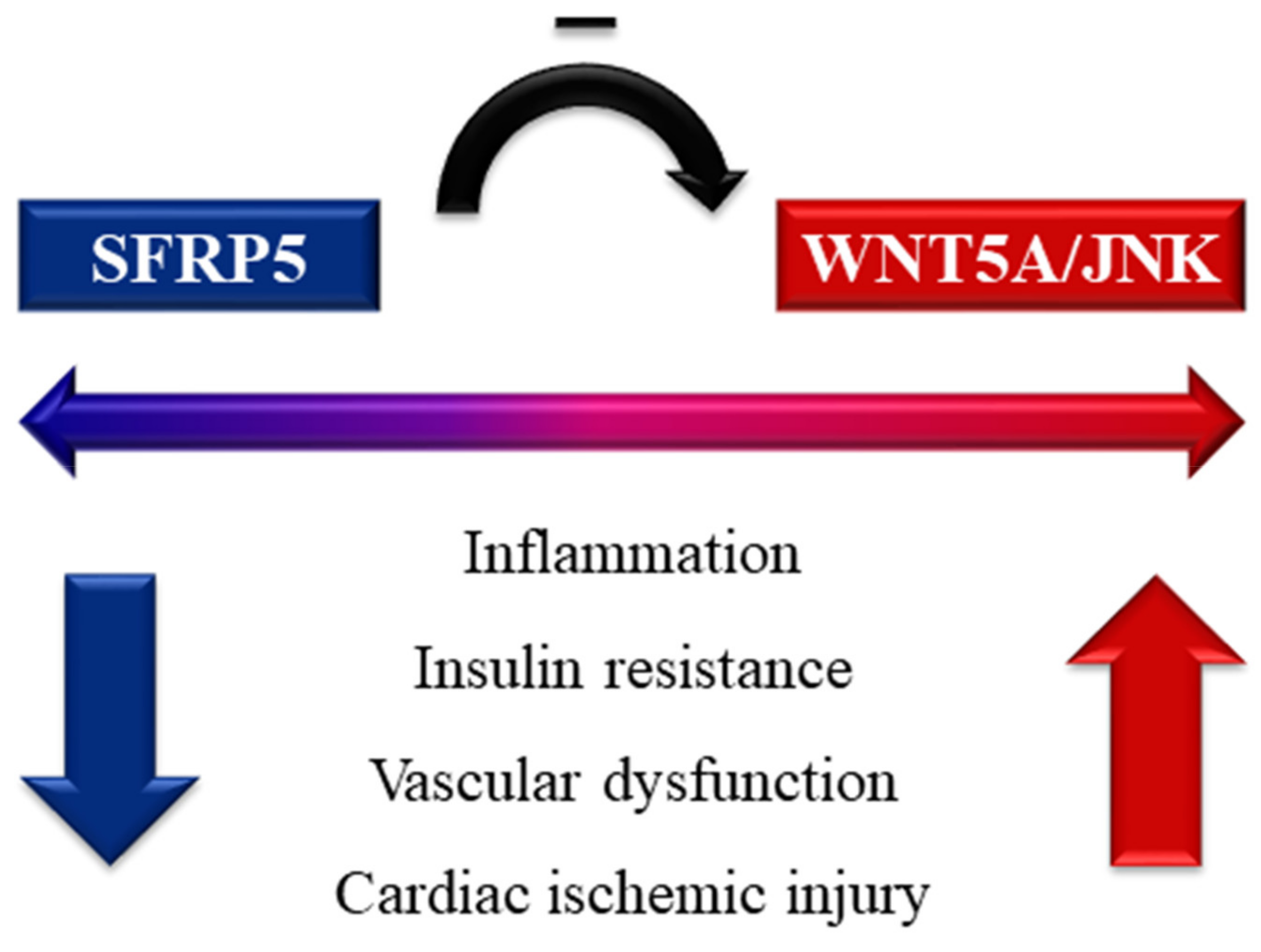
5. Secreted Frizzled-Related Protein 5 (SFRP5)
5.1. Biological Actions
5.2. Human Relevance
6. Omentin-1
6.1. Biological Actions
6.2. Human Relevance
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Blüher, M. Adipokines—Removing road blocks to obesity and diabetes therapy. Mol. Metab. 2014, 3, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Tam, C.S.; Ban, L.A.; Ehrenfeld-Slater, P.; Mclennan, S.V.; Twigg, S.M. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism 2020, 102, 154008. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Salloum, F.N.; Fisher, B.J.; Kukreja, R.C.; Fowler, A.A. Hypoxia Inducible Factor-1 Upregulates Adiponectin in Diabetic Mouse Hearts and Attenuates Post-Ischemic Injury. J. Cardiovasc. Pharmacol. 2008, 51, 178–187. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2011, 8, 1031–1063. [Google Scholar]
- Xuan, D.; Han, Q.; Tu, Q.; Zhang, L.; Yu, L.; Murry, D.; Tu, T.; Tang, Y.; Lian, J.B.; Stein, G.S.; et al. Epigenetic Modulation in Periodontitis: Interaction of Adiponectin and JMJD3-IRF4 Axis in Macrophages. J. Cell. Physiol. 2015, 231, 1090–1096. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Bourlier, V.; Bouloumie, A. Role of macrophage tissue infiltration in obesity and insulin resistance. Diabetes Metab. 2009, 35, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin Reduces Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation 2002, 106, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Combs, T.P.; Berg, A.H.; Obici, S.; Scherer, P.E.; Rossetti, L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Investig. 2001, 108, 1875–1881. [Google Scholar] [CrossRef]
- Combs, T.P.; Marliss, E.B. Adiponectin signaling in the liver. Rev. Endocr. Metab. Disord. 2014, 15, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Pendzialek, M.; Grybel, K.J.; Seeling, T.; Gürke, J.; Fischer, B.; Santos, A.N. Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts. Hum. Reprod. 2017, 32, 1382–1392. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Rakatzi, I.; Mueller, H.; Ritzeler, O.; Tennagels, N.; Eckel, J. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 2004, 47, 249–258. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Deng, G.; Long, Y.; Yu, Y.R.; Li, M.R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int. J. Obes. 2010, 34, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, X.; Li, L.; Huang, X.Z.; Liu, Y.S.; Chen, L.; Zhang, K.; Wang, L.; Li, X.; Song, J.; et al. Adiponectin reduces carotid atherosclerotic plaque formation in ApoE-/- mice: Roles of oxidative and nitrosative stress and inducible nitric oxide synthase. Mol. Med. Rep. 2015, 11, 1715–1721. [Google Scholar] [CrossRef]
- Nour-Eldine, W.; Ghantous, C.M.; Zibara, K.; Dib, L.; Issaa, H.; Itani, H.A.; El-Zein, N.; Zeidan, A. Adiponectin Attenuates Angiotensin II-Induced Vascular Smooth Muscle Cell Remodeling through Nitric Oxide and the RhoA/ROCK Pathway. Front. Pharmacol. 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Bodles, A.M.; Banga, A.; Rasouli, N.; Ono, F.; Kern, P.A.; Owens, R.J. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am. J. Physiol. Metab. 2006, 291, E1100–E1105. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.T.K.; Hosaka, T.; Yoshida, M.; Harada, N.; Sakaue, H.; Sakai, T.; Nakaya, Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun 2009, 390, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors: Part 2. Antidiabetic effects in type 2 diabetic mice. J. Pharmacol. Sci. 2016, 131, 198–208. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Bethel, M.A.; Patel, R.A.; Merrill, P.; Lokhnygina, Y.; Buse, J.B.; Mentz, R.J.; Pagidipati, N.J.; Chan, J.C.; Gustavson, S.M.; Iqbal, N.; et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol. 2017, 6, 105–113. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. Fibroblast growth factor 21: From pharmacology to physiology. Am. J. Clin. Nutr. 2010, 91, S254–S257. [Google Scholar] [CrossRef]
- Holland, W.L.; Adams, A.C.; Brozinick, J.T.; Bui, H.H.; Miyauchi, Y.; Kusminski, C.M.; Bauer, S.M.; Wade, M.; Singhal, E.; Cheng, C.C.; et al. An FGF21-Adiponectin-Ceramide Axis Controls Energy Expenditure and Insulin Action in Mice. Cell Metab. 2013, 17, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tian, H.; Lam, K.S.; Lin, S.; Hoo, R.C.; Konishi, M.; Itoh, N.; Wang, Y.; Bornstein, S.R.; Xu, A.; et al. Adiponectin Mediates the Metabolic Effects of FGF21 on Glucose Homeostasis and Insulin Sensitivity in Mice. Cell Metab. 2013, 17, 779–789. [Google Scholar] [CrossRef]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Total adiponectin in overweight and obese subjects and its response to visceral fat loss. BMC Endocr. Disord. 2019, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef]
- Lindberg, S.; Jensen, J.S.; Pedersen, S.H.; Galatius, S.; Frystyk, J.; Flyvbjerg, A.; Bjerre, M.; Mogelvang, R. Low Adiponectin Levels and Increased Risk of Type 2 Diabetes in Patients with Myocardial Infarction. Diabetes Care 2014, 37, 3003–3008. [Google Scholar] [CrossRef]
- Kou, H.; Deng, J.; Gao, D.; Song, A.; Han, Z.; Wei, J.; Jin, X.; Ma, R.; Zheng, Q. Relationship among adiponectin, insulin resistance and atherosclerosis in non-diabetic hypertensive patients and healthy adults. Clin. Exp. Hypertens. 2018, 40, 656–663. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, C.; Ding, E.L.; Townsend, M.K.; Lipsitz, L.A. Adiponectin levels and the risk of hypertension: A systematic review and meta-analysis. Hypertension 2013, 62, 27–32. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirowatari, Y.; Kurosawa, H.; Tada, N. Implications of decreased serum adiponectin for type IIb hyperlipidaemia and increased cholesterol levels of very-low-density lipoprotein in type II diabetic patients. Clin. Sci. 2005, 109, 297–302. [Google Scholar] [CrossRef]
- Tomono, Y.; Hiraishi, C.; Yoshida, H. Age and sex differences in serum adiponectin and its association with lipoprotein fractions. Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 55, 165–171. [Google Scholar] [CrossRef]
- Marsche, G.; Zelzer, S.; Meinitzer, A.; Kern, S.; Meissl, S.; Pregartner, G.; Weghuber, D.; Almer, G.; Mangge, H. Adiponectin Predicts High-Density Lipoprotein Cholesterol Efflux Capacity in Adults Irrespective of Body Mass Index and Fat Distribution. J. Clin. Endocrinol. Metab. 2017, 102, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Doumatey, A.P.; Bentley, A.R.; Zhou, J.; Huang, H.; Adeyemo, A.; Rotimi, C.N. Paradoxical Hyperadiponectinemia is Associated with the Metabolically Healthy Obese (MHO) Phenotype in African Americans. J. Endocrinol. Metab. 2012, 2, 51–65. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ouchi, N.; Kihara, S.; Walsh, K.; Kumada, M.; Abe, Y.; Funahashi, T.; Matsuzawa, Y. Selective Suppression of Endothelial Cell Apoptosis by the High Molecular Weight Form of Adiponectin. Circ. Res. 2004, 94, e27–e31. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Abargouei, A.; Izadi, V.; Azadbakht, L. The Effect of Low Calorie Diet on Adiponectin Concentration: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2015, 47, 549–555. [Google Scholar] [CrossRef]
- De Vincentis, A.; Pedone, C.; Gentilucci, U.V.; Picardi, A.; Derosa, G.; Maffioli, P.; Sahebkar, A. Effect of Sibutramine on Plasma C-Reactive Protein, Leptin and Adipon ectin Concentrations: A Systematic Review and Meta-Analysis of Randomized Contr olled Trials. Curr. Pharm. Des. 2017, 23, 870–878. [Google Scholar] [CrossRef]
- Garvey, W.T.; Ryan, D.H.; Henry, R.; Bohannon, N.J.; Toplak, H.; Schwiers, M.; Troupin, B.; Day, W.W. Prevention of Type 2 Diabetes in Subjects with Prediabetes and Metabolic Syndrome Treated with Phentermine and Topiramate Extended Release. Diabetes Care 2013, 37, 912–921. [Google Scholar] [CrossRef]
- Khosravi-Largani, M.; Nojomi, M.; Aghili, R.; Otaghvar, H.A.; Tanha, K.; Seyedi, S.H.S.; Mottaghi, A. Evaluation of all Types of Metabolic Bariatric Surgery and its Consequences: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 651–690. [Google Scholar] [CrossRef]
- Woodward, L.; Akoumianakis, I.; Antoniades, C. Unravelling the adiponectin paradox: Novel roles of adiponectin in the reg-ulation of cardiovascular disease. Br. J. Pharmacol. 2017, 174, 4007–4020. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Whincup, P.; Lennon, L.; Sattar, N. Circulating Adiponectin Levels and Mortality in Elderly Men with and Without Cardiovascular Disease and Heart Failure. Arch. Intern. Med. 2007, 167, 1510–1517. [Google Scholar] [CrossRef]
- McEntegart, M.B.; Awede, B.; Petrie, M.C.; Sattar, N.; Dunn, F.G.; Macfarlane, N.G.; McMurray, J.J. Increase in serum adiponectin concentration in patients with heart failure and cachexia: Relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur. Heart J. 2007, 28, 829–835. [Google Scholar] [CrossRef]
- Tindall, C.A.; Dommel, S.; Riedl, V.; Ulbricht, D.; Hanke, S.; Sträter, N.; Heiker, J.T. Membrane Phospholipids and Polyphosphates as Cofactors and Binding Molecules of SERPINA12 (vaspin). Molecules 2020, 25, 1992. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Zieger, K.; Pippel, J.; Heiker, J.T. Molecular Mechanisms of Vaspin Action—From Adipose Tissue to Skin and Bone, from Blood Vessels to the Brain. Adv. Exp. Med. Biol. 2019, 1111, 159–188. [Google Scholar] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Jurek, M.; Klimczyk, D.; Dupont, J.; Rak, A. Review: Vaspin (SERPINA12) Expression and Function in Endocrine Cells. Cells 2021, 10, 1710. [Google Scholar] [CrossRef]
- Heiker, J.T.; Klöting, N.; Kovacs, P.; Kuettner, E.B.; Sträter, N.; Schultz, S.; Kern, M.; Stumvoll, M.; Blüher, M.; Beck-Sickinger, A.G. Vaspin inhibits kallikrein 7 by serpin mechanism. Experientia 2013, 70, 2569–2583. [Google Scholar] [CrossRef]
- Ulbricht, D.; Tindall, C.A.; Oertwig, K.; Hanke, S.; Sträter, N.; Heiker, J.T. Kallikrein-related peptidase 14 is the second KLK protease targeted by the serpin vaspin. Biol. Chem. 2018, 399, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Shirai, R.; Yamaguchi, M.; Yamashita, T.; Shibata, K.; Okano, T.; Mori, Y.; Matsuyama, T.A.; Ishibashi-Ueda, H.; Hirano, T.; et al. Anti-Atherogenic Effects of Vaspin on Human Aortic Smooth Muscle Cell/Macrophage Responses and Hyper-lipidemic Mouse Plaque Phenotype. Int. J. Mol. Sci. 2018, 19, 1732. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, K.; Zhang, C.; Yang, G.; Yang, M.; Jia, Y.; Zhang, L.; Ma, Z.A.; Boden, G.; Li, L. Central administration of vaspin inhibits glucose production and augments hepatic insulin signaling in high-fat-diet-fed rat. Int. J. Obes. 2016, 40, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dong, Y.; Wang, T.; Zhao, S.; Yang, K.; Chen, X.; Zheng, C. Vaspin inhibited proinflammatory cytokine induced activation of nuclear factor-kappa B and its downstream mol-ecules in human endothelial EA.hy926 cells. Diabetes Res. Clin. Pract. 2014, 103, 482–488. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; La Lee, Y.; Yoon, H.K.; Kang, S.-W.; Lee, W.J.; Park, J.-Y. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc. Diabetol. 2014, 13, 41. [Google Scholar] [CrossRef]
- Zieger, K.; Weiner, J.; Krause, K.; Schwarz, M.; Kohn, M.; Stumvoll, M.; Blüher, M.; Heiker, J.T. Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFκB pathway. Mol. Cell. Endocrinol. 2018, 460, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kameshima, S.; Kakuda, C.; Okamura, Y.; Kodama, T.; Okada, M.; Yamawaki, H. Visceral adipose tissue-derived serine protease inhibitor prevents the development of monocrotaline-induced pulmonary arterial hypertension in rats. Pflugers Arch. 2017, 469, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wang, D.; Zhang, C.; Tang, X.; He, J.; Zhao, Y.; Deng, W.; Deng, X. Vaspin protects against LPS-induced ARDS by inhibiting inflammation, apoptosis and reactive oxygen species gen-eration in pulmonary endothelial cells via the Akt/GSK-3β pathway. Int. J. Mol. Med. 2017, 40, 1803–1817. [Google Scholar]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Seol, S.M.; Kim, Y.M.; La Lee, Y.; Park, J.-Y. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem. Biophys. Res. Commun. 2011, 413, 264–269. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Chen, Y.; Chen, B.; Huang, H.; Lv, J.; Hu, S.; Shen, J. Vaspin protects mouse mesenchymal stem cells from oxidative stress-induced apoptosis through the MAPK/p38 pathway. Mol. Cell. Biochem. 2019, 462, 107–114. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Visceral adipose tissue-derived serine proteinase inhibitor inhibits apoptosis of endothelial cells as a ligand for the cell-surface GRP78/voltage-dependent anion channel complex. Circ. Res. 2013, 112, 771–780. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Lee, M.J.; Seol, S.M.; Kim, Y.M.; La Lee, Y.; Kim, H.S.; Kim, M.-S.; Park, J.-Y. Vaspin Increases Nitric Oxide Bioavailability through the Reduction of Asymmetric Dimethylarginine in Vascular Endothelial Cells. PLoS ONE 2012, 7, e52346. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Blüher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Di Nisio, C.; Recinella, L.; Chiavaroli, A.; Leone, S.; Ferrante, C.; Orlando, G.; Vacca, M. Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides 2011, 32, 1866–1871. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Zeng, M.Y.; Yu, X.H.; Zeng, G.F.; He, L.H.; Zheng, X.L.; Zhang, D.W.; Ouyang, X.P.; Tang, C.K. Visceral adipose tissue-derived serine protease inhibitor accelerates cholesterol efflux by up-regulating ABCA1 expression via the NF-κB/miR-33a pathway in THP-1 macropahge-derived foam cells. Biochem. Biophys. Res. Commun. 2018, 500, 318–324. [Google Scholar] [CrossRef]
- Taheri, E.; Hosseini, S.; Qorbani, M.; Mirmiran, P. Association of adipocytokines with lipid and glycemic profiles in women with normal weight obesity. BMC Endocr. Disord. 2020, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Zhang, H.; Zhu, J.; Kuang, H.; Yu, Q.; Bai, M.; Mu, J. Association between vaspin level and coronary artery disease in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 113, 26–32. [Google Scholar] [CrossRef]
- Yang, H.; Huang, Y.; Gai, C.; Chai, G.; Lee, S. Serum vaspin levels are positively associated with diabetic retinopathy in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2020, 12, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.Y.; Han, T.; Gao, L.; Wang, L.; Wang, S.C.; Yang, L.; Zhang, J.; Guan, Y.Y.; Yan, N.N.; Yu, H.Y.; et al. Effect of aerobic exercise and resistance exercise in improving non-alcoholic fatty liver disease: A randomized controlled trial. Zhonghua Gan Zang Bing Za Zhi 2018, 26, 34–41. [Google Scholar]
- Tan, B.K.; Heutling, D.; Chen, J.; Farhatullah, S.; Adya, R.; Keay, S.D.; Kennedy, C.R.; Lehnert, H.; Randeva, H.S. Metformin Decreases the Adipokine Vaspin in Overweight Women with Polycystic Ovary Syndrome Concomitant with Improvement in Insulin Sensitivity and a Decrease in Insulin Resistance. Diabetes 2008, 57, 1501–1507. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Yang, M.; Liu, H.; Yang, G. Elevated circulating vaspin levels were decreased by rosiglitazone therapy in T2DM patients with poor glycemic control on metformin alone. Cytokine 2011, 56, 399–402. [Google Scholar] [CrossRef]
- Golpaie, A.; Tajik, N.; Masoudkabir, F.; Karbaschian, Z.; Talebpour, M.; Hosseini, M.; Hosseinzadeh-Attar, M.J. Short-term effect of weight loss through restrictive bariatric surgery on serum levels of vaspin in morbidly obese subjects. Eur. Cytokine Netw. 2011, 22, 181–186. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Zhu, Y.; Wang, Y.; Gao, C.; Li, X.; Ji, T.; Bai, S. Association of vaspin rs2236242 gene variants with type 2 diabetes and obesity in a Chinese population: A prospective, single-center study. J. Cell. Physiol. 2019, 234, 16097–16101. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Kassimis, G.; Patsourakos, N.; Kanonidis, I.; Valsami, G. Omentin-1 and vaspin serum levels in patients with pre-clinical carotid atherosclerosis and the effect of statin therapy on them. Cytokine 2021, 138, 155364. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Buhadilly, A.K. Rosuvastatin Improves Vaspin Serum Levels in Obese Patients with Acute Coronary Syndrome. Diseases 2018, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Kastl, S.P.; Katsaros, K.M.; Krychtiuk, K.A.; Jägersberger, G.; Kaun, C.; Huber, K.; Wojta, J.; Speidl, W.S. The adipokine vaspin is associated with decreased coronary in-stent restenosis in vivo and inhibits migration of human coronary smooth muscle cells in vitro. PLoS ONE 2020, 15, e0232483. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, W.; Wang, K.; Li, H.; Xu, Y. Vaspin as a Prognostic Marker in Patients with Acute Myocardial Infarction. Heart Lung Circ. 2016, 25, 257–264. [Google Scholar] [CrossRef]
- Ji, S.; Kou, W.; Luan, P.; Jian, W.; Zhuang, J.; Xu, X.; Zhao, Y.; Li, H.; Peng, W. Plasma vaspin is an effective biomarker for evaluation of future cardiovascular events in patients with chest pain: A 5-year retrospective observational study. Ann. Transl. Med. 2020, 8, 479. [Google Scholar] [CrossRef]
- Rashad, N.M.; Ahmed, H.S.; Ashour, W.M.R.; Yousef, M.S. Association of vaspin gene expression and its serum level on the risk of ischemic stroke in type 2 diabetic Egyptian patients: Prospective case-control study. Biotechnol. Appl. Biochem. 2020, 67, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Cakal, E.; Ustun, Y.; Engin-Ustun, Y.; Ozkaya, M.; Kilinç, M. Serum vaspin and C-reactive protein levels in women with polycystic ovaries and polycystic ovary syndrome. Gynecol. Endocrinol. 2011, 27, 491–495. [Google Scholar] [CrossRef]
- Esteghamati, A.; Noshad, S.; Mousavizadeh, M.; Zandieh, A.; Nakhjavani, M. Association of vaspin with metabolic syndrome: The pivotal role of insulin resistance. Diabetes Metab. J. 2014, 38, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Buyukinan, M.; Atar, M.; Can, U.; Pirgon, O.; Guzelant, A.; Deniz, I. The Association Between Serum Vaspin and Omentin-1 Levels in Obese Children with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 76–81. [Google Scholar] [CrossRef]
- Si, Y.; Fan, W.; Sun, L. A Review of the Relationship Between CTRP Family and Coronary Artery Disease. Curr. Atheroscler. Rep. 2020, 22, 1–7. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, Z.; Gao, J.; Liu, C.; Wang, J.; Guo, R.; Zhao, J.; Lopez, B.; Christopher, T.; Lee, D.; et al. C1q Complement/Tumor Necrosis Factor-Associated Proteins in Cardiovascular Disease and COVID-19. Proteomes 2021, 9, 12. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Ge, G.; Spooner, E.; Hug, C.; Gimeno, R.; Lodish, H.F. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009, 23, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lau, W.B.; Su, H.; Sun, Y.; Yi, W.; Du, Y.; Christopher, T.; Lopez, B.; Wang, Y.; Ma, X.L. C1q-TNF-related protein-9, a novel cardioprotetcive cardiokine, requires proteolytic cleavage to generate a bio-logically active globular domain isoform. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E891–E898. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yuan, Y.; Wang, X.M.; Lau, W.B.; Wang, Y.; Wang, X.; Gao, E.; Koch, W.J.; Ma, X.L. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFα-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res. Cardiol. 2013, 108, 315. [Google Scholar] [CrossRef]
- Yu, X.-H.; Zhang, D.; Zheng, X.-L.; Tang, C.-K. C1q tumor necrosis factor-related protein 9 in atherosclerosis: Mechanistic insights and therapeutic potential. Atherosclerosis 2018, 276, 109–116. [Google Scholar] [CrossRef]
- Yan, W.; Guo, Y.; Tao, L.; Lau, W.B.; Gan, L.; Yan, Z.; Guo, R.; Gao, E.; Wong, G.W.; Koch, W.L.; et al. C1q/Tumor Necrosis Factor-Related Protein-9 Regulates the Fate of Implanted Mesenchymal Stem Cells and Mobilizes Their Protective Effects Against Ischemic Heart Injury via Multiple Novel Signaling Pathways. Circulation 2017, 136, 2162–2177. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Lin, J.; Zhang, R.; Chen, S.; Liu, W.; Sun, M.; Du, W.; Hou, J.; Yu, B. C1q/TNF-related protein 9 inhibits the cholesterol-induced Vascular smooth muscle cell phenotype switch and cell dysfunction by activating AMP-dependent kinase. J. Cell. Mol. Med. 2017, 21, 2823–2836. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; La Lee, Y.; Seol, S.M.; Yoon, H.K.; Kang, S.-W.; Lee, W.J.; Park, J.-Y. C1q/TNF-related protein-9 inhibits cytokine-induced vascular inflammation and leukocyte adhesiveness via AMP-activated protein kinase activation in endothelial cells. Mol. Cell. Endocrinol. 2016, 419, 235–243. [Google Scholar] [CrossRef]
- Liu, M.; Yin, L.; Li, W.; Hu, J.; Wang, H.; Ye, B.; Tang, Y.; Huang, C. C1q/TNF-related protein-9 promotes macrophage polarization and improves cardiac dysfunction after myocardial infarction. J. Cell. Physiol. 2019, 234, 18731–18747. [Google Scholar] [CrossRef]
- Wei, Z.; Lei, X.; Petersen, P.S.; Aja, S.; Wong, G.W. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E779–E790. [Google Scholar] [CrossRef]
- Yang, G.; Qin, C.; Wang, B.; Jia, J.; Yuan, X.; Sun, C.; Li, W. Molecular identification and functional analysis of Ctrp9 in Epinephelus coioides. J. Mol. Endocrinol. 2017, 58, 179–191. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Zhang, H.; Wang, X.-D.; Chen, S.-Y.; Yang, Y.; Lv, H.; Hou, J.-B.; Yu, B. C1q/TNF-Related Protein 9 Inhibits THP-1 Macrophage Foam Cell Formation by Enhancing Autophagy. J. Cardiovasc. Pharmacol. 2018, 72, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Chen, J.; Song, C.; Li, J.; Zuo, A.; Xu, D.; Li, T.; Guo, Y. CTRP9 alleviates foam cells apoptosis by enhancing cholesterol efflux. Mol. Cell. Endocrinol. 2021, 522, 111138. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Shibata, R.; Ohashi, K.; Enomoto, T.; Kambara, T.; Yamamoto, T.; Ogura, Y.; Yuasa, D.; Joki, Y.; Matsuo, K.; et al. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013, 27, 25–33. [Google Scholar] [CrossRef]
- Li, Y.X.; Run, L.; Shi, T.; Zhang, Y.J. CTRP9 regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation, apoptosis and migration via TGF-β1/ERK1/2 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 490, 1319–1325. [Google Scholar] [CrossRef]
- Wang, W.; Lau, W.B.; Wang, Y.; Ma, X.; Li, R. Reduction of CTRP9, a novel anti-platelet adipokine, contributes to abnormal platelet activity in diabetic animals. Cardiovasc. Diabetol. 2016, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yuan, Y.; Yi, W.; Lau, W.B.; Wang, Y.; Wang, X.; Sun, Y.; Lopez, B.L.; Christopher, T.A.; Peterson, J.M.; et al. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Shibata, R.; Ohashi, K.; Enomoto, T.; Ogawa, H.; Otaka, N.; Hiramatsu-Ito, M.; Masutomi, T.; Kawanishi, H.; Murohara, T.; et al. C1q/TNF-Related Protein 9 Promotes Revascularization in Response to Ischemia via an eNOS-Dependent Manner. Front. Pharmacol. 2020, 11, 1313. [Google Scholar] [CrossRef]
- Li, Y.; Geng, X.; Wang, H.; Cheng, G.; Xu, S. CTRP9 Ameliorates Pulmonary Arterial Hypertension Through Attenuating Inflammation and Improving Endothelial Cell Survival and Function. J. Cardiovasc. Pharmacol. 2016, 67, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, B.; Chen, X.; Su, J.; Wang, H.; Yu, S.; Zheng, Q. CTRP9 induces mitochondrial biogenesis and protects high glucose-induced endothelial oxidative damage via AdipoR1 -SIRT1- PGC-1α activation. Biochem. Biophys. Res. Commun. 2016, 477, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Zhou, Y.; Cai, W.; Qiu, L. C1q/TNF-Related Protein-9 Ameliorates Ox-LDL-Induced Endothelial Dysfunction via PGC-1α/AMPK-Mediated Antioxidant Enzyme Induction. Int. J. Mol. Sci. 2017, 18, 1097. [Google Scholar] [CrossRef]
- Niemann, B.; Li, L.; Siegler, D.; Siegler, B.H.; Knapp, F.; Hanna, J.; Aslam, M.; Kracht, M.; Schulz, R.; Rohrbach, S. CTRP9 Mediates Protective Effects in Cardiomyocytes via AMPK- and Adiponectin Receptor-Mediated Induction of Anti-Oxidant Response. Cells 2020, 9, 1229. [Google Scholar] [CrossRef]
- Song, C.X.; Chen, J.Y.; Li, N.; Guo, Y. CTRP9 Enhances Efferocytosis in Macrophages via MAPK/Drp1-Mediated Mitochondrial Fission and Adi-poR1-Induced Immunometabolism. J. Inflamm. Res. 2021, 14, 1007–1017. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.H.; Kim, H.S.; Cho, Y.K.; La Lee, Y.; Lee, W.J.; Park, J.-Y.; Jung, C.H. C1q/TNF-related protein-9 attenuates palmitic acid-induced endothelial cell senescence via increasing autophagy. Mol. Cell. Endocrinol. 2021, 521, 111114. [Google Scholar] [CrossRef]
- Sun, Y.; Yi, W.; Yuan, Y.; Lau, W.B.; Yi, D.; Wang, X.; Wang, Y.; Su, H.; Wang, X.; Gao, E.; et al. C1q/Tumor Necrosis Factor–Related Protein-9, a Novel Adipocyte-Derived Cytokine, Attenuates Adverse Remodeling in the Ischemic Mouse Heart via Protein Kinase a Activation. Circulation 2013, 128 (Suppl. 1), S113–S120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hang, T.; Cheng, X.M.; Li, D.M.; Zhang, Q.G.; Wang, L.J.; Peng, Y.P.; Gong, J.B. Associations of C1q/TNF-Related Protein-9 Levels in Serum and Epicardial Adipose Tissue with Coronary Atherosclerosis in Humans. Biomed. Res. Int. 2015, 2015, 971683. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Fadaei, R.; Emamgholipour, S.; Kazemian, E.; Panahi, G.; Vahedi, S.; Saed, L.; Fallah, S. Association of circulating CTRP9 with soluble adhesion molecules and inflammatory markers in patients with type 2 diabetes mellitus and coronary artery disease. PLoS ONE 2018, 13, e0192159. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhao, S.; Lian, K.; Mi, B.; Si, R.; Tan, Z.; Fu, F.; Wang, S.; Wang, R.; Ma, X.; et al. C1q/TNF-related protein 3 (CTRP3) and 9 (CTRP9) concentrations are decreased in patients with heart failure and are associated with increased morbidity and mortality. BMC Cardiovasc. Disord. 2019, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, N.; Adachi, H.; Nomura-Nakayama, K.; Okada, K.; Okino, K.; Hayashi, N.; Fujimoto, K.; Furuichi, K.; Yokoyama, H. Circulating CTRP9 correlates with the prevention of aortic calcification in renal allograft recipients. PLoS ONE 2020, 15, e0226526. [Google Scholar] [CrossRef]
- Pan, J.; Cui, X.; Wang, G.; Xue, K.; Hu, J.; Zhou, L. Predictive value of serum CTRP9 and STIM1 for restenosis after cerebrovascular stent implantation and its relationship with vasoactive substances and inflammatory cytokines. Exp. Ther. Med. 2020, 20, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Luo, X.; Ji, Y.; Xie, J.; Jiang, H.; Fu, M.; Li, X. Circulating CTRP9 levels are increased in patients with newly diagnosed type 2 diabetes and correlated with insulin resistance. Diabetes Res. Clin. Pract. 2017, 131, 116–123. [Google Scholar] [CrossRef]
- Hwang, Y.-C.; Oh, S.W.; Park, S.-W.; Park, C.-Y. Association of serum C1q/TNF-Related Protein-9 (CTRP9) concentration with visceral adiposity and metabolic syndrome in humans. Int. J. Obes. 2014, 38, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.M.; Steele, K.E.; Peterson, L.A.; Zeng, X.; Jaffe, A.; Schweitzer, M.A.; Magnuson, T.H.; Wong, G.W. C1q/TNF-Related Protein-9 (CTRP9) Levels Are Associated with Obesity and Decrease Following Weight Loss Surgery. J. Clin. Endocrinol. Metab. 2016, 101, 2211–2217. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, H.; Shi, Q.; Zhang, X.; Wang, C.; Lin, G. Protective Role of CTRP3 and CTRP9 in the Development of Gestational Diabetes Mellitus. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, D.; Chen, Y.; Ma, Y.; Shi, X.; Wang, X.; Lv, Y.; Yuan, H. Association of serum CTRP9 levels with cardiac autonomic neuropathy in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2021, 12, 1442–1451. [Google Scholar] [CrossRef]
- Asada, M.; Morioka, T.; Yamazaki, Y.; Kakutani, Y.; Kawarabayashi, R.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shioi, A.; Shoji, T.; et al. Plasma C1q/TNF-Related Protein-9 Levels are Associated with Atherosclerosis in Patients with Type 2 Diabetes without Renal Dysfunction. J. Diabetes Res. 2016, 2016, 8624313. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Jang, J.E.; Leem, J.; La Lee, Y.; Seol, S.M.; Yoon, H.K.; Lee, W.J.; Park, J.-Y. Association of Serum C1q/TNF-Related Protein-9 Concentration with Arterial Stiffness in Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E2477–E2484. [Google Scholar] [CrossRef][Green Version]
- Na, N.; Ji, M. Role of First-Trimester Serum C1q/TNF-Related Protein 9 in Gestational Diabetes Mellitus. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, L.; Liu, X.; Meng, X. CTRP3 Alleviates Ox-LDL-Induced Inflammatory Response and Endothelial Dysfunction in Mouse Aortic Endo-thelial Cells by Activating the PI3K/Akt/eNOS Pathway. Inflammation 2019, 42, 1350–1359. [Google Scholar] [CrossRef]
- Fadaei, R.; Moradi, N.; Kazemi, T.; Chamani, E.; Azdaki, N.; Moezibady, S.A.; Shahmohamadnejad, S.; Fallah, S. Decreased serum levels of CTRP12/adipolin in patients with coronary artery disease in relation to inflammatory cytokines and insulin resistance. Cytokine 2019, 113, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, J.-J.; Deng, W.-Y.; Ren, K.; Yin, S.-H.; Yu, X.-H. CTRP12 ameliorates atherosclerosis by promoting cholesterol efflux and inhibiting inflammatory response via the miR-155-5p/LXRα pathway. Cell Death Dis. 2021, 12, 254. [Google Scholar] [CrossRef]
- Peterson, J.; Wei, Z.; Wong, G.W. C1q/TNF-related Protein-3 (CTRP3), a Novel Adipokine That Regulates Hepatic Glucose Output. J. Biol. Chem. 2010, 285, 39691–39701. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Peterson, J.M.; Wong, G.W. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): Activation of AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J. Biol. Chem. 2011, 286, 15652–15665. [Google Scholar] [CrossRef]
- Wei, Z.; Peterson, J.; Lei, X.; Cebotaru, L.; Wolfgang, M.J.; Baldeviano, G.C.; Wong, G.W. C1q/TNF-related Protein-12 (CTRP12), a Novel Adipokine That Improves Insulin Sensitivity and Glycemic Control in Mouse Models of Obesity and Diabetes*. J. Biol. Chem. 2012, 287, 10301–10315. [Google Scholar] [CrossRef]
- Ogawa, H.; Ohashi, K.; Ito, M.; Shibata, R.; Kanemura, N.; Yuasa, D.; Kambara, T.; Matsuo, K.; Hayakawa, S.; Hiramatsu-Ito, M.; et al. Adipolin/CTRP12 protects against pathological vascular remodelling through suppression of smooth muscle cell growth and macrophage inflammatory response. Cardiovasc. Res. 2020, 116, 237–249. [Google Scholar] [CrossRef]
- Wang, C.; Xu, W.; Liang, M.; Huang, D.; Huang, K. CTRP13 inhibits atherosclerosis via autophagy-lysosome-dependent degradation of CD36. FASEB J. 2019, 33, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, L.; Feng, P.; Tan, Y.; Zhang, B.; Gao, E.; Wang, X.; Fan, C.; Wang, X.; Yi, W.; et al. C1q/tumor necrosis factor-related protein-3-engineered mesenchymal stromal cells attenuate cardiac impairment in mice with myocardial infarction. Cell Death Dis. 2019, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lei, H.; Wang, J.Y.; Zhang, C.L.; Feng, H.; Fu, F.Y.; Li, L.; Wu, L.L. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J. Mol. Med. 2015, 93, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Sun, Y.; Yuan, Y.; Lau, W.B.; Zheng, Q.; Wang, X.; Wang, Y.; Shang, X.; Gao, E.; Koch, W.J.; et al. C1q/Tumor Necrosis Factor-Related Protein-3, a Newly Identified Adipokine, Is a Novel Antiapoptotic, Proangiogenic, and Cardioprotective Molecule in the Ischemic Mouse Heart. Circulation 2012, 125, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-G.; Yuan, Y.-P.; Zhang, X.; Xu, S.-C.; Kong, C.-Y.; Song, P.; Li, N.; Tang, Q.-Z. C1q-tumour necrosis factor-related protein-3 exacerbates cardiac hypertrophy in mice. Cardiovasc. Res. 2019, 115, 1067–1077. [Google Scholar] [CrossRef]
- Takikawa, T.; Ohashi, K.; Ogawa, H.; Otaka, N.; Kawanishi, H.; Fang, L.; Ozaki, Y.; Eguchi, S.; Tatsumi, M.; Takefuji, M.; et al. Adipolin/C1q/Tnf-related protein 12 prevents adverse cardiac remodeling after myocardial infarction. PLoS ONE 2020, 15, e0243483. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Wu, Y.; Yang, J.; Xu, L.; Yang, Y. Serum CTRP3 level is inversely associated with nonalcoholic fatty liver disease: A 3-y longitudinal study. Clin. Chim. Acta 2018, 479, 79–83. [Google Scholar] [CrossRef]
- Choi, K.M.; Hwang, S.Y.; Hong, H.C.; Yang, S.J.; Choi, H.Y.; Yoo, H.J.; Lee, K.W.; Nam, M.S.; Park, Y.S.; Woo, J.T.; et al. C1q/TNF-Related Protein-3 (CTRP-3) and Pigment Epithelium-Derived Factor (PEDF) Concentrations in Patients with Type 2 Diabetes and Metabolic Syndrome. Diabetes 2012, 61, 2932–2936. [Google Scholar] [CrossRef]
- Moradi, N.; Fadaei, R.; Khamseh, M.E.; Nobakht, A.; Rezaei, M.J.; Aliakbari, F.; Vatannejad, A.; Hosseini, J. Serum levels of CTRP3 in diabetic nephropathy and its relationship with insulin resistance and kidney function. PLoS ONE 2019, 14, e0215617. [Google Scholar] [CrossRef]
- Flehmig, G.; Scholz, M.; Klöting, N.; Fasshauer, M.; Tönjes, A.; Stumvoll, M.; Youn, B.-S.; Blüher, M. Identification of Adipokine Clusters Related to Parameters of Fat Mass, Insulin Sensitivity and Inflammation. PLoS ONE 2014, 9, e99785. [Google Scholar] [CrossRef]
- Tan, B.K.; Chen, J.; Hu, J.; Amar, O.; Mattu, H.S.; Ramanjaneya, M.; Patel, V.; Lehnert, H.; Randeva, H.S. Circulatory changes of the novel adipokine adipolin/CTRP12 in response to metformin treatment and an oral glucose challenge in humans. Clin. Endocrinol. 2014, 81, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Shanaki, M.; Fadaei, R.; Moradi, N.; Emamgholipour, S.; Poustchi, H. The Circulating CTRP13 in Type 2 Diabetes and Non-Alcoholic Fatty Liver Patients. PLoS ONE 2016, 11, e0168082. [Google Scholar] [CrossRef]
- Choi, K.M.; Hwang, S.Y.; Hong, H.C.; Choi, H.Y.; Yoo, H.J.; Youn, B.-S.; Baik, S.H.; Seo, H.S. Implications of C1q/TNF-related protein-3 (CTRP-3) and progranulin in patients with acute coronary syndrome and stable angina pectoris. Cardiovasc. Diabetol. 2014, 13, 14. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, M.; Ye, J.; Liu, J.; Wang, Z.; Xu, Y.; Ye, D.; Wan, J. Serum Levels of Complement-C1q/Tumor Necrosis Factor-Related Protein-3 Decreased in Patients with Acute Aortic Dissection. Am. J. Cardiol. 2018, 122, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Sumbul, H.E.; Koca, H.; Kucukosmanoglu, M.; Icen, Y.K.; Koc, M. Complement C1q/Tumor Necrosis Factor-Related Protein-3 (CTRP3) is Significantly Decreased in Patients with Heart Failure and Closely Related with Ventricular Tachycardia. Acta. Cardiol. Sin. 2021, 37, 278–285. [Google Scholar] [PubMed]
- Shahraki, Z.N.; Azimi, H.; Ilchi, N.; Borj, M.R.; Pourghadamyari, H.; Mosallanejad, S.; Abbaszadeh-Goudarzi, K.; Sahebkar, A. Circulating C1q/TNF-related protein-12 levels are associated with the severity of coronary artery disease. Cytokine 2021, 144, 155545. [Google Scholar] [CrossRef]
- Schumaker, V.N.; Zavodszky, P.; Poon, P.H. Activation of the first component of complement. Annu. Rev. Immunol. 1987, 5, 21–42. [Google Scholar] [CrossRef]
- Hirata, A.; Kishida, K.; Kobayashi, H.; Nakatsuji, H.; Funahashi, T.; Shimomura, I. Correlation between serum C1q-adiponectin/total adiponectin ratio and polyvascular lesions detected by vascular ultrasonography in Japanese type 2 diabetics. Metabolism 2013, 62, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Kishida, K.; Nakatsuji, H.; Kobayashi, H.; Funahashi, T.; Shimomura, I. High serum C1q-adiponectin/total adiponectin ratio correlates with coronary artery disease in Japanese type 2 diabetics. Metabolism 2013, 62, 578–585. [Google Scholar] [CrossRef]
- Kishida, K.; Nakagawa, Y.; Kobayashi, H.; Yanagi, K.; Funahashi, T.; Shimomura, I. Increased serum C1q-binding adiponectin complex to total-adiponectin ratio in men with multi-vessel coronary disease. Diabetol. Metab. Syndr. 2014, 6, 64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, E.S.; Lim, C.; Choi, H.Y.; Ku, E.J.; Kim, K.M.; Moon, J.H.; Lim, S.; Park, K.S.; Jang, H.C.; Choi, S.H. The amount of C1q–adiponectin complex is higher in the serum and the complex localizes to perivascular areas of fat tissues and the intimal–medial layer of blood vessels of coronary artery disease patients. Cardiovasc. Diabetol. 2015, 14, 50. [Google Scholar] [CrossRef]
- Bilkovski, R.; Schulte, D.M.; Oberhauser, F.; Mauer, J.; Hampel, B.; Gutschow, C.; Krone, W.; Laudes, M. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int. J. Obes. 2011, 35, 1450–1454. [Google Scholar] [CrossRef]
- Pashirzad, M.; Shafiee, M.; Rahmani, F.; Behnam-Rassouli, R.; Hoseinkhani, F.; Ryzhikov, M.; Binabaj, M.M.; Parizadeh, M.R.; Avan, A.; Hassanian, S.M. Role of Wnt5a in the Pathogenesis of Inflammatory Diseases. J. Cell. Physiol. 2017, 232, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 Is an Anti-Inflammatory Adipokine That Modulates Metabolic Dysfunction in Obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Rydzewska, M.; Nikołajuk, A.; Matulewicz, N.; Stefanowicz, M.; Karczewska-Kupczewska, M. Serum secreted frizzled-related protein 5 in relation to insulin sensitivity and its regulation by insulin and free fatty acids. Endocrine 2021, 74, 300–307. [Google Scholar] [CrossRef]
- Tong, S.; Ji, Q.; Du, Y.; Zhu, X.; Zhu, C.; Zhou, Y. Sfrp5/Wnt Pathway: A Protective Regulatory System in Atherosclerotic Cardiovascular Disease. J. Interferon Cytokine Res. 2019, 39, 472–482. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Shen, C. Research update on the association between SFRP5, an anti-inflammatory adipokine, with obesity, type 2 diabetes mellitus and coronary heart disease. J. Cell. Mol. Med. 2020, 24, 2730–2735. [Google Scholar] [CrossRef]
- Sun, M.; Wang, W.; Min, L.; Chen, C.; Li, Q.; Weng, W. Secreted frizzled-related protein 5 (SFRP5) protects ATDC5 cells against LPS-induced inflammation and apoptosis via inhibiting Wnt5a/JNK pathway. J. Orthop. Surg. Res. 2021, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Prestwich, T.C.; Reid, M.; Longo, K.A.; Gerin, I.; Cawthorn, W.; Susulic, V.S.; Krishnan, V.; Greenfield, A.; MacDougald, O. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J. Clin. Investig. 2012, 122, 2405–2416. [Google Scholar] [CrossRef]
- Carstensen-Kirberg, M.; Röhrig, K.; Niersmann, C.; Ouwens, D.M.; Belgardt, B.F.; Roden, M.; Herder, C. Sfrp5 increases glucose-stimulated insulin secretion in the rat pancreatic beta cell line INS-1E. PLoS ONE 2019, 14, e0213650. [Google Scholar] [CrossRef]
- Li, Y.; Tian, M.; Yang, M.; Yang, G.; Chen, J.; Wang, H.; Liu, D.; Wang, H.; Deng, W.; Zhu, Z.; et al. Central Sfrp5 regulates hepatic glucose flux and VLDL-triglyceride secretion. Metabolism 2020, 103, 154029. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, Q.; Jiang, F.; Xue, L.; Li, J.; Fan, Z.; Chen, P.; Chen, G.; Cai, Y. Secreted frizzled-related protein 5 protects against oxidative stress-induced apoptosis in human aortic endothelial cells via downregulation of Bax. J. Biochem. Mol. Toxicol. 2017, 31, e21978. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ji, Y.; Chu, H.; Wang, M.; Yang, B.; Yin, C. SFRP5 mediates downregulation of the wnt5a/caveolin-1/JNK signaling pathway. J. Endocrinol. 2020, 247, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Kang, Y.M.; Lee, S.E.; La Lee, Y.; Seol, S.M.; Lee, W.J.; Park, J.-Y.; Jung, C.H. Effect of SFRP5 (Secreted Frizzled–Related Protein 5) on the WNT5A (Wingless-Type Family Member 5A)-Induced Endothelial Dysfunction and Its Relevance with Arterial Stiffness in Human Subjects. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Teliewubai, J.; Ji, H.; Lu, Y.; Bai, B.; Yu, S.; Chi, C.; Xu, Y.; Zhang, Y. SFRP5 serves a beneficial role in arterial aging by inhibiting the proliferation, migration and inflammation of smooth muscle cells. Mol. Med. Rep. 2018, 18, 4682–4690. [Google Scholar] [CrossRef]
- Nakamura, K.; Sano, S.; Fuster, J.J.; Kikuchi, R.; Shimizu, I.; Ohshima, K.; Katanasaka, Y.; Ouchi, N.; Walsh, K. Secreted Frizzled-related Protein 5 Diminishes Cardiac Inflammation and Protects the Heart from Ischemia/Reperfusion Injury. J. Biol. Chem. 2016, 291, 2566–2575. [Google Scholar] [CrossRef]
- Bie, Z.-D.; Sun, L.-Y.; Geng, C.-L.; Meng, Q.-G.; Lin, X.-J.; Wang, Y.-F.; Wang, X.-B.; Yang, J. MiR-125b regulates SFRP5 expression to promote growth and activation of cardiac fibroblasts. Cell Biol. Int. 2016, 40, 1224–1234. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Sanna, F.; Margaritis, M.; Badi, I.; Akawi, N.; Herdman, L.; Coutinho, P.; Fagan, H.; Antonopoulos, A.S.; Oikonomou, E.K.; et al. Adipose tissue-derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci. Transl. Med. 2019, 11, eaav5055. [Google Scholar] [CrossRef] [PubMed]
- Carstensen-Kirberg, M.; Kannenberg, J.M.; Huth, C.; Meisinger, C.; Koenig, W.; Heier, M.; Peters, A.; Rathmann, W.; Roden, M.; Herder, C.; et al. Inverse associations between serum levels of secreted frizzled-related protein-5 (SFRP5) and multiple cardiometabolic risk factors: KORA F4 study. Cardiovasc. Diabetol. 2017, 16, 109. [Google Scholar] [CrossRef]
- Bai, Y.; Du, Q.; Jiang, R.; Zhang, L.; Du, R.; Wu, N.; Li, P.; Li, L. Secreted Frizzled-Related Protein 5 is Associated with Glucose and Lipid Metabolism Related Metabolic Syndrome Components Among Adolescents in Northeastern China. Diabetes Metab. Syndr. Obes. 2021, 14, 2735–2742. [Google Scholar] [CrossRef]
- Tan, X.; Wang, X.; Chu, H.; Liu, H.; Yi, X.; Xiao, Y. SFRP5 correlates with obesity and metabolic syndrome and increases after weight loss in children. Clin. Endocrinol. 2014, 81, 363–369. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, H. Correlation Between Circulating Levels of Secreted Frizzled-Related Protein 5 and Type 2 Diabetic Patients and Subjects with Impaired-Glucose Regulation. Diabetes Metab. Syndr. Obes. 2020, 13, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, L.; Yang, M.; Luo, X.; Ran, W.; Liu, D.; Xiong, Z.; Liu, H.; Yang, G. Circulating Sfrp5 Is a Signature of Obesity-Related Metabolic Disorders and Is Regulated by Glucose and Liraglutide in Humans. J. Clin. Endocrinol. Metab. 2013, 98, 290–298. [Google Scholar] [CrossRef]
- Almario, R.U.; Karakas, S.E. Roles of Circulating WNT-Signaling Proteins and WNT-Inhibitors in Human Adiposity, Insulin Resistance, Insulin Secretion, and Inflammation. Horm. Metab. Res. 2015, 47, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, H.; Li, Y.; Wang, J.; Lai, Y.; Gao, L.; Lei, L.; Yang, G.; Liao, X.; Fang, X.; et al. Plasma Sfrp5 levels correlate with determinants of the metabolic syndrome in Chinese adults. Diabetes Metab. Res. Rev. 2017, 33, e2896. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Wang, C.P.; Hsu, C.C.; Chiu, C.A.; Yu, T.H.; Hung, W.C.; Lu, L.F.; Chung, F.M.; Tsai, I.T.; Lin, H.C.; et al. Circulating secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2013, 29, 551–556. [Google Scholar]
- Wang, B.; Pan, Y.; Yang, G.; Yu, W.; Liu, H.; Bai, B. Sfrp5/Wnt5a and leptin/adiponectin levels in the serum and the periarterial adipose tissue of patients with peripheral arterial occlusive disease. Clin. Biochem. 2021, 87, 46–51. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, H.; Kim, A.J.; Ro, H.; Chang, J.H.; Lee, H.H.; Chung, W.; Jun, H.-S.; Jung, J.Y. Reduction of Secreted Frizzled-Related Protein 5 Drives Vascular Calcification through Wnt3a-Mediated Rho/ROCK/JNK Signaling in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 3539. [Google Scholar] [CrossRef]
- Tong, S.; Du, Y.; Ji, Q.; Dong, R.; Cao, J.; Wang, Z.; Li, W.; Zeng, M.; Chen, H.; Zhu, X.; et al. Expression of Sfrp5/Wnt5a in human epicardial adipose tissue and their relationship with coronary artery disease. Life Sci. 2020, 245, 117338. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Y.; Zhu, Y.; Hu, C.; Zhang, J.; Ji, Q.; Liu, W.; Han, H.; Yang, L.; Zhang, D.; et al. High Serum Secreted Frizzled-Related Protein 5 Levels Associates with Early Improvement of Cardiac Function Following ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. J. Atheroscler. Thromb. 2019, 26, 868–878. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, H.; Liu, X.; Chen, P.; Zhang, Y.; Luo, J.; Kuang, J.; Li, J.; Yang, Y.; Ma, T.; et al. Prognostic Value of Secreted Frizzled-Related Protein 5 in Heart Failure Patients with and Without Type 2 Diabetes Mellitus. Circ. Heart Fail. 2020, 13, e007054. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, H.; Zhuang, J.; Su, Y.; Wen, J.; Zhang, J.; Zhao, D.; Zhang, Y.; Xu, Y. High serum level of secreted frizzled-related protein 5 (sfrp5) is associated with future cardiovascular events. Cardiovasc. Ther. 2017, 2, e115. [Google Scholar]
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef] [PubMed]
- De Souza Batista, C.M.; Yang, R.-Z.; Lee, M.-J.; Glynn, N.M.; Yu, D.-Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr. Physiol. 2017, 7, 765–781. [Google Scholar] [PubMed]
- Hiramatsu-Ito, M.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kanemura, N.; Kambara, T.; Enomoto, T.; Yuasa, D.; Matsuo, K.; Ito, M.; et al. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2016, 110, 107–117. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.-A.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; et al. Counteractive effects of omentin-1 against atherogenesis. Cardiovasc. Res. 2016, 110, 118–128. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Kanemura, N.; Ohashi, K.; Kambara, T.; Hiramatsu-Ito, M.; Enomoto, T.; Yuasa, D.; Joki, Y.; Matsuo, K.; et al. Adipose-derived protein omentin prevents neointimal formation after arterial injury. FASEB J. 2015, 29, 141–151. [Google Scholar] [CrossRef]
- Xie, H.; Xie, P.-L.; Wu, X.-P.; Chen, S.-M.; Zhou, H.-D.; Yuan, L.-Q.; Sheng, Z.-F.; Tang, S.-Y.; Luo, X.-H.; Liao, E.-Y. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc. Res. 2011, 92, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Queiroz, M.; Azul, L.; Seiça, R.; Sena, C.M. Omentin: A novel therapeutic approach for the treatment of endothelial dysfunction in type 2 diabetes. Free. Radic. Biol. Med. 2021, 162, 233–242. [Google Scholar] [CrossRef]
- Yamawaki, H.; Tsubaki, N.; Mukohda, M.; Okada, M.; Hara, Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem. Biophys. Res. Commun. 2010, 393, 668–672. [Google Scholar] [CrossRef]
- Maruyama, S.; Shibata, R.; Kikuchi, R.; Izumiya, Y.; Rokutanda, T.; Araki, S.; Kataoka, Y.; Ohashi, K.; Daida, H.; Kihara, S.; et al. Fat-derived Factor Omentin Stimulates Endothelial Cell Function and Ischemia-induced Revascularization via Endothelial Nitric Oxide Synthase-dependent Mechanism. J. Biol. Chem. 2012, 287, 408–417. [Google Scholar] [CrossRef]
- Kataoka, Y.; Shibata, R.; Ohashi, K.; Kambara, T.; Enomoto, T.; Uemura, Y.; Ogura, Y.; Yuasa, D.; Matsuo, K.; Nagata, T.; et al. Omentin Prevents Myocardial Ischemic Injury Through AMP-Activated Protein Kinase- and Akt-Dependent Mechanisms. J. Am. Coll. Cardiol. 2014, 63, 2722–2733. [Google Scholar] [CrossRef]
- Matsuo, K.; Shibata, R.; Ohashi, K.; Kambara, T.; Uemura, Y.; Hiramatsu-Ito, M.; Enomoto, T.; Yuasa, D.; Joki, Y.; Ito, M.; et al. Omentin functions to attenuate cardiac hypertrophic response. J. Mol. Cell. Cardiol. 2015, 79, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xia, F.; Dong, J.; Lin, T.; Cai, Y.; Chen, J.; Chen, X.; Huang, Z.; Wang, Q.; Chen, H.; et al. Omentin-1 attenuates glucocorticoid-induced cardiac injury by phosphorylating GSK3β. J. Mol. Endocrinol. 2021, 66, 273–283. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, L.; Zheng, M.; Fan, C.; Li, Y.; Zhang, D.; He, Y.; Yang, H. Changes of serum omentin-1 levels in normal subjects, type 2 diabetes and type 2 diabetes with overweight and obesity in Chinese adults. Ann. Endocrionol 2014, 75, 171–175. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Kaur, H.; Adams-Huet, B.; Bremer, A.A. Increased Chemerin and Decreased Omentin-1 in Both Adipose Tissue and Plasma in Nascent Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E514–E517. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cano, M.I.; Valencia-Ortega, J.; Morales-Ávila, E.; Díaz-Velázquez, M.F.; Gómez-Díaz, R.; Saucedo, R. Omentin-1 and its relationship with inflammatory factors in maternal plasma and visceral adipose tissue of women with gestational diabetes mellitus. J. Endocrinol. Investig. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.H.E.; Anwar, S.; Gautham, K.S.; Kadurei, F.; Ojo, R.O.; Hafizyar, F.; Haroon, D.M.; Rakesh, F.; Talpur, A.S. Association of Plasma Omentin-1 Levels with Diabetes and Its Complications. Cureus 2021, 13, e18203. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Bonadia, N.; Angelini, F.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Association between plasma omentin-1 levels in type 2 diabetic patients and peripheral artery disease. Cardiovasc. Diabetol. 2019, 18, 74–77. [Google Scholar] [CrossRef]
- Senthilkumar, G.P.; Anithalekshmi, M.S.; Yasir, M.; Parameswaran, S.; Packirisamy, R.M.; Bobby, Z. Role of omentin 1 and IL-6 in type 2 diabetes mellitus patients with diabetic nephropathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 23–26. [Google Scholar] [CrossRef] [PubMed]
- El-Mesallamy, H.O.; El-Derany, M.O.; Hamdy, N.M. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabet. Med. 2011, 28, 1194–1200. [Google Scholar] [CrossRef]
- Bai, P.; Abdullah, F.; Lodi, M.; Sarhadi, M.; Dilip, A.; Shahab, S.; Yasir, F.; Jahangir, M. Association between Coronary Artery Disease and Plasma Omentin-1 Levels. Cureus 2021, 13, e17347. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, H.-Y.; Tan, H.; Zhou, Y.; Liu, F.-L.; Chen, F.-Q.; Shang, D.-Y. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol. Sin. 2011, 32, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Angelini, F.; Cina, A.; Iezzi, R.; Filipponi, M.; Santoliquido, A.; Pitocco, D.; et al. Association between omentin-1 and major cardiovascular events after lower extremity endovascular revascu-larization in diabetic patients: A prospective cohort study. Cardiovasc. Diabetol. 2020, 19, 170. [Google Scholar] [CrossRef]
- Narumi, T.; Watanabe, T.; Kadowaki, S.; Kinoshita, D.; Yokoyama, M.; Honda, Y.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; et al. Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovasc. Diabetol. 2014, 13, 84. [Google Scholar] [CrossRef]
- Çelik, M.; Nar, R.; Nar, G.; Sökmen, E.; Günver, G. Serum omentin-1 levels in hypertensive patients. J. Hum. Hypertens 2021, 35, 290–295. [Google Scholar] [CrossRef]
- Xu, T.; Zuo, P.; Wang, Y.; Gao, Z.; Ke, K. Serum omentin-1 is a novel biomarker for predicting the functional outcome of acute ischemic stroke patients. Clin. Chem. Lab. Med. 2018, 56, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, Y. Clinical relevance of serum omentin-1 levels as a biomarker of prognosis in patients with acute cerebral infarction. Brain Behav. 2020, 10, e01678. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zuo, P.; Cao, L.; Gao, Z.; Ke, K. Omentin-1 is Associated with Carotid Plaque Instability among Ischemic Stroke Patients. J. Atheroscler. Thromb. 2018, 25, 505–511. [Google Scholar] [CrossRef]
- Xu, T.; Li, Y.; Su, Y.; Zuo, P.; Gao, Z.; Ke, K. Serum omentin-1 and risk of one-year mortality in patients with ischemic stroke. Clin. Chim. Acta 2020, 505, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-H.; Ye, Z.-H.; Guan, H.-J.; Guo, M.; Zhou, X.-X.; Xu, Y.-Y. Impact of serum omentin-1 concentrations on functional outcome among acute intracerebral hemorrhage patients. Clin. Chim. Acta 2020, 503, 169–174. [Google Scholar] [CrossRef]
- Onur, I.; Oz, F.; Yildiz, S.; Oflaz, H.; Sigirci, S.; Elitok, A.; Pilten, S.; Karaayvaz, E.B.; Cizgici, A.Y.; Kaya, M.G.; et al. Serum Omentin 1 Level Is Associated with Coronary Artery Disease and Its Severity in Postmenopausal Women. Angiology 2014, 65, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-P.; Tong, X.-Y.; Zhu, L.-P.; Luo, J.-M.; Luo, Y.; Bai, Y.-P.; Li, C.-C.; Zhang, G.-G. Plasma Omentin-1 Level as a Predictor of Good Coronary Collateral Circulation. J. Atheroscler. Thromb. 2017, 24, 940–948. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hwang, S.Y.; Hong, H.C.; Choi, H.Y.; Yang, S.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Onur, I.; Oz, F.; Yildiz, S.; Kuplay, H.; Yucel, C.; Sigirci, S.; Elitok, A.; Pilten, S.; Kasali, K.; Cizgici, A.Y.; et al. A decreased serum omentin-1 level may be an independent risk factor for peripheral arterial disease. Int Angiol. 2014, 33, 455–460. [Google Scholar]
- Yasir, M.; Senthilkumar, G.P.; Jayashree, K.; Babu, K.R.; Vadivelan, M.; Palanivel, C. Association of serum omentin-1, apelin and chemerin concentrations with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus patients. Arch. Physiol. Biochem. 2019, 1–8. [Google Scholar] [CrossRef]
- Siegrist, M.; Heitkamp, M.; Braun, I.; Vogg, N.; Haller, B.; Langhof, H.; Koenig, W.; Halle, M. Changes of omentin-1 and chemerin during 4 weeks of lifestyle intervention and 1 year follow-up in children with obesity. Clin. Nutr. 2021, 40, 5648–5654. [Google Scholar] [CrossRef]
- Atashak, S.; Stannard, S.R.; Daraei, A.; Soltani, M.; Saeidi, A.; Moradi, F.; Laher, I.; Hackney, A.C.; Zouhal, H. High-intensity Interval Training Improves Lipocalin-2 and Omentin-1 Levels in Men with Obesity. Int. J. Sports Med. 2021, 1–16. [Google Scholar] [CrossRef]
- De Luis, D.A.; Calvo, S.G.; Gomez, J.J.L.; Izaola, O.; Primo, D.; Pacheco, D.; Aller, R. Omentin-1 Changes following Biliopancreatic Diversion and Relationship with Cardiovascular Risk Factors. Ann. Nutr. Metab. 2018, 73, 106–112. [Google Scholar] [CrossRef]
- Alkuraishy, H.M.; Al-Gareeb, A.I. New Insights into the Role of Metformin Effects on Serum Omentin-1 Levels in Acute My-ocardial Infarction: Cross-Sectional Study. Emerg. Med. Int. 2015, 2015, 283021. [Google Scholar] [CrossRef] [PubMed]

| Author, Year [ref] * | Study Design † | Participants | Mean Age (years) | Men (%) | Ethnicity ‡ | Outcomes or Parameters | Cardiometabolic Health Association § | |
|---|---|---|---|---|---|---|---|---|
| Adiponectin | Gariballa et al., 2019 [35] | PC | 193 overweight and obese subjects | 36.0 | 7.0 | Asian | Visceral fat | Positive |
| Lindberg et al., 2014 [37] | PC | 666 patients with STEMI, without diabetes | 63.5 | 74.3 | White | Incident T2DM | Positive | |
| Kou et al., 2018 [38] | CC | 309 subjects | N/R | N/R | Asian | Atherogenic index of plasma | Positive | |
| Yoshida et al., 2005 [40] | CS | 56 patients with T2DM | N/R | N/R | Asian | VLDL levels | Positive | |
| Tomono et al., 2018 [41] | CS | 174 subjects without diabetes | 67.9 | 45.4 | Asian | HDL levels | Positive | |
| Doumatey et al., 2012 [44] | CS | 822 subjects | 43.3 | 44.3 | Black | Obesity and MetS | Positive | |
| Garvey et al., 2014 [48] | RC | 475 subjects with prediabetes and/or MetS | 52.0 | 35.2 | Multiracial | Phentermine/topiramate | Positive | |
| Wannamethee et al., 2007 [51] | PC | 4046 men | 68.7 | 100 | White | All-cause and CVD mortality | Negative | |
| McEntegart et al., 2007 [52] | CS | 47 patients with CAD | 67.3 | 83.0 | White | HF and cachexia | Negative | |
| Vaspin | Taheri et al., 2020 [75] | CS | 70 women | 29.0 | 0 | Asian | Obesity | Negative |
| Hao et al., 2016 [77] | CS | 348 subjects | 52.8 | 58.0 | Asian | T2DM and CAD | Negative | |
| Yang et al., 2021 [78] | CS | 372 patients with T2DM | 53.0 | 55.6 | Asian | Diabetic retinopathy | Negative | |
| Jia et al., 2018 [79] | RC | 474 patients with NAFLD | N/R | N/R | Asian | Exercise | Negative | |
| Tan et al., 2008 [80] | NRI | 21 women with PCOS | 28.0 | 0 | White | Metformin | Negative | |
| Zhang et al., 2011 [81] | NRI | 31 patients with T2DM | 55.3 | 35.5 | Asian | Rosiglitazone | Negative | |
| Golpaie et al., 2011 [82] | NRI | 30 obese subjects | 32.5 | 30.0 | Asian | Restrictive bariatric surgery | Negative | |
| Kadoglou et al., 2021 [84] | NRI | 84 subjects with preclinical carotid atherosclerosis | 62.0 | 46.4 | White | Atorvastatin | Positive | |
| Al-Kuraishy et al., 2018 [85] | RC | 110 patients with ACS | 48.6 | 65.5 | Asian | Rosuvastatin | Positive | |
| Kastl et al., 2020 [86] | PC | 85 patients with CAD | 64.0 | 77.6 | White | In-stent restenosis | Positive | |
| Zhang et al., 2016 [87] | PC | 80 patients with MI | 68.0 | 81.2 | Asian | MACE | Positive | |
| Ji et al., 2020 [88] | RC | 197 subjects with chest pain | 65.0 | 56.9 | Asian | MACE | Positive | |
| Rashad et al., 2020 [89] | PCC | 90 patients with T2DM | 58.7 | 55.6 | Asian | IS | Negative | |
| Cakal et al., 2011 [90] | CS | 71 women | N/R | 0 | Asian | Diabetogenic risk | Negative | |
| Esteghamati et al., 2014 [91] | CS | 145 subjects | 49.4 | 42.8 | Asian | MetS | Negative | |
| Buyukinan et al., 2018 [92] | CS | 121 obese children | N/R | 34.7 | Asian | MetS | Negative | |
| CTRP9 | Wang et al., 2015 [119] | CS | 362 subjects | 62.1 | 72.1 | Asian | CAD | Positive |
| Moradi et al., 2018 [120] | CS | 337 subjects | 58.0 | 70.0 | Asian | CAD and T2DM | Negative | |
| Gao et al., 2019 [121] | PC | 344 subjects | 56.2 | 69.2 | Asian | HFrEF | Positive | |
| Miyatake et al., 2020 [122] | RC | 50 recipients of kidney transplantation | 31.5 | 66.0 | Asian | Aortic calcification | Positive | |
| Pan et al., 2020 [123] | CS | 128 CI patients with cerebrovascular stent | 54.0 | 58.6 | Asian | Restenosis after cerebrovascular stent | Positive | |
| Jia et al., 2017 [124] | CS | 306 subjects | 52.0 | 48.0 | Asian | Incident T2DM and obesity | Negative | |
| Hwang et al., 2014 [125] | CS | 221 subjects | 46.0 | 63.3 | Asian | MetS | Positive | |
| Wolf et al., 2016 [126] | NRI | 21 obese subjects | N/R | 14.0 | Multiracial | Bariatric surgery | Negative | |
| Xia et al., 2020 [127] | CS | 259 pregnant women | N/R | 0 | Asian | GDM | Positive | |
| Yang et al., 2021 [128] | CS | 262 patients with T2DM | 55.0 | 68.3 | Asian | Cardiac autonomic neuropathy | Positive | |
| Asada et al., 2016 [129] | CS | 258 patients with T2DM without CKD | 62.0 | 54.3 | Asian | Carotid IMT | Negative | |
| Jung et al., 2014 [130] | CS | 278 patients with T2DM | 58.3 | 60.8 | Asian | baPWV | Negative | |
| Na et al., 2020 [131] | CS | 133 pregnant women | N/R | 0 | Asian | GDM | Positive | |
| CTRP3 | Zhou et al., 2018 [145] | PC | 313 subjects | N/R | N/R | Asian | Incident NAFLD | Positive |
| Choi et al., 2012 [146] | CS | 345 subjects | 51.8 | 38.2 | Asian | Prediabetes, T2DM, and MetS | Negative | |
| Moradi et al., 2019 [147] | CS | 164 subjects | 58.0 | 62.8 | Asian | T2DM and diabetic nephropathy | Positive | |
| Flehmig et al., 2014 [148] | CS | 141 obese subjects | 48.0 | 47.5 | White | Metformin | Positive | |
| Choi et al., 2014 [151] | CS | 362 subjects | 60.4 | 67.4 | Asian | ACS and SAP | Positive | |
| Jiang et al., 2018 [152] | CS | 108 subjects | 56.3 | 71.3 | Asian | Acute aortic dissection | Positive | |
| Yildirim et al., 2021 [153] | CS | 118 subjects | 64.4 | 66.1 | Asian | HFrEF and VT | Positive | |
| CTRP12 | Tan et al., 2014 [149] | NRI | 21 women with PCOS | 28.0 | 0 | White | Metformin | Positive |
| Nadimi et al., 2021 [154] | CS | 250 subjects | 58.5 | 54.8 | Asian | CAD severity | Positive | |
| CTRP13 | Shanaki et al., 2016 [150] | CS | 86 men | 54.0 | 100 | Asian | BMI, visceral fat, and IMT | Positive |
| SFRP5 | Akoumianakis et al., 2019 [177] | CC | 140 subjects | 64.0 | 45.0 | White | CAD | Positive |
| Carstensen-Kirberg et al., 2017 [178] | CS | 1096 subjects | 70.2 | 51.5 | White | Prediabetes, T2DM, BMI, and HDL-C | Positive | |
| Bai et al., 2021 [179] | CS | 684 adolescents | 13.7 | 54.2 | Asian | FPG and TC | Positive | |
| Tan et al., 2014 [180] | NRI | 31 obese children | 11.0 | 71.0 | Asian | Lifestyle intervention | Positive | |
| He et al., 2020 [181] | NRI | 111 patients with T2DM | 57.0 | 46.8 | Asian | Metformin | Positive | |
| Hu et al., 2013 [182] | NRI | 30 patients with T2DM | N/R | N/R | Asian | Liraglutide | Positive | |
| Almario et al., 2015 [183] | CS | 84 women | 36.1 | 0 | White | Weight and cholesterol | Positive | |
| Xu et al., 2017 [184] | CS | 284 subjects | 53.4 | 53.9 | Asian | MetS | Positive | |
| Lu et al., 2013 [185] | CS | 124 subjects | 59.5 | 56.5 | Asian | T2DM | Negative | |
| Wang et al., 2021 [186] | CS | 114 subjects | 67.3 | 51.8 | Asian | PAD | Positive | |
| Oh et al., 2020 [187] | CS | 120 subjects | 51.7 | 19.3 | Asian | Vascular calcification | Positive | |
| Cho et al., 2018 [173] | CS | 282 patients with T2DM | 58.0 | 63.6 | Asian | baPWV | Negative | |
| Tong et al., 2020 [188] | CS | 87 subjects | 61.5 | 56.3 | Asian | CAD | Positive | |
| Du et al., 2019 [189] | PC | 85 patients with STEMI | 55.7 | 76.5 | Asian | Early improvement of LVEF | Positive | |
| Wu et al., 2020 [190] | PC | 833 patients with HF | 65.9 | 57.4 | Asian | Composite of all-cause mortality or HF rehospitalization | Positive | |
| Ji et al., 2017 [191] | PC | 168 subjects | 65.0 | 55.4 | Asian | MACE | Negative | |
| Omentin-1 | Batista et al., 2007 [193] | CS | 91 subjects | 43.7 | 42.9 | White | Obesity | Positive |
| Zhang et al., 2014 [206] | CS | 120 subjects | 66.3 | 51.7 | Asian | T2DM and obesity | Positive | |
| Jialal et al., 2013 [207] | CS | 75 subjects | 51.6 | 78.7 | White | MetS | Positive | |
| Peña-Cano et al., 2021 [208] | CS | 231 pregnant women | 29.5 | 0 | Hispanic | GDM | Positive | |
| Latif et al., 2021 [209] | CS | 500 patients with T2DM | 53.0 | 52.0 | Asian | Diabetic complications | Positive | |
| Biscetti et al., 2019 [210] | S | 600 patients with T2DM | 74.7 | 68.2 | White | PAD | Positive | |
| Senthilkumar et al., 2018 [211] | CS | 82 patients with T2DM | 48.5 | N/R | Asian | Diabetic nephropathy | Positive | |
| El-Mesallamy et al., 2011 [212] | CS | 90 subjects | 57.3 | 74.4 | Asian | T2DM | Positive | |
| Bai et al., 2021 [213] | CS | 600 subjects | 52.5 | 61.2 | Asian | CAD | Positive | |
| Zhong et al., 2011 [214] | CS | 207 subjects | 61.2 | 69.1 | Asian | ACS and SAP | Positive | |
| Biscetti et al., 2020 [215] | PC | 207 patients with T2DM and CTLI | 75.0 | 69.6 | White | MACE and MALE | Positive | |
| Kataoka et al., 2014 [203] | CS | 20 patients with acute MI | 62.5 | 65.0 | Asian | Myocardial salvage index and EF | Positive | |
| Narumi et al., 2014 [216] | PC | 136 patients with HF | 72.0 | 55.9 | Asian | Cardiac death or HF rehospitalization | Positive | |
| Çelik et al., 2021 [217] | CS | 121 subjects | 49.6 | 48.8 | Asian | Hypertension | Positive | |
| Xu et al., 2018 [218] | PC | 266 patients with IS | N/R | N/R | Asian | Functional outcome | Positive | |
| Yang et al., 2020 [219] | PC | 109 patients with CI | 62.8 | 62.4 | Asian | Functional prognosis | Positive | |
| Xu et al., 2018 [220] | CS | 173 patients with IS | N/R | N/R | Asian | Unstable carotid plaque | Positive | |
| Xu et al., 2020 [221] | PC | 303 patients with IS | 66.8 | 64.7 | Asian | 1 year mortality | Positive | |
| Zhang et al., 2020 [222] | PC | 104 patients with hemorrhagic stroke | 68.0 | 54.8 | Asian | Functional outcome | Positive | |
| Onur et al., 2014 [223] | CS | 193 women | 67.3 | 0 | Asian | Angiographic CAD | Positive | |
| Zhou et al., 2017 [224] | CS | 142 patients with CAD | N/R | N/R | Asian | Coronary collateral circulation | Positive | |
| Yoo et al., 2011 [225] | CS | 90 subjects | 54.5 | 41.1 | Asian | T2DM and carotid plaque | Positive | |
| Onur et al., 2014 [226] | CS | 173 subjects | N/R | N/R | Asian | PAD | Positive | |
| Yasir et al., 2019 [227] | CS | 167 patients with T2DM | N/R | N/R | Asian | Diabetic retinopathy | Positive | |
| Siegrist et al., 2021 [228] | NRI | 156 overweight and obese children | 14.0 | 44.9 | White | Lifestyle intervention | Positive | |
| Atashak et al., 2021 [229] | RC | 30 obese men | N/R | 100 | Multiracial | Exercise | Positive | |
| Luis et al., 2018 [230] | NRI | 24 obese subjects | N/R | N/R | White | Biliopancreatic diversion surgery | Positive | |
| Kadoglou et al., 2021 [84] | NRI | 84 subjects with preclinical carotid atherosclerosis | 62.0 | 46.4 | White | Atorvastatin | Positive | |
| Alkuraishy et al., 2015 [231] | CS | 85 patients with T2DM and acute MI | 57.5 | 60.0 | White | Metformin | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.N.; Jung, C.H. The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin. Int. J. Mol. Sci. 2021, 22, 13529. https://doi.org/10.3390/ijms222413529
Jung HN, Jung CH. The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin. International Journal of Molecular Sciences. 2021; 22(24):13529. https://doi.org/10.3390/ijms222413529
Chicago/Turabian StyleJung, Han Na, and Chang Hee Jung. 2021. "The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin" International Journal of Molecular Sciences 22, no. 24: 13529. https://doi.org/10.3390/ijms222413529
APA StyleJung, H. N., & Jung, C. H. (2021). The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin. International Journal of Molecular Sciences, 22(24), 13529. https://doi.org/10.3390/ijms222413529






